Abstract
The transcription factor Math1 (encoded by the gene Atoh1, also called Math1) is required for the formation of mechanosensory hair cells in the inner ear; however, its specific molecular role is unknown. Here we show that absence of Math1 in mice results in a complete disruption of formation of the sensory epithelium of the cochlea, including the development of both hair cells and associated supporting cells. In addition, ectopic expression of Math1 in nonsensory regions of the cochlea is sufficient to induce the formation of sensory clusters that contain both hair cells and supporting cells. The formation of these clusters is dependent on inhibitory interactions mediated, most probably, through the Notch pathway, and on inductive interactions that recruit cells to develop as supporting cells through a pathway independent of Math1. These results show that Math1 functions in the developing cochlea to initiate both inductive and inhibitory signals that regulate the overall formation of the sensory epithelia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bryant, J., Goodyear, R.J. & Richardson, G.P. Sensory organ development in the inner ear: molecular and cellular mechanisms. Br. Med. Bull. 63, 39–57 (2002).
Colvin, J.S., Bohne, B.A., Harding, G.W., McEwen, D.G. & Ornitz, D.M. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat. Genet. 12, 390–397 (1996).
Bermingham, N.A. et al. Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1841 (1999).
Zheng, J.L. & Gao, W.Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 3, 580–586 (2000).
Shailam, R. et al. Expression of proneural and neurogenic genes in the embryonic mammalian vestibular system. J. Neurocytol. 28, 809–819 (1999).
Lanford, P.J., Shailam, R., Norton, C.R., Gridley, T. & Kelley, M.W. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J. Assoc. Res. Otolaryngol. 1, 161–171 (2000).
Chen, P., Johnson, J.E., Zoghbi, H.Y. & Segil, N. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129, 2495–2505 (2002).
Rubel, E.W. Ontogeny of structure and function in the vertebrate auditory system. in Handbook of Sensory Physiology, Vol. IX (ed. Jacobson, M.) 135–237 (Springer, New York, 1978).
Rio, C., Dikkes, P., Liberman, M.C. & Corfas, G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J. Comp. Neurol. 442, 156–162 (2002).
Peters, K., Ornitz, D., Werner, S. & Williams, L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev. Biol. 155, 423–430 (1993).
Mueller, K.L., Jacques, B.E. & Kelley, M.W. Fibroblast growth factor signaling regulates pillar cell development in the organ of Corti. J. Neurosci. 22, 9368–9377 (2002).
von Bartheld, C.S. et al. Expression of nerve growth factor (NGF) receptors in the developing inner ear of chick and rat. Development 113, 455–470 (1991).
Bianchi, L.M., Liu, H., Krug, E.L. & Capehart, A.A. Selective and transient expression of a native chondroitin sulfate epitope in Deiters' cells, pillar cells, and the developing tectorial membrane. Anat. Rec. 256, 64–71 (1999).
Morrison, A., Hodgetts, C., Gossler, A., Hrabe de Angelis, M. & Lewis, J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech. Dev. 84, 169–172 (1999).
Coppens, A.G., Kiss, R., Heizmann, C.W., Schafer, B.W. & Poncelet, L. Immunolocalization of the calcium binding S100A1, S100A5 and S100A6 proteins in the dog cochlea during postnatal development. Brain Res. Dev. Brain Res. 126, 191–199 (2001).
Rau, A., Legan, P.K. & Richardson, G.P. Tectorin mRNA expression is spatially and temporally restricted during mouse inner ear development. J. Comp. Neurol. 405, 271–280 (1999).
El-Amraoui, A., Cohen-Salmon, M., Petit, C. & Simmler, M.C. Spatiotemporal expression of Otogelin in the developing and adult mouse inner ear. Hear. Res. 158, 151–159 (2001).
Hasson, T. et al. Unconventional myosins in inner-ear sensory epithelia. J. Cell. Biol. 137, 1287–1307 (1997).
Cernuda-Cernuda, R. & Garcia-Fernandez, J.M. Structural diversity of the ordinary and specialized lateral line organs. Microsc. Res. Tech. 34, 302–312 (1996).
Helms, A.D., Abney, A.L., Ben-Arie, N., Zoghbi, H.Y. & Johnson, J.E. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development 127, 1185–1196 (2000).
Littlewood, T.D., Hancock, D.C., Danielian, P.S., Parker, M.G. & Evan, G.I. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 23, 1686–1690 (1995).
Mattioni, T., Louvion, J.F. & Picard, D. Regulation of protein activities by fusion to steroid binding domains. Methods Cell Biol. 43, 335–352 (1994).
