Abstract
Severe sleep deprivation (SD) has been highly associated with systemic energy wasting, such as lipid loss and glycogen depletion. Despite immune dysregulation and neurotoxicity observed in SD animals, whether and how the gut-secreted hormones participate in SD-induced disruption of energy homeostasis remains largely unknown. Using Drosophila as a conserved model organism, we characterize that production of intestinal Allatostatin A (AstA), a major gut-peptide hormone, is robustly increased in adult flies bearing severe SD. Interestingly, the removal of AstA production in the gut using specific drivers significantly improves lipid loss and glycogen depletion in SD flies without affecting sleep homeostasis. We reveal the molecular mechanisms whereby gut AstA promotes the release of an adipokinetic hormone (Akh), an insulin counter-regulatory hormone functionally equivalent to mammalian glucagon, to mobilize systemic energy reserves by remotely targeting its receptor AstA-R2 in Akh-producing cells. Similar regulation of glucagon secretion and energy wasting by AstA/galanin is also observed in SD mice. Further, integrating single-cell RNA sequencing and genetic validation, we uncover that severe SD results in ROS accumulation in the gut to augment AstA production via TrpA1. Altogether, our results demonstrate the essential roles of the gut-peptide hormone AstA in mediating SD-associated energy wasting.
Similar content being viewed by others
Introduction
Sleep plays a fundamental role in maintaining systemic metabolic homeostasis in many species1,2. Severe sleep deprivation (SD) is established to be associated with increased energy expenditure, weight loss, and decline of carbo-lipid storage in mammals2. Many groups have uncovered the participation of immune-response impairment, neuronal disturbance, and neurotoxicity in the brain, as well as oxidative stress and local catabolism in individual peripheral tissues based on rodent models and clinical investigations3,4,5,6,7. However, despite the gained knowledge, whether and how secreted factors mediate signals between metabolic organs and promote systemic energy catabolism under SD remains largely unknown.
Recent studies indicate that severe SD causes deleterious ROS accumulation predominantly in the intestine and perturbs intestinal homeostasis in both Drosophila and mice8. The gastrointestinal tract is a key endocrinal organ that modulates systemic energy homeostasis in response to environmental and physiological cues across species. In mammals, enteroendocrine cells (EEs) secrete distinct peptide hormones like CCK, GLP-1, and ghrelin to target multiple metabolic tissues and orchestrate systemic carbohydrate and lipid metabolism9. However, mammalian physiological complexity and gene redundancy have complicated functional investigations in the intestine. Drosophila has emerged as an evolutionary conserved model with similar but simple anatomy and physiology of the intestine. Researchers using the Drosophila have addressed significant aspects of intestinal endocrinal impacts, including EE heterogeneities, stress and nutrient sensing of EEs, types and functions of peptide hormones derived from EEs and other cells, as well as intestinal endocrinal regulation in diverse disease models10,11,12,13,14,15.
Two major subtypes of EEs are present in the Drosophila intestinal epithelium, tachykinin (Tk) and allatostatin A/C (AstA/C) EEs, which produce more than 20 mature peptide hormones16,17,18. Many groups and ours have demonstrated the essential metabolic roles of gut-peptide hormones that are produced by Tk EEs, including Tk, Neuropeptide F (NPF), Bursicon (Burs), and Activin-β (Actβ) to target enterocytes, ovary, neuroendocrine cells, and fat body to modulate systemic carbo-lipid balance19,20,21,22. In contrast, the physiological functions of peptide hormones derived from the other subtype AstA/C EEs are not well understood. AstA is a major peptide hormone that is expressed in AstA/C EEs23, as well as in the nervous systems24. Recent studies using different AstA-GAL4 lines and mutations in AstA to manipulate systemic AstA expression, revealed that brain-derived AstA affects sleep, feeding, as well as animal development, via activation of certain neurons24,25,26,27. Nevertheless, AstA expression in the gut was inevitably perturbed in those studies, and the physiological roles of EE-derived AstA was not characterized so far.
In this study, we employed specific drivers that target AstA EEs in the midgut and demonstrated that gut-derived AstA is essential for the depletion of carbohydrate and lipid storages caused by SD. The molecular mechanisms include that gut-derived AstA remotely promotes Akh release by targeting its receptor AstA-R2 in Akh-producing cells (APCs). Interestingly, we also confirmed similar regulation of glucagon release by AstA/galanin in SD mice. Moreover, we show that AstA EEs express TrpA1 to sense accumulated ROS in the gut under SD and enhance AstA production, leading to energy wasting.
