Abstract
Synaptic adhesion molecules regulate synapse development and plasticity through mechanisms that include trans-synaptic adhesion and recruitment of diverse synaptic proteins. We found that the immunoglobulin superfamily member 11 (IgSF11), a homophilic adhesion molecule that preferentially expressed in the brain, is a dual-binding partner of the postsynaptic scaffolding protein PSD-95 and AMPA glutamate receptors (AMPARs). IgSF11 required PSD-95 binding for its excitatory synaptic localization. In addition, IgSF11 stabilized synaptic AMPARs, as determined by IgSF11 knockdown–induced suppression of AMPAR-mediated synaptic transmission and increased surface mobility of AMPARs, measured by high-throughput, single-molecule tracking. IgSF11 deletion in mice led to the suppression of AMPAR-mediated synaptic transmission in the dentate gyrus and long-term potentiation in the CA1 region of the hippocampus. IgSF11 did not regulate the functional characteristics of AMPARs, including desensitization, deactivation or recovery. These results suggest that IgSF11 regulates excitatory synaptic transmission and plasticity through its tripartite interactions with PSD-95 and AMPARs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Yuzaki, M. Cbln1 and its family proteins in synapse formation and maintenance. Curr. Opin. Neurobiol. 21, 215–220 (2011).
Takahashi, H. & Craig, A.M. Protein tyrosine phosphatases PTPdelta, PTPsigma, and LAR: presynaptic hubs for synapse organization. Trends Neurosci. 36, 522–534 (2013).
Krueger, D.D., Tuffy, L.P., Papadopoulos, T. & Brose, N. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr. Opin. Neurobiol. 22, 412–422 (2012).
de Wit, J., Hong, W., Luo, L. & Ghosh, A. Role of leucine-rich repeat proteins in the development and function of neural circuits. Annu. Rev. Cell Dev. Biol. 27, 697–729 (2011).
Shen, K. & Scheiffele, P. Genetics and cell biology of building specific synapse connectivity. Annu. Rev. Neurosci. 33, 473–507 (2010).
Dalva, M.B., McClelland, A.C. & Kayser, M.S. Cell adhesion molecules: signalling functions at the synapse. Nat. Rev. Neurosci. 8, 206–220 (2007).
Biederer, T. & Stagi, M. Signaling by synaptogenic molecules. Curr. Opin. Neurobiol. 18, 261–269 (2008).
Um, J.W. & Ko, J. LAR-RPTPs: synaptic adhesion molecules that shape synapse development. Trends Cell Biol. 23, 465–475 (2013).
Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Nuriya, M. & Huganir, R.L. Regulation of AMPA receptor trafficking by N-cadherin. J. Neurochem. 97, 652–661 (2006).
Saglietti, L. et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron 54, 461–477 (2007).
Budreck, E.C. et al. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proc. Natl. Acad. Sci. USA 110, 725–730 (2013).
Herrera-Molina, R. et al. Structure of excitatory synapses and GABAA receptor localization at inhibitory synapses are regulated by neuroplastin-65. J. Biol. Chem. 289, 8973–8988 (2014).
Suzu, S. et al. Molecular cloning of a novel immunoglobulin superfamily gene preferentially expressed by brain and testis. Biochem. Biophys. Res. Commun. 296, 1215–1221 (2002).
Harada, H., Suzu, S., Hayashi, Y. & Okada, S. BT-IgSF, a novel immunoglobulin superfamily protein, functions as a cell adhesion molecule. J. Cell. Physiol. 204, 919–926 (2005).
Eom, D.S. et al. Melanophore migration and survival during zebrafish adult pigment stripe development require the immunoglobulin superfamily adhesion molecule Igsf11. PLoS Genet. 8, e1002899 (2012).
Brennand, K.J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011).
Raschperger, E., Engstrom, U., Pettersson, R.F. & Fuxe, J. CLMP, a novel member of the CTX family and a new component of epithelial tight junctions. J. Biol. Chem. 279, 796–804 (2004).