Picard, D. Regulation of protein function through expression of chimaeric proteins. Curr. Opin. Biotechnol. 5, 511–515 (1994).
Lanford, P.J. et al. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat. Genet. 21, 289–292 (1999).
Zine, A., Van De Water, T.R. & de Ribaupierre, F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development 127, 3373–3383 (2000).
Haddon, C., Jiang, Y.J., Smithers, L. & Lewis, J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development 125, 4637–4644 (1998).
Haddon, C. et al. Hair cells without supporting cells: further studies in the ear of the zebrafish mind bomb mutant. J. Neurocytol. 28, 837–850 (1999).
Riley, B.B., Chiang, M., Farmer, L. & Heck, R. The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development 126, 5669–78 (1999).
Zheng, J.L., Shou, J., Guillemot, F., Kageyama, R. & Gao, W.Q. Hes1 is a negative regulator of inner ear hair cell differentiation. Development 127, 4551–4560 (2000).
Zine, A. et al. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J. Neurosci. 21, 4712–4720 (2001).
Lin, M.H. and Kopan, R. Long-range, nonautonomous effects of activated Notch1 on tissue homeostasis in the nail. Dev. Biol. 263, 343–359 (2003).
Geling, A., Steiner, H., Willem, M., Bally-Cuif, L. & Haass, C. A γ-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 3, 688–694 (2002).
Micchelli, C.A. et al. γ-Secretase/presenilin inhibitors for Alzheimer's disease phenocopy Notch mutations in Drosophila. FASEB J. 17, 79–81 (2003).
Cheng, H.T. et al. γ-Secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 130, 5031–5042 (2003).
Lewis, J. Rules for the production of sensory cells. Ciba Found. Symp. 160, 25–39 (1991).
Corwin, J.T., Jones, J.E., Katayama, A., Kelley, M.W. & Warchol, M.E. Hair cell regeneration: the identities of progenitor cells, potential triggers and instructive cues. Ciba Found. Symp. 160, 103–120 (1991).
Adam, J. et al. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development 125, 4645–4654 (1998).
Goodyear, R., Holley, M. & Richardson, G. Hair and supporting-cell differentiation during the development of the avian inner ear. J. Comp. Neurol. 351, 81–93 (1995).
Kelley, M.W., Talreja, D.R. & Corwin, J.T. Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. J. Neurosci. 15, 3013–3026 (1995).
Kelley, M.W., Xu, X.M., Wagner, M.A., Warchol, M.E. & Corwin, J.T. The developing organ of Corti contains retinoic acid and forms supernumerary hair cells in response to exogenous retinoic acid in culture. Development 119, 1041–1053 (1993).
Kimberly, W.T. & Wolfe, M.S. Identity and function of γ-secretase. J. Neurosci. Res. 74, 353–360 (2003).
Cole, L.K. et al. Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate1, and lunatic fringe. J. Comp. Neurol. 424, 509–520 (2000).
Kelley, M.W. & Bianchi, L.M. Development and neuronal innervation of the organ of Corti. in Handbook of the Mouse Auditory Research: From Behavior to Molecular Biology (ed. Willott, J.F.) 137–156 (CRC, New York, 2001).
Chen, P. & Segil, N. p27Kip1 links proliferation to morphogenesis in the developing organ of Corti. Development 126, 1581–1590 (1999).
Morsli, H., Choo, D., Ryan, A., Johnson, R. & Wu, D.K. Development of the mouse inner ear and origin of its sensory organs. J. Neurosci. 18, 3327–3335 (1998).
Montcouquiol, M. & Kelley, M.W. Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J. Neurosci. 23, 9469–9478 (2003).