Results
Severe sleep loss results in energy wasting
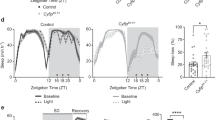
Due to various degrees of sleep loss in different fly models, previous studies focusing on metabolic regulation under SD remain controversial28,29,30. To investigate whether severe SD impairs systemic energy homeostasis, we examined the levels of triglycerides (TAG) and glycogen, the major forms of stored lipids and carbohydrates, respectively, in adult flies with consistent sleep loss. We first performed thermogenetic stimulation by overexpressing heat-activated cation channel TrpA1 (TrpA1-A) to manually activate specific neurons at 29 °C using 50A07-LexA, 68A07-LexA, or 11H05-GAL4 drivers that had caused > 85% sleep loss8 (Fig. 1a). Interestingly, as compared to their control flies, both 50A07 > TrpA1 and 68A07 > TrpA1 flies exhibited a significant decrease in systemic TAG and glycogen levels after SD for 10 days (Fig. 1d and Supplementary Fig. S1h). 11H05 > TrpA1 flies also showed a strong decrease in TAG storage but a mild decline in glycogen content (Fig. 1e). As a consequence of the TAG decrease, abdomens of 50A07 > TrpA1 and 68A07 > TrpA1 flies appeared more translucent with loss of the lipid layer (Fig. 1h). We examined the lipid content in the abdominal fat body using Bodipy staining and consistently observed a decreased mass of lipid droplets in 50A07 > TrpA1 and 68A07 > TrpA1 flies (Fig. 1i). We also examined sleepless (sssΔ40) mutant flies that showed > 85% sleep loss (Fig. 1b)8,31,32 and similarly observed loss of TAG and glycogen storages, translucent abdomens, as well as smaller lipid droplets, as compared to their control flies after SD for 8 days (Fig. 1f, h, i). However, another sleep-loss mutant flies, DATfmn33, exhibited moderate sleep loss (< 50%) and no changes in both TAG and glycogen storages (Supplementary Fig. S1a, b). To further validate SD-induced metabolic imbalance, we examined flies that were mechanically deprived of sleep by ~80% using the vibration method as well (Fig. 1c)8. Consistently, flies with mechanical sleep deprivation (mSD) for 8 days exhibited loss of TAG and glycogen storages (Fig. 1g–i). These results collectively indicate that SD results in energy wasting in Drosophila.
a–c SD in 7-day-old 50A07 > TrpA1 (n = 25) (a), sssΔ40 (n = 31) (b), mSD (n = 16) (c), and matched control female flies that were normally cultured (n = 20, left, sleep in every 30 min per day; right, total sleep per day). d–g TAG and glycogen storages of 7-day-old females of the indicated genotypes after SD for 10 days (d, e, TrpA1), 8 days (f, mutant), or 8 days (g, mechanical) (n = 4, 5 flies/replicate). h, i Abdomen appearance (h) and lipid droplets indicated by Bodipy staining (i, green) in abdominal fat bodies of the indicated genotypes after SD. Data were presented as means ± SEM. *P < 0.05.
SD increases AstA production in the gut
Drosophila gut-peptide hormones have been shown to extensively modulate systemic energy homeostasis11, we thus wonder whether SD modulates the production of certain gut-peptide hormones to execute the impacts on carbo-lipid metabolism. We examined a few known gut-peptide hormones and found robust changes in the production of gut AstA under SD. AstA was found to be majorly produced in the posterior region of the midgut (Fig. 2a). Importantly, intracellular AstA protein levels in the gut of these SD flies were potently decreased (Fig. 2b, c), while the gut AstA mRNA levels remained unchanged or slightly increased (Fig. 2d), in 50A07 > TrpA1, 68A07 > TrpA1, sssΔ40, and mSD flies, indicating enhanced AstA release. We noticed an increase in the numbers of gut AstA EEs as well (Fig. 2b). By contrast, DATfmn flies with moderate sleep loss exhibited no changes in gut AstA production (Supplementary Fig. S1c, d).
a Distribution of AstA EEs as indicated by AstA immunostaining (red) in the posterior region of the midgut of 7-day-old WT flies. b–d Immunostaining of AstA (b, red), quantification of intracellular AstA signal in each single cell (c, n > 80), and AstA mRNA levels (d, n = 3, 15 flies/genotype) in the midguts of 7-day-old female flies after SD for 10 days (TrpA1), 8 days (mutant), or 8 days (mSD). e, f Immunostaining indicating intestinal expressions of AstA (e) and Tk (f) in 8-day-old AstA33 > GFP flies. g, h mRNA (g, n = 3, 15 flies/replicate) and protein (h, up, posterior midgut; bottom, superior medial protocerebrum of the brain) levels of AstA in the midguts and brains in indicated 7-day-old female flies. Data were presented as means ± SEM. *P < 0.05.
We next investigated whether gut AstA production is essential for energy wasting-associated SD by manipulating AstA expression specifically in the gut. Similar to the previous characterization of Tk-gut-GAL4 that predominantly targets Tk EEs in the gut19, we screened multiple AstA-GAL4 lines and managed to identify one, referred to as AstA33-GAL4, that is expressed in all AstA EEs as the other heterologous pair of EEs opposite to Tk in the posterior midgut (Fig. 2e, f) and in very few AstA neurons (two in the protocerebrum, several in the optic lobe, one in the ventral nerve cord (VNC), but not in gnathal ganglia (GNG) region) (Supplementary Fig. S2a, b). Because pros-GAL4 is a well-established driver targeting all gut EEs in Drosophila34, we also examined it and found its expression in all AstA EEs of the gut but rarely in AstA neurons in both brain and VNC (Supplementary Fig. S2c, d). We next expressed double-stranded RNAs against AstA (RNAi) to knock down gut AstA expression under the control of AstA33- or pros-GAL4. As compared with control, AstA expression was only diminished in the midgut, not the brain, as indicated by both quantitive RT-PCR (qPCR) and immunostaining (Fig. 2g, h and Supplementary Fig. S3a, b).