Opazo, P., Sainlos, M. & Choquet, D. Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr. Opin. Neurobiol. 22, 453–460 (2012).
Giannone, G. et al. Dynamic superresolution imaging of endogenous proteins on living cells at ultra-high density. Biophys. J. 99, 1303–1310 (2010).
Schnell, E. et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl. Acad. Sci. USA 99, 13902–13907 (2002).
Schreiber, J., Langhorst, H., Jüttner, R. & Rathjen, F.G. The IgCAMs CAR, BT-IgSF and CLMP: structure, function and diseases. in Cell Adhesion Molecules: Implications in Neurological Diseases (eds. Berezin, V. & Walmod, P.S.) (Springer, 2014).
Siddiqui, T.J. et al. An LRRTM4-HSPG complex mediates excitatory synapse development on dentate gyrus granule cells. Neuron 79, 680–695 (2013).
Jackson, A.C. & Nicoll, R.A. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 70, 178–199 (2011).
Chih, B., Engelman, H. & Scheiffele, P. Control of excitatory and inhibitory synapse formation by neuroligins. Science 307, 1324–1328 (2005).
Soler-Llavina, G.J., Fuccillo, M.V., Ko, J., Sudhof, T.C. & Malenka, R.C. The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. Proc. Natl. Acad. Sci. USA 108, 16502–16509 (2011).
Chubykin, A.A. et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54, 919–931 (2007).
Jung, S.Y. et al. Input-specific synaptic plasticity in the amygdala is regulated by neuroligin-1 via postsynaptic NMDA receptors. Proc. Natl. Acad. Sci. USA 107, 4710–4715 (2010).
Blundell, J. et al. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 8, 114–126 (2009).
Shipman, S.L. & Nicoll, R.A. A subtype-specific function for the extracellular domain of neuroligin 1 in hippocampal LTP. Neuron 76, 309–316 (2012).
Kim, J. et al. Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc. Natl. Acad. Sci. USA 105, 9087–9092 (2008).
Mondin, M. et al. Neurexin-neuroligin adhesions capture surface-diffusing AMPA receptors through PSD-95 scaffolds. J. Neurosci. 31, 13500–13515 (2011).
Shipman, S.L. et al. Functional dependence of neuroligin on a new non-PDZ intracellular domain. Nat. Neurosci. 14, 718–726 (2011).
Cingolani, L.A. et al. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron 58, 749–762 (2008).
Pozo, K. et al. beta3 integrin interacts directly with GluA2 AMPA receptor subunit and regulates AMPA receptor expression in hippocampal neurons. Proc. Natl. Acad. Sci. USA 109, 1323–1328 (2012).
Tai, C.Y., Kim, S.A. & Schuman, E.M. Cadherins and synaptic plasticity. Curr. Opin. Cell Biol. 20, 567–575 (2008).
Song, M. et al. Slitrk5 mediates BDNF-dependent TrkB receptor trafficking and signaling. Dev. Cell 33, 690–702 (2015).
Taniguchi, H. et al. Silencing of neuroligin function by postsynaptic neurexins. J. Neurosci. 27, 2815–2824 (2007).
Schwenk, J. et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323, 1313–1319 (2009).
Shanks, N.F. et al. Differences in AMPA and kainate receptor interactomes facilitate identification of AMPA receptor auxiliary subunit GSG1L. Cell Reports 1, 590–598 (2012).
von Engelhardt, J. et al. CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science 327, 1518–1522 (2010).
Kalashnikova, E. et al. SynDIG1: an activity-regulated, AMPA receptor–interacting transmembrane protein that regulates excitatory synapse development. Neuron 65, 80–93 (2010).
Chen, L. et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408, 936–943 (2000).
Schwenk, J. et al. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron 74, 621–633 (2012).