Acknowledgements
We thank R. Hertzano for technical advice; H. Zoghbi and K. Barald for the Math1 mutant mice; T. Friedman, R. Wenthold, N. Sans, A. Chitnis and R. Hertzano for reading an earlier version of this manuscript; M. Pryor for technical assistance; A. Capehart, L. Bianchi, M. Cohen-Salmon and C. Petit for antibodies; and M. Rivolta and A. McMahon for cDNAs. This research was supported by the Intramural Program at the National Institute on Deafness and Other Communication Disorders.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
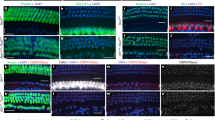
Transfection with Math1ORF induces hair cell formation in the GER. (a) Low magnification view of an E13 explant transfected with Math1ORF after 6 DIV. Hair cells located in the sensory epithelium and in KO are labeled with an antibody for Myosin VI (red). Note the presence of ectopic hair cells in KO (arrows). (b) The same image as in a, except with cells transfected with Math1ORF labeled in green. (c) Merged image of panels a and b. (d) The same image as in a, however each ectopic hair cell as been circled in white. (e) The same image as in b, with each transfected cell circled. (f) The same image as in c, except with circles. Only three transfected cells (purple circles) are not positive for Myosin 6. (g) High magnification view of Myosin VI-positive cells (red) in an E13 explant transfected as in a. The edge of the sensory epithelium is located in the upper left hand corner of the image. Ectopic hair cells are present in KO. (h) The same image as in g, except with cells transfected with Math1ORF labeled in green. (i) Merged image of panels h and i. (j) The same image as in g, except with ecotpic hair cells circled in white. (k) The same image as in h, except with transfected cells circled in white. (l) The same image as in i, except with circles. Only a single transfected cell (purple circle) is not positive for Myosin VI. Scale bar in a (same in b-f), 200 µm. Scale bar in g (same in h-l), 40 µm. (PDF 432 kb)
Supplementary Fig. 2
Transfection with Math1ER induces hair cells only in the presence of tamoxifen. (a) Low magnification image of an E13 explant transfected with Math1ERand maintained for 6 DIV in the absence of tamoxifen. Myosin VI-positive hair cells in the sensory epithelium are labeled in red. (b) The same image as in a. Cells expressing Math1ER are illustrated in green. (c) Merged image of a and b. Note that none of the transfected cells in KO have developed as hair cells. (d) Low magnification image of an E13 explant transfected with Math1ERand maintained for 6 DIV in the absence of tamoxifen. Jagged1-positive supporting cells in the sensory epithelium are labeled in red. (e) The same image as in d. Cells expressing Math1ER are illustrated in green. (f) Merged image of d and e. Note that none of the transfected cells in KO have developed as supporting cells. (g) Low magnification image of an E13 explant transfected with Math1ER and maintained for 6 DIV in the presence of 15 nM tamoxifen. Myosin VI-positive hair cells (red) are present in both the sensory epithelium and KO. (h) The same image as in g. Cells expressing Math1ER are illustrated in green. (i) Merged image of g and h. Note that transfected cells in KO are also positive for Myosin VI, indicating that these cells have developed as hair cells. (j) Magnified image of KO from the sample illustrated in g. Transfected cells that have developed as hair cells are circled. Note that many of the green cells express Myosin VI at the lumenal surface. See Figure 6a-d for a higher magnification view. Scale bar in b (same in a, c-f), 100 µm. Scale bar in h (same in g,i) 200 µm. Scale bar in j, 200 µm. (PDF 364 kb)
Supplementary Fig. 3
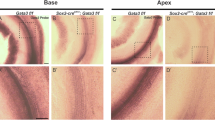
Detection of Math1-promoter activity using anti-β-galactosidase. Confocal image of the lumenal surface of the sensory epithelium from an E13.5 explant established from a Math1+/bgal embryo and maintained for 6 DIV. Inner (IHC) and outer (numbered) hair cells are positive for β-galactosidase, while supporting cells are negative. Scale bar, 20 µm. (PDF 119 kb)
Supplementary Fig. 4
Expression of Math1ORF induces expression of supporting cell markers in explants from Math1-nulls. (a) Low magnification image of a large region of Math1ORF-transfected cells (green). Jag1 (red) is expressed in cells surrounding the transfected cells. (b) High magnification image of a small cluster of Math1ORF-transfected cells (green). Cells surrounding the cluster are positive for Jag1 (red). (c) High magnification image of a small cluster of Math1ORF-transfected cells (green). Surrounding cells express Otogelin (red). Scale bar in a 100 µ. Scale bar in b (same in c), 20 µm. (PDF 229 kb)
Rights and permissions
About this article
Cite this article
Woods, C., Montcouquiol, M. & Kelley, M. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci 7, 1310–1318 (2004). https://doi.org/10.1038/nn1349
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1349
This article is cited by
-
Stem Cell-Based Hair Cell Regeneration and Therapy in the Inner Ear
Neuroscience Bulletin (2024)
-
Fgf8P2A-3×GFP/+: A New Genetic Mouse Model for Specifically Labeling and Sorting Cochlear Inner Hair Cells
Neuroscience Bulletin (2023)
-
GFI1 regulates hair cell differentiation by acting as an off-DNA transcriptional co-activator of ATOH1, and a DNA-binding repressor
Scientific Reports (2022)
-
Towards maturation of human otic hair cell–like cells in pluripotent stem cell–derived organoid transplants
Cell and Tissue Research (2021)
-
Combinatorial Atoh1 and Gfi1 induction enhances hair cell regeneration in the adult cochlea
Scientific Reports (2020)