Gut AstA is required for SD-induced energy wasting
These genetic tools allowed us to manipulate AstA expression specifically in the gut and investigate its metabolic roles in SD-associated energy wasting. First, we found that gut AstA knockdown in control flies driven by either AstA33- or pros-GAL4 did not affect sleep homeostasis (Fig. 3a). In 50A07 > TrpA1 SD flies, gut AstA deficiency did not improve severe sleep loss either (Fig. 3b, c), excluding the impacts of gut AstA on sleep regulation. We next examined the metabolic changes and found that gut AstA removal using either AstA33- or pros-GAL4 in control files with normal sleep hardly affected the systemic storages of TAG and glycogen (Fig. 3d). Strikingly, gut AstA deficiency dramatically improved the loss of TAG and glycogen reserves in 50A07 > TrpA1 flies and restored them to the levels close to control flies (Fig. 3d). We further confirmed these results using another independent AstA RNAi line as well as 68A07 > TrpA1 SD flies (Fig. 3e). Note that, we hardly observed the expression of 50A07-LexA, 68A07-LexA and 11H05-GAL4 in either AstA EEs or neurons (Supplementary Fig. S3a–d). Further, flies bearing only UAS-TrpA1, UAS-AstA-RNAi, or LexAop-TrpA1 insertion exhibited normal sleep and carbo-lipid metabolism (Supplementary Fig. S1f–h). These results excluded the possibilities that leaky expression of TrpA1 in AstA neurons leads to energy wasting. Finally, we consistently observed that, in flies with mSD, gut AstA deficiency using either AstA33- or pros-GAL4 potently rescued the loss of TAG and glycogen reserves without affecting sleep loss (Fig. 3f, g). By contrast, the knockdown of AstA in the brain by > 50%, but not in the gut, using a weak strain of elav-GAL4 failed to affect energy wasting in mSD flies (Supplementary Fig. S1i–k). Taken together, our results demonstrate that gut AstA production is essential for SD-induced energy wasting.
a Sleep of 6-day-old female flies with indicated genotypes (n = 31 for AstA33>, n = 16 for pros>, left, sleep in every 30 min per day; right, total sleep per day). b Immunostaining of intestinal AstA (red) in flies of indicated genotypes after SD for 10 days. c Sleep in every 30 min per day of indicated flies (+, n = 30; AstA-i, n = 25). d, e TAG and glycogen storages of 7-day-old females of the indicated genotypes with or without SD for 10 days (n = 4, 5 flies/replicate). f Sleep of 5-day-old female flies with indicated genotypes (n = 15 for AstA33>, n = 16 for pros>, left, sleep in every 30 min per 24/48 h). g TAG and glycogen storages of 5-day-old females of the indicated genotypes with or without mSD for 8 days (n = 4, 5 flies/replicate). Data were presented as means ± SEM. *P < 0.05.
Gut AstA remotely promotes Akh release
Since it is well established that nutrients regulate the production of gut-peptide hormones, we also investigated the metabolic roles of gut AstA under nutrient availability. Similar to SD, starvation for 36 h significantly increased gut AstA release as indicated by the decline of intracellular AstA protein (Supplementary Fig. S4a) and systemic storages of TAG and glycogen (Supplementary Fig. S4b). Interestingly, gut AstA deficiency using two different RNA lines (Supplementary Fig. S4b–d), as well as Rpr-induced AstA-EE ablation (Supplementary Fig. S4e–h), potently restored systemic TAG and glycogen storages after starvation and increased fly survival rates during starvation.
We asked how AstA modulates systemic metabolic homeostasis and exploited the potential molecular mechanisms. Akh is a metabolic hormone that is released from APCs upon nutrient deprivation to activate AkhR/cAMP/Ca2+ cascade in the fat body, leading to the carbo-lipid utilization by promoting target gene expression associated with lipolysis and glycogenolysis35,36,37,38. As the metabolic phenotypes associated with gut AstA deficiency are reminiscent of the phenotype of Akh mutants, we tested whether gut AstA regulates carbo-lipid metabolism through Akh. To monitor Akh release, we generated a polyclonal antibody from a rabbit against Drosophila Akh (see Materials and Methods). The immunostaining results indicated that the signals of the Akh antibody overlap with APCs only in control but not Akh-null mutant flies (AkhA and AkhSAP)39 (Supplementary Fig. S5a, b). No obvious signals of Akh antibody were found in the brain as well, indicating no interactions between our Akh antibody and Wamide, Akh-related, or other peptides (Supplementary Fig. S5c). We also observed increased Akh amounts in the hemolymph of control but not Akh-null mutant flies under starvation using dot-blot assays (Supplementary Fig. S5d), demonstrating the specificity of our Akh antibody. Interestingly, gut AstA removal robustly suppressed Akh release, as indicated by decreased Akh amounts in the hemolymph and increased intracellular Akh accumulation in APCs (Supplementary Fig. S5e, f), and suppressed systemic expression of Akh-target gene tobi (Supplementary Fig. S6g) under starvation. These results indicate that gut AstA remotely promotes Akh release.