Lee, K. et al. MDGAs interact selectively with neuroligin-2, but not other neuroligins to regulate inhibitory synapse development. Proc. Natl. Acad. Sci. USA 110, 336–341 (2013).
Pettem, K.L., Yokomaku, D., Takahashi, H., Ge, Y. & Craig, A.M. Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development. J. Cell Biol. 200, 321–336 (2013).
Fogel, A.I., Stagi, M., Perez de Arce, K. & Biederer, T. Lateral assembly of the immunoglobulin protein SynCAM 1 controls its adhesive function and instructs synapse formation. EMBO J. 30, 4728–4738 (2011).
Song, Y.S., Lee, H.J., Prosselkov, P., Itohara, S. & Kim, E. Trans-induced cis interaction in the tripartite NGL-1, netrin-G1 and LAR adhesion complex promotes development of excitatory synapses. J. Cell Sci. 126, 4926–4938 (2013).
Patzke, C. et al. The coxsackievirus-adenovirus receptor reveals complex homophilic and heterophilic interactions on neural cells. J. Neurosci. 30, 2897–2910 (2010).
Lucic´, V., Yang, T., Schweikert, G., Forster, F. & Baumeister, W. Morphological characterization of molecular complexes present in the synaptic cleft. Structure 13, 423–434 (2005).
Kim, E., Niethammer, M., Rothschild, A., Jan, Y.N. & Sheng, M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature 378, 85–88 (1995).
Choi, J. et al. Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases. J. Neurosci. 25, 869–879 (2005).
Neve, R.L., Neve, K.A., Nestler, E.J. & Carlezon, W.A. Jr. Use of herpes virus amplicon vectors to study brain disorders. Biotechniques 39, 381–391 (2005).
Kim, J., Kwon, J.T., Kim, H.S., Josselyn, S.A. & Han, J.H. Memory recall and modifications by activating neurons with elevated CREB. Nat. Neurosci. 17, 65–72 (2014).
Yang, J. et al. DGKiota regulates presynaptic release during mGluR-dependent LTD. EMBO J. 30, 165–180 (2011).
Kim, M.H. et al. Enhanced NMDA receptor-mediated synaptic transmission, enhanced long-term potentiation, and impaired learning and memory in mice lacking IRSp53. J. Neurosci. 29, 1586–1595 (2009).
Sheng, M., Cummings, J., Roldan, L.A., Jan, Y.N. & Jan, L.Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368, 144–147 (1994).
Wyszynski, M., Kim, E., Yang, F.C. & Sheng, M. Biochemical and immunocytochemical characterization of GRIP, a putative AMPA receptor anchoring protein, in rat brain. Neuropharmacology 37, 1335–1344 (1998).
Han, S. et al. Altered expression of synaptotagmin 13 mRNA in adult mouse brain after contextual fear conditioning. Biochem. Biophys. Res. Commun. 425, 880–885 (2012).
Huttner, W.B., Schiebler, W., Greengard, P. & De Camilli, P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 96, 1374–1388 (1983).
Cho, K.O., Hunt, C.A. & Kennedy, M.B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 9, 929–942 (1992).
Han, K. et al. Regulated RalBP1 binding to RalA and PSD-95 controls AMPA receptor endocytosis and LTD. PLoS Biol. 7, e1000187 (2009).
Jo, J. et al. Abeta(1–42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nat. Neurosci. 14, 545–547 (2011).
Giannone, G. et al. Dynamic super-resolution imaging of endogenous proteins on living cells at ultra-high density. Biophys. J. 99, 1303–1310 (2010).
Groc, L. et al. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat. Neurosci. 7, 695–696 (2004).
Skarnes, W.C. et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474, 337–342 (2011).
O'Gorman, S., Dagenais, N.A., Qian, M. & Marchuk, Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl. Acad. Sci. USA 94, 14602–14607 (1997).
Carlin, R.K., Grab, D.J., Cohen, R.S. & Siekevitz, P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J. Cell Biol. 86, 831–845 (1980).