AstA-R2, a GPCR of AstA, has been previously reported to be expressed in the APCs25. We confirmed this result using a specific AstA-R2-GAL4 driver that expresses GFP in all endogenous AstA-R2-expressing cells (Supplementary Fig. S6a). Consistent with gut AstA deficiency, knockdown of AstA-R2 expression in APCs using Akh-GAL4 led to a lower intracellular Ca2+ level as indicated by CaLexA reporter40, increased intracellular Akh accumulation, reduced tobi expression, as well as elevated TAG and glycogen storages, under starvation and decreased starvation-induced mortality (Supplementary Fig. S6b–d, g, h).
To investigate whether the metabolic actions of AstA is Akh dependent, we tested the effect of gut AstA removal in AkhA/SAP mutant flies. Similar to the effect of loss of gut AstA, TAG storages after starvation were increased by ~50% in AkhA/SAP flies (Supplementary Fig. S6e). Strikingly, gut AstA knockdown using AstA33-GAL4 in AkhA/SAP flies did not cause a further increase in TAG levels as compared to either AkhA/SAP or AstA33 > AstA-i alone (Supplementary Fig. S6e). We also simultaneously knocked down AstA in the midgut and AstA-R2 in the APCs using AstA33-GAL4 and Akh-GAL4, respectively. Again, TAG storages and tobi expression after starvation, as well as starvation resistance, were not further increased by gut AstA deficiency plus AstA-R2 knockdown in APCs (Supplementary Fig. S6f–h). Altogether, these results demonstrate that gut AstA regulates metabolic homeostasis by remotely enhancing Akh release.
SD augments Akh release via gut AstA production
We wonder whether AstA–Akh axis contributes to SD-induced energy wasting. To address this hypothesis, we first examined the Akh release under SD. Interestingly, decreased intracellular Akh accumulation in APCs, elevated circulating Akh amounts in the hemolymph, and increased tobi expression were consistently observed in different SD flies (Fig. 4a–c), indicating enhancement of Akh secretion and systemic Akh response. Note that, we failed to observe any changes in systemic expression of 4EBP, an indicator of insulin signaling, in SD flies (Fig. 4c). We next knocked down gut AstA expression in SD flies and, as expected, observed suppression of systemic Akh responses as indicated by tobi expression (Fig. 4d). Furthermore, in order to examine whether Akh is essential for SD-induced energy wasting, we performed SD in AkhA/SAP mutants using both thermogenetic and mechanical methods. We found that Akh deficiency potently restored TAG and glycogen storages in 50A07 > TrpA1, 68A07 > TrpA1, and mSD flies (Fig. 4e, f). Even though Akh deficiency elevated TAG and glycogen storages at basal conditions (Fig. 4e, f), we still observed that both thermogenetic and mSD resulted in TAG and glycogen loss to a much lesser extent in AkhA/SAP mutant flies as compared to that in control flies (Fig. 4e, f). Collectively, our data demonstrate that SD augments Akh release via gut AstA production to cause energy wasting.
a, b Confocal images (Z-stack projection) of intracellular Akh (a, green) and hemolymph Akh amounts (b, loading protein amounts were indicated by Ponceau S staining) of the indicated 6-day-old flies after SD for 10 days (TrpA1) or 8 days (mutant). c–f Whole-body gene expression (c, d, n = 4, 5 flies/replicate) and TAG and glycogen storages (e, f, n = 4, 5 flies/replicate) of indicated flies after SD for 10 days (TrpA1), 8 days (mutant), or 8 days (mSD). Data were presented as means ± SEM. *P < 0.05.
Gut galanin promotes glucagon release to impair carbo-lipid metabolism in SD mice
Given the fact that Akh is functionally equivalent to mammalian glucagon mobilizing systemic energy storages41, we next ask whether a similar AstA/Akh axis also exists in SD mice to impair energy balance. Galanin receptors (GALR1/2/3), the mammalian homologs of AstA-R2, were shown to be activated by the gut hormone galanin42,43. To examine whether GALR signaling directly enhances glucagon release, we treated cultured mouse pancreatic α-cells (αTC1) with galanin and measured glucagon secretion. Interestingly, galanin increased glucagon release into the medium potently and mildly under low- and high-glucose conditions, respectively, without significantly affecting the expression of its receptors (Fig. 5a and Supplementary Fig. S7a). Incubation with M35, an established inhibitor of galanin receptors44, significantly suppressed galanin-induced glucagon secretion (Fig. 5a). We also examined the in vivo regulation of glucagon release by GALR signaling. We injected intraperitoneally (IP) galanin into C57BL/6 mice that were fasted overnight and observed a significant increase in serum glucagon levels, which was further suppressed by M35 IP injection (Fig. 5b). Consistently, acute IP injection of galanin also caused hyperglycemia in wild-type (WT), but not Gcg−/− mice (Supplementary Fig. S7b, c), indicating a glucagon-dependent role. Further, blockade of GALR signaling by M35 IP injection potently improved hyperglycemia, hyperglucagonemia, as well as glucagon-associated hepatic glycogen depletion, of streptozotocin (STZ)-treated mice with insulin deficiency45 (Fig. 5c and Supplementary Fig. S7d–g), excluding the participation of insulin. Taken together, our results demonstrate that, like AstA/Akh axis in Drosophila, mammalian galanin potentiates glucagon release in a conserved manner.