Won, H. et al. GIT1 is associated with ADHD in humans and ADHD-like behaviors in mice. Nat. Med. 17, 566–572 (2011).
Schoch, S. et al. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature 415, 321–326 (2002).
Patzke, C. et al. The coxsackievirus-adenovirus receptor reveals complex homophilic and heterophilic interactions on neural cells. J. Neurosci. 30, 2897–2910 (2010).
Arnold, K., Bordoli, L., Kopp, J. & Schwede, T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 (2006).
Lyskov, S. & Gray, J.J. The RosettaDock server for local protein-protein docking. Nucleic Acids Res. 36, W233–W238 (2008).
Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.3r1, (http://pymol.org) (2010).
Acknowledgements
We would like to thank R. Neve for the kind gift of the HSV system, J.-R. Lee and K. Han for the PSD fraction sample, J.W. Um and J. Ko for the subcellular fraction sample, M.-H. Kim for the mini analysis program, and Mihyeon Kim for helping with IgSF11 antibody generation. This work was supported by the Institute for Basic Science (to E.K.), the Brain Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Science, ICT & Future Planning (2013-056732 to H.K.), National Honor Scientist Program of the NRF (to B.K.K.), and Wellcome Trust and UK MRC Neurodegenerative Disease Initiative Programme (to K.C.).
Author information
Authors and Affiliations
Contributions
D.L. and H.K. performed the in situ hybridization analysis. S.J., D.O., Y.L., H.S., D.K. and S.G.K. performed immunoblot and co-immunoprecipitation experiments. D.O., S.K.K., J.W. and H.S. performed neuron culture, co-culture, co-clustering experiments, homophilic binding assay and antibody feeding experiments. D.O., Y.L. and H.S. performed neuronal shRNA knockdown experiments. S.J., C.V.R., D.W., J.M.W., J.J., S.G.K., S.M.U., S.K.K., M.H.K., J.D.R., H.J., W.M. and K.C. performed electrophysiological experiments and analyzed the data. E.H. performed uPAINT experiments. Y.L. and J.D.R. carried out HSV experiments. S.J. generated and characterized Igsf11–/– (LacZ) and Igsf11–/– mice. D.K. modeled IgSF11 structures. H.K., B.K.K., K.C., J.S.R., D.C. and E.K. supervised the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
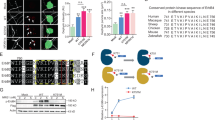
Supplementary Figure 1 Amino acid sequence alignment of IgSF11 proteins from various vertebrate species.
Amino acids in black and gray backgrounds indicate fully and partially conserved residues. The following amino acid sequences were used for comparison; human (NP_001015887.1), chimpanzee (XP_001161500.1), macaque monkey (NP_001244835.1), mouse (NP_733548.2), rat (NP_001013138.1), chicken (XP_416568.3), finch (ENSTGUP00000013797), lizard (ENSACAP00000002845), Xenopus (ENSXETP00000049789), and zebrafish (XP_001343768.4).
Supplementary Figure 2 Amino acid sequence alignment of CAR subgroup members and their in situ hybridization patterns in the brain.
(a) Amino acids in black and gray backgrounds indicate fully and partially conserved residues. The following mouse amino acid sequences were used for comparison; IgSF11 (NP_733548.2), CAR (NP_001020363.1), ESAM (NP_081378.1), and CLMP (NP_598494.2). (b) In situ mRNA distribution patterns of IgSF11, CAR, ESAM, and CLMP in adult (8 weeks) male mouse brains obtained from the Allen Brain Atlas (IgSF11, http://mouse.brain-map.org/experiment/show?id=68861816, CAR, http://mouse.brain-map.org/experiment/show?id=77454980, ESAM, http://mouse.brain-map.org/experiment/show?id=69262331, and CLMP, http://mouse.brain-map.org/experiment/show?id=70445538). Scale bar, 1 mm.
Supplementary Figure 3 IgSF11 interacts with PSD-95 in yeast two-hybrid assays.
The C-terminal PDZ-binding motif of IgSF11 interacts with PDZ domains of PSD-95 family proteins (PSD-95, PSD-93/chapsyn-110 and SAP97) in yeast two-hybrid assays. pBHA, bait vector; pGAD10, prey vector; WT, wild type (last seven residues); VA, a mutant IgSF11 in which the last residue (valine) was changed to alanine. PDZ domains from GRIP2, Shank1 and NHERF were used as controls. β-Galactosidase (β-gal) activity: +++, < 45 min; ++, 45–90 min; +, 90–240 min; –, no significant β-gal activity. Three independent experiments were performed.
Supplementary Figure 4 IgSF11 does not induce clustering of pre- or postsynaptic proteins in contacting axons and dendrites, and does not interact with other synaptic adhesion molecules.
(a-c) Cultured rat hippocampal neurons were cocultured with HEK293T cells expressing rat IgSF11 (untagged), or EGFP (control; DIV 10–13), followed by staining for synapsin I (a presynaptic protein), PSD-95 (an excitatory postsynaptic protein), and gephyrin (an inhibitory postsynaptic protein). Scale bar, 30 μm. (d) IgSF11 does not interact with other synaptic adhesion molecules, as shown by the lack of binding of the ectodomain of IgSF11 fused to human Fc (IgSF11-ecto-Fc) to known synaptic adhesion molecules expressed in HEK293T cells. IgSF11-expressing HEK293T cells were used as a control. Scale bar, 10 μm. Three independent experiments were performed.
Supplementary Figure 5 The transmembrane domain of IgSF11 is important for GluA1 binding.
(a and b) HEK293T cells were cotransfected with GluA1 and the indicated deletion variants of HA-IgSF11, followed by immunoprecipitation with HA antibodies and immunoblotting with HA and GluA1 antibodies. Three independent experiments were performed. Full-length blots are presented in Supplementary Figure 17c.
Supplementary Figure 6 Characterization of IgSF11-knockdown and IgSF11 rescue constructs.
(a) Acute knockdown of IgSF11 expression in heterologous cells. HEK293T cells were doubly transfected with rIgSF11 (rat IgSF11) and sh-vec (empty vector), sh-IgSF11#1, or sh-IgSF11#1* (a mutant incapable of knocking down IgSF11) for two days, and the cell lysates were characterized by immunoblotting for IgSF11 and α-tubulin (loading control). n = 3, * p < 0.05, ** p < 0.01, ns, not significant, one-way ANOVA. Full-length blots are presented in Supplementary Figure 17d.
(b) Acute knockdown of IgSF11 expression by an independent IgSF11 knockdown construct (sh-IgSF11#2). HEK293T cells were transfected with doubly transfected with IgSF11 and sh-vec (empty vector) or sh-IgSF11#2 for two days, and the cell lysates were characterized by immunoblotting for IgSF11 and α-tubulin (loading control). n = 3, *** p < 0.001, Student’s t-test. Full-length blots are presented in Supplementary Figure 17e.
(c and d) Characterization of an sh-IgSF11#1-resistant IgSF11 expression construct. HEK293T cells were doubly transfected with IgSF11 (rat) + sh-vec, IgSF11 + sh-IgSF11#1, or sh-IgSF11#1-resistant IgSF11 (WT or ΔC3) + sh-IgSF11#1, followed by immunoblotting with IgSF11 antibodies. Three independent experiments were performed. Full-length blots are presented in Supplementary Figure 17f,g.
Supplementary Figure 7 IgSF11 knockdown in cultured neurons suppresses surface clustering of GluA1 and GluA2 but has no effect on excitatory synapses.