a Glucagon secretion (R&D ELISA kit) in αTC1 cells that were treated with 100 nM galanin (Gal) or 1 nM M35 for 4 h in DMEM containing different doses of glucose. b Plasma glucagon levels (R&D ELISA kit) in 8-week male C57BL/6 mice that were IP injected with galanin (0.32 mg/kg) with or without M35 (22 μg/kg) (n = 10). c Plasma glucagon levels (R&D ELISA kit) of STZ-treated mice that were IP injected with or without M35 (0.5 mg/kg/day) (n = 14 per group) as compared to control mice treated with PBS (n = 8). d Strategies of SD in mice using the water bath method. e–i Gut GAL mRNA (e), serum glucagon (f) (Sangon ELISA kit), hepatic G6p mRNA levels (g), body weight changes (h), and eWAT weights (i) of indicated 10-week mice. C57BL/6 mice were IP injected with M35 (0.5 mg/kg/day) and GRA Ex-25 (GRA) (5 mg/kg/day) for 3 days and 1 day, respectively, before being transferred into a water bath platform for SD for 2 days (Con, n = 7; SD, n = 10; SD + M35, n = 11; SD + GRA, n = 10). j, k Body weight changes (j) and eWAT weights (k) of indicated mice with or without SD for 2 days (Gcg+/+, n = 8; Gcg+/++ SD, n = 8; Gcg−/−+ SD, n = 8). l, m Hepatic PAS staining to indicate glycogen level (l) and H&E staining to indicate adipocyte mass (m) of indicated mice with SD and pharmaceutical intervention. Data were presented as means ± SEM. *P < 0.05.
Finally, we assessed the impact of the galanin/glucagon axis on energy homeostasis in SD mice using a multi-platform of water bath46 (Fig. 5d). Interestingly, the intestinal expression of Galanin (GAL) gene, serum glucagon level, as well as hepatic target gene expression of glucagon, were significantly increased in the mice after 2-day SD (Fig. 5e–g). In line with the catabolic effects of glucagon on carbo-lipid storages, mice also exhibited weight loss, hepatic glycogen depletion, as well as lipid loss, under SD (Fig. 5h, i, l, m). Importantly, blockade of GALR signaling by M35 IP injection for 3 days prior to SD sufficiently decreased serum glucagon level and its associated metabolic activity, including hepatic target gene expression, hepatic glycogen depletion, lipid loss, and weight decline (Fig. 5f–i, l, m). To further verify the essential roles of glucagon, we deprived sleep of Gcg−/− mice or blocked glucagon response using a small-molecule inhibitor, GRA Ex-25 (GRA). Consistent with M35 injection, either deficiency of glucagon or GRA IP injection sufficiently restored energy homeostasis, including lipid and glycogen contents and body weight, in the context of SD (Fig. 5h–m). Note that, glucagon deficiency in control mice with normal sleep hardly caused loss of body weight, adipose tissues, and hepatic glycogen (Supplementary Fig. S7h–k). Altogether, our results demonstrate that SD promotes galanin production, at least in the gut, to enhance glucagon release, leading to energy wasting.
SD increases gut AstA production via ROS
To explore the mechanisms of aberrant production in gut AstA by SD, we analyzed the gene expression pattern of AstA EEs from a published dataset of single-cell RNA sequencing (scRNA-seq)16. Among the 22 distinct cell clusters identified in the adult midgut, the AstA-EE cluster exhibits 1555 marker gene expression as compared to others (Supplementary Table S1). Gene Ontology enrichment analysis of these marker genes revealed a significant enrichment of genes involved in EE-associated cellular processes like “peptide secretion”, “vesicle endocytosis”, “cell adhesion”, and “glucose metabolism” (Fig. 6a). Interestingly, genes that are involved in “response to oxidative stress” are also significantly enriched (Fig. 6a). Since only severe (50A07 > TrpA1, 68A07 > TrpA1, sssΔ40, mSD flies), but not moderate (DATfmn) sleep loss triggers oxidative stress and ROS production in the adult fly midgut8 (Supplementary Figs. S1e, S8a–d), we wondered whether ROS triggers gut AstA production under SD. To address it, we fed different SD flies with the antioxidant lipoic acid (LA), which diminishes ROS accumulation8. Interestingly, as compared to controls, LA feeding significantly decreased gut ROS amount and increased intracellular AstA level in 50A07 > TrpA1, 68A07 > TrpA1, and sssΔ40 flies (Fig. 6b, c and Supplementary Fig. S8a–c), indicating decreased AstA release. LA feeding also decreased Akh release, systemic Akh response indicated by tobi expression, and loss of systemic glycogen and lipid in SD flies, but not in normal flies (Fig. 6d–g and Supplementary Fig. S8e).
a Gene Ontology enrichment analysis of genes that are specifically expressed in the AstA-EE cluster. b, c Immunostaining of AstA (b) and quantification of intracellular AstA signal in each single cell (c, n > 100) in the posterior midgut of female flies of the indicated flies with SD for 6 days (TrpA1) or 6 days (mutant). d–g Whole-body gene expression (d, e) and TAG and glycogen storages (f, g) of indicated flies with 2 mM LA feeding in the food together with SD for 10 days (TrpA1) or 8 days (mutant) (n = 4, 5 flies/replicate). Data were presented as means ± SEM. *P < 0.05.
TrpA1 mediates ROS-induced gut AstA production
Through scRNA-seq analysis, we further identified TrpA1, a major ROS sensor controlling gut hormone release47, as a top gene that is specifically expressed in AstA EEs (Fig. 7a). We examined GFP expression driven by a TrpA1-GAL4 and observed that it is co-localized with AstA expression in the posterior midgut (Fig. 7b). Using knock-in T2A-GAL4 lines that report the expression of individual TrpA1 isoforms48, we also revealed the expression of TrpA1-C and -D that respond to ROS in AstA EEs (Supplementary Fig. S8f)49. To examine whether TrpA1 regulates AstA production in response to ROS, we knocked down TrpA1 specifically in the AstA EEs of both thermogenetic 50A07 > TrpA1 and mSD flies. We strikingly found that AstA release and Akh-induced tobi induction were decreased and that energy-wasting phenotypes, including TAG and glycogen depletion, were remarkably improved (Fig. 7c–g). Note that, TrpA1 knockdown in AstA EEs of control flies with normal sleep failed to elevate systemic TAG and glycogen storages (Fig. 7f). Taken together, these results indicated that ROS promotes gut AstA production via TrpA1 in SD flies.
a UMAP of TrpA1 expression in different cell clusters of adult midguts. b Immunostaining of AstA (red) and TrpA1>GFP (green) expression in the posterior region of the adult midgut. c–g Immunostaining of AstA (c) and quantification of intracellular AstA signal in each single cell (d, n > 100) in the posterior midgut, gut AstA mRNA (e, n = 3, 15 flies/replicate), whole-body gene expression (f, g, left, n = 4, 5 flies/replicate), and TAG and glycogen storages (f, g, right, n = 4, 5 flies/replicate) of the indicated genotypes after SD for 10 days (TrpA1) or 8 days (mSD). Data were presented as means ± SEM. *P < 0.05.
Discussion
In this study, we uncover that SD enhances the production of gut AstA to cause energy wasting. The molecular mechanisms include that, at least, gut AstA remotely targets APCs to promote Akh release, leading to systemic loss of lipid and glycogen. We also show similar regulation in SD mammals whereby galanin signaling enhances glucagon secretion to cause energy wasting. Finally, we demonstrate that gut AstA EEs sense the intestinal ROS through TrpA1 to increase AstA production (Fig. 8).
Fly AstA EEs express TrpA1 to sense SD-induced ROS in the gut lumen and enhance AstA production to remotely target Akh-producing cells via AstA-R2, leading to increased Akh secretion and systemic energy mobilization. Similar AstA/galanin-triggered Akh/glucagon secretion and energy loss is observed in sleep-deprived mice.
Sleep loss has been highly associated with metabolic dysregulation1; however, the metabolic output in different SD flies remains controversial in Drosophila. Specifically, a decline of whole-body TAG storages has only been observed in severe sleep-loss flies (50A07 > TrpA1, 68A07 > TrpA1, sssΔ40, and mSD), but not in moderate sleep-loss (DATfmn) flies. A recent study reported extensive ROS accumulation only in the guts of flies under severe, but not moderate, sleep loss8. In this study, we consistently found that the diminishment of gut ROS by feeding of LA, a ROS scavenger, sufficiently restores energy storages in severe sleep-loss flies, indicating the important roles of gut ROS in systemic energy maintenance. We further elucidated the mechanisms that gut ROS promotes the production of a gut-peptide hormone AstA to affect systemic carbo-lipid metabolism in an endocrinal manner. In support of our notion, intestinal AstA production was found to be increased only in severe sleep-loss flies. Thus, our results demonstrate ROS/AstA axis in the gut as the key pathogenic factor mediating metabolic dysregulation in various SD flies.
Genes encoding gut-peptide hormones are also expressed in the brain of Drosophila18. It is essential to use specific drivers to investigate the functions and regulations of EE-derived gut hormones such as Tk and NPF, distinguishing from the ones produced in the brain. Many studies managed to use pros-GAL4 that targets all EEs in the gut to knock down the expression of peptide hormones21,34. However, it is difficult to perform loss-of-function in certain subtype EEs for investigating physiological regulations such as nutrient or stress signals of gut-peptide production. We have previously generated a specific Tk-g-GAL4 that predominantly expresses in the gut Tk EEs, and that allows us and other groups to reveal the metabolic functions and regulations of Tk in the gut19,20,22,50,51. In this study, we further generated a specific AstA33-GAL4 that predominantly targets AstA EEs in the gut. We characterized the critical roles of gut AstA as mobilizing systemic lipid and carbohydrate storages under SD integrating AstA33- and pros-GAL4, even though the participation of AstA derived by certain neurons was not completely excluded. Moreover, we used this AstA33-GAL4 to uncover TrpA1 as an important regulator sensing intracellular ROS to promote AstA production under SD. Since multiple functional receptors and stress sensors are found to be expressed in the gut EEs to orchestrate the hormone production in the context of physiological and environmental cues11, we believe our AstA33-Gal4 will offer opportunities to exploit diverse regulation of AstA release under SD and beyond.