(a-c) Acute knockdown of IgSF11 decreases surface clustering of GluA1 in cultured hippocampal neurons. Cultured neurons were transfected with sh-vec (empty vector), sh-IgSF11#1, or sh-IgSF11#1* (DIV 14–17), and stained for surface GluA1. au, arbitrary unit; n = 18 for sh-vec, 17 for sh-IgSF11#1, and 17 for sh-IgSF11#1*, *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA. Scale bar, 10 μm.
(d-g) IgSF11 knockdown has no effect on excitatory synapses, defined by synapsin I-positive PSD-95 clusters. Cultured neurons were transfected with sh-vec, sh-IgSF11#1, or sh-IgSF11#1* (DIV14–17), and immunostained for PSD-95 and synapsin I. n = 22 for sh-vec, 23 for sh-IgSF11#1, and 21 for sh-IgSF11#1*, ns, not significant, one-way ANOVA. Scale bar, 10 μm.
(h-k) Independent IgSF11 knockdown suppresses surface GluA2 clustering but has no effect on PSD-95 clusters. Cultured hippocampal neurons were transfected with sh-vec (empty vector) or sh-IgSF11#2 (DIV 14–17), and stained for surface GluA2 or PSD-95. au, arbitrary unit; n = 18 for sh-vec, 17 for sh-IgSF11#2, ***p < 0.001, ns, not significant, Student’s t-test. Scale bar, 10 μm.
Supplementary Figure 8 IgSF11 knockdown reduces steady-state levels of surface GluA2.
Cultured hippocampal neurons doubly transfected with N-terminally HA-tagged GluA2 (HA-GluA2) and sh-vec, sh-IgSF11#1, or IgSF11#1* (DIV 14–17) were measured of surface and internal levels of GluA2. n = 19 for sh-vec, 19 for sh-IgSF11#1, and 18 for sh-IgSF11#1*, *p < 0.05, **p < 0.01, one-way ANOVA. Scale bar, 30 μm.
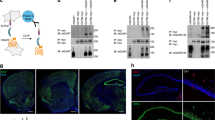
Supplementary Figure 9 Generation and characterization of Igsf11−/− (LacZ) and Igsf11−/− mice.
(a) Schematic diagram showing the strategy for the generation of the gene-trapped Igsf11−/− (LacZ) mice and exon 3-deleted Igsf11−/− mice. Note that exon 2 is captured by the inserted cassette to yield a truncated protein fused to β-geo (β-galactosidase + neomycin), and that crossing of Igsf11 floxed mice with protamine-Cre mice leads to the deletion of exon 3 in the IgSF11 gene. Primer locations for genotyping are indicated. (b) Genotyping of wild-type (Igsf11+/+; WT), Igsf11+/− (LacZ), and Igsf11−/− (LacZ) mice by PCR. Full-length gels are presented in Supplementary Figure 17h. (c) Igsf11−/− (LacZ) mice show no detectable levels of IgSF11 proteins in the brain. α-Tubulin blot was used as a control. Full-length blots are presented in Supplementary Figure 17i.
Supplementary Figure 10 Spatiotemporal IgSF11 expression revealed by X-Gal staining.
(a and b) The spatial expression pattern of IgSF11 was characterized by X‑Gal staining of sagittal brain slices from Igsf11−/− (LacZ) mice at P5 and postnatal weeks 2, 3, and 12. Scale bars, 1 mm for whole brain, 200 μm for hippocampus. Three independent experiments were performed.(c) No differences are observed in left and right Igsf11−/− (LacZ) hippocampal regions at 8 weeks. (d) IgSF11 protein levels in mouse brains are rapidly increased during the first and second weeks and are maintained at later stages. Three independent experiments were performed. Full-length blots are presented in Supplementary Figure 17j.
Supplementary Figure 11 Normal expression levels of synaptic proteins in the Igsf11−/− brain.