Even though AstA-R2 was found to be expressed in APCs and to regulate Akh release25, the origin(s) of functional AstA was not well characterized. Here we confirmed the interaction between gut AstA and Akh in APCs genetically, as AstA-R2 knockdown in APCs plus AstA knockdown in EEs failed to further impair energy balance as compared to the knockdown of each of them individually. Similar results were obtained by performing Akh deficiency plus AstA knockdown in EEs. On the other hand, it has been reported that the production of Akh, which functions as a central metabolic hormone, is both positively and negatively modulated by multiple gut-peptide hormones like NPF and Burs21,34,51, highlighting a sophisticated Yin-Yang maintenance of Akh release and systemic energy homeostasis. It will be important to investigate whether other gut-peptide hormones also participate in SD-induced energy loss.
TrpA1 encodes an ion channel sensing extracellular stresses, including ROS, to activate Ca2+ signaling and regulate diverse biological processes in Drosophila47,49. In this study, we found that both AstA release and AstA-EE numbers were significantly increased by gut ROS in SD flies. Note that, TrpA1 knockdown in AstA EEs using AstA33-GAL4 diminished the increase only in AstA release but not in AstA-EE numbers of SD flies, demonstrating the essential roles of TrpA1 in AstA EEs to sense ROS and promote AstA release. It is in line with previous studies of TrpA1 expression in EEs and TrpA1 regulation of DH31 release47. Previous studies have shown that TrpA1-associated Ca2+ signaling in ISCs is required for both self-proliferation and differentiation into EEs49,52; therefore, it is possible that ROS impacts ISCs via TrpA1 to increase AstA-EE number. We performed TrpA1 knockdown in ISCs of 50A07 > TrpA1 flies and, however, failed to observe any changes of AstA-EE numbers, AstA production, or carbo-lipid metabolism (data not shown), excluding TrpA1 roles in ISC differentiation into AstA EEs in SD flies.
Similar to AstA/Akh axis in Drosophila, we also confirmed that galanin-mediated glucagon release is essential for SD-induced energy loss in mammals, indicating an evolutionarily conserved phenomenon. Previous studies have suggested that galanin directly targets β-cells and inhibits the secretion of insulin that potently suppresses glucagon release53, generating a plausible hypothesis that galanin indirectly enhances glucagon secretion via diminishing insulin-leashed glucagon release. Here, we provide evidence that galanin directly impacts glucagon secretion. First, galanin promotes glucagon secretion in cultured α-cells and triggers glucagon-dependent hyperglycemia in mice. Second, blocking galanin signaling sufficiently impairs glucagon secretion and glycemic elevation under conditions with impaired insulin response, including chronic fasting and STZ-induced β-cell loss. In support of our conclusion, elevations of blood galanin, glucagon, and glucose levels were observed in both type 1 and 2 diabetic patients with impaired insulin response54,55,56. Nevertheless, mammalian Kiss 1 receptor (KISS1R) also shares homology (albeit lower than GALRs) with AstA-R2 as indicated by DIOPT Ortholog Finder57; it would be interesting to study the roles of KISS1R signaling in SD-induced energy wasting as well. Altogether, our results reveal that gut galanin modulates glucagon release to augment energy wasting in SD mice and provide novel pharmaceutical opportunities for the treatment of metabolic disorders in SD patients.
Materials and methods
Drosophila Strains
UAS-Rpr (#5823), Akh-GAL4 (#25684), UAS-srcGFP (#5432), UAS-CaLexA-GFP (#66542), UAS-TrpA1 (#26264), UAS-AstA-RNAi-JF (JF01905, #25866), TrpA1-GAL4 (#36922), UAS-AR2-RNAi-JF (JF01955, #25935), elav-GAL4/CyO,GFP (#8765), AstA-R2-GAL4 (#76727), 11H05-GAL4 (#45016), 50A07-LexA (#61586), and 68A07-LexA (#53538) were obtained from Bloomington Drosophila Stock Center (BDSC). UAS-AstA-R2-i-NIG (NIG10001R-1) and UAS-AstA-i-v (v103215) were obtained from Fly Stocks of the National Institute of Genetics (NIG, Japan) and Vienna Drosophila Resource Center (VDRC), respectively. AkhSAP and AkhA were kind gifts from Dr. Ronald Kühnlein (University of Graz);39 sssΔ40, DATfmn, and matched control flies wiso31 from Dr. Chang Liu (Shenzhen Institutes of Advanced Technology, China); LexAop-TrpA1 from Dr. Yufeng Pan (Southeast University, China); KI-T2A-GAL4 lines for different TrpA1 isoforms from Drs. Yang Xiang (UMass Chan Medical School) and Pengyu Gu (Southeast University, China)48. Negative controls, w1118 and yv;attp2, exhibited similar phenotypes. More information on flies used in this study was included in Supplementary Table S2.
The promoter region of the AstA gene was cloned by classical PCR using primers, 5′-GCGCAATTGGGGAAAAATCTCCGAAAACC-3′ and 5′-GCCGGATCCAGAGGTTCCGCGGACTAAAT-3′, to generate different AstA-GAL4 flies. We crossed these AstA-GAL4 lines to UAS-srcGFP to examine the expression patterns and used AstA33-GAL4 that targets all AstA EEs but very limited AstA neurons in this study.