(a and b) Hippocampal samples from WT and Igsf11−/− (LacZ) brains (3 weeks) were immunoblotted for the synaptic proteins indicated, followed by quantification. Protein amounts in Igsf11−/− brains normalized to α-tubulin were normalized to those of WT brains. n = 3 mice for WT and KO, ns, not significant, Student’s t-test. Full-length blots are presented in Supplementary Figure 17k.
Supplementary Figure 12 Igsf11−/− hippocampal CA1 pyramidal neurons display unaltered levels extrasynaptic AMPAR currents.
(a and b) Igsf11−/− (LacZ) DG granule cells in acute hippocampal slices (3 weeks) were treated with AMPA (1 μM), a condition known to induce extrasynaptic AMPAR-mediated currents, and measured of evoked EPSCs. n = 8 slices (2 mice) for WT and KO, ns, not significant, Student’s t-test.
Supplementary Figure 13 Normal levels of basal transmission and paired pulse ratio at Igsf11−/− MPP-DG and SC-CA1 synapses.
(a and b) Basal transmission (input-out relationship) and paired pulse ratio at WT and Igsf11−/− (LacZ) MPP-DG synapses (3–6 months). n = 12 slices (6 mice) for WT, and 10, 6 for KO. (c and d) Basal transmission and paired pulse ratio at WT and Igsf11−/− (LacZ) SC-CA1 synapses (3–6 months). n = 10, 6 for WT and KO.
Supplementary Figure 14 The Igsf11−/− hippocampus shows normal levels of immature GluA2.
Hippocampal lysates from WT and Igsf11−/− (LacZ) mice were digested with endoglycosidase H (Endo H) or peptide N-glycosidase F (PNGase F), and measured of the ratio of Endo H-sensitive GluA2 over total (Endo H-resistant + Endo H-sensitive) GluA2. PNGase F, which completely removes all types of N-linked oligosaccharides on GluA2, was used as a control. n = 3 mice for WT and KO (3 weeks), ns, not significant, Student’s t-test. Full-length blots are presented in Supplementary Figure 17l.
Supplementary Figure 15 Molecular modeling of IgSF11-dependent homophilic adhesion.
Three possible molecular models of homophilic and trans-cellular adhesion mediated by the two Ig domains of IgSF11. These models were based on the X-ray crystal structure of CAR (a relative of IgSF11) 49.
Supplementary Figure 17 Full-length images of immunoblots and gels.
(a) A full-length gel for Fig. 8a. (b) Full-length immunoblots for Fig. 8b. (c) Supplementary Fig. 5b. (d) Supplementary Fig. 6a. (e) Supplementary Fig. 6b. (f) Supplementary Fig. 6c. (g) Supplementary Fig. 6d. (h) A full-length gel for Supplementary Fig. 9b. (i) Full-length immunoblots for Supplementary Fig. 9c. (j) Supplementary Fig. 10d. (k) Supplementary Fig. 11a. The blackened immunoblot panels indicate overexposed films, which could be properly exposed in other immunoblot experiments. (l) A full-length immunoblot for Supplementary Fig. 14a.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–17 (PDF 3161 kb)
Rights and permissions
About this article
Cite this article
Jang, S., Oh, D., Lee, Y. et al. Synaptic adhesion molecule IgSF11 regulates synaptic transmission and plasticity. Nat Neurosci 19, 84–93 (2016). https://doi.org/10.1038/nn.4176
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4176
This article is cited by
-
VISTA and its ligands: the next generation of promising therapeutic targets in immunotherapy
Cancer Cell International (2023)
-
IgSF11-mediated phosphorylation of pyruvate kinase M2 regulates osteoclast differentiation and prevents pathological bone loss
Bone Research (2023)
-
IgSF11 deficiency alleviates osteoarthritis in mice by suppressing early subchondral bone changes
Experimental & Molecular Medicine (2023)
-
A shared neural basis underlying psychiatric comorbidity
Nature Medicine (2023)
-
IGSF11 and VISTA: a pair of promising immune checkpoints in tumor immunotherapy
Biomarker Research (2022)