Flies were maintained on food, which contained 30 g yeast, 50 g corn flour, and 50 g Maltose in 1 L distilled water, in 65% humidity and 25 °C incubators in a 12-h light 12-h dark (12:12 LD) cycle. For LA treatment, virgin flies were transferred onto food containing 2 mM LA (A506197, Sangon Biotech) every 2 days. The genotypes of flies used in this study were included in Supplementary Table S3.
SD and sleep measurement in flies
Thermogenetic SD was performed using the Gal4/UAS and LexA/LexAop systems. Flies were raised in 12:12 LD cycles at 18 °C until 6–7-day post eclosion. The temperature was raised to a constant 29 °C, which triggered SD. Deprivation was done in 12:12 LD cycles.
mSD was performed at 25 °C using a multi-tubes vortexer. Flies were raised at 25 °C (12:12 LD) until day 4–5 post eclosion. After a day of baseline sleep recording, the multi-tube vortexer delivered 10-s vibrations at intervals centered around 50 s. The intensity of the vortexer was set to 4 (~1500 rpm).
5-day old female flies were transferred to separated glass tubes containing 5% sucrose agar food, then the tubes were sealed with a ventilated rubber plug. The tubes were inserted into Drosophila activity monitors to measure activities under a 12:12 LD at 25 °C or 29 °C.
Mice
Male Gcg−/− (T014382, Gempharmatech) and C57BL/6 mice were maintained at room temperature (21 ± 2 °C), the humidity of 50% ± 15%, and a 12-h cycle of light and dark in the animal facility of Medical Research Institute, Wuhan University and fed either a normal chow diet ad libitum with free access to water. All animal experiments were performed under animal ethical regulations and the study protocol was approved by the Institutional Animal Care and Use Committee of Wuhan University.
We IP injected 0.32 mg/kg galanin (T510180, Sangon Biotech) with or without 22 μg/kg M35 (GWTLNSAGYLLGPPPGFSPFR-NH2, purity: 98%, Cusabio) dissolved in PBS into 8–10-week-old C57BL/6 and Gcg−/− male mice at the overnight-fasting state. To disrupt the integrity of pancreatic β-cells, we IP injected 40 mg/kg/day STZ (T1507, TargetMol, USA) dissolved in 0.1 M citrate buffer (pH 4.5) into C57BL/6 mice under a fasting state for five consecutive days. Mice with blood glucose higher than 16.7 mM or 300 mg/dL were characterized as diabetic subjects for subsequent IP injection of 0.5 mg/kg/day M35 dissolved in PBS. As for SD, mice were transferred to a multiple-platform water bath with free access to water and food for 48 h under controlled conditions of temperature (22–24 °C), humidity (55%–60%), and a 12:12 LD cycle. Multiple small platforms (2.5 cm) were placed in a tank (40 × 30 cm) filled with water to within 1–4 cm of the upper surface of the platforms and spaced 4 cm. Mice were IP injected with 0.5 mg/kg/day M35 to block galanin response for 3 days or 5 mg/kg/day GRA-Ex25 (T3422 TargetMol, USA) to block glucagon resonse for 1 day prior to SD. Orbital blood samples were collected under anesthesia to examine serum glucagon levels using an ELISA kit (DGCG0, R&D Systems; D721189, Sangon Biotech) according to the standard protocol. Each group contained at least eight mice.
Generation of anti-Akh antibodies
Polyclonal antibodies against Drosophila Akh were prepared by Dai-An Biotech in Wuhan, China. The Akh peptides (CQLTFSPDWa), which were conjugated to keyhole limpet hemocyanin (KLH) through the added N-terminal cysteine, were immunized in rabbits four times. The antisera were purified by an affinity column coupled with Akh peptides. The polyclonal Akh antibodies have been diluted and sourced to a commercialized company as an OEM product (A22867, Abclonal).
Immunostaining, lipid staining, and microscopy
Gut, brain, and fat-body tissues from adult flies were dissected in PBS and fixed for 15 min in 4% formaldehyde/PBS. After fixation, the samples were washed with 0.2% Triton/PBS and incubated in primary antibody overnight at 4 °C. After incubation of second antibody (1:1000, Alexa Fluor, Thermo Fisher) plus DAPI (1:1000, Thermo Fisher Scientific) at room temperature for 1 h, tissues were washed and mounted in Antifade Mounting Medium (P0128M, Beyotime). Primary antibodies used in this study included anti-AstA (1:2000)58, anti-Tk (1:5000)19, anti-Prosper (1:100, MR1A, Developmental Studies Hybridoma Bank), anti-Akh (1:1000, A22867, ABclonal). Bodipy 493/503 (1 mg/mL, D3922, Thermo Fisher Scientific) were used for staining of neutral lipids.
For DHE staining, adult Drosophila guts were dissected with PBS containing 2 μM DHE (S0063, Beyotime) medium at room temperature in darkness for 15 min. Before mounting, samples were briefly washed with PBS three times and fixed with 4% paraformaldehyde at room temperature for 20 min.
Dissected mouse liver samples were fixed in 10% neutral buffered formalin for 48 h and performed Periodic acid Schiff (PAS) staining to detect glycogen by Wuhan Servicebio Biotechnology according to a standard protocol. Microscopy was performed on an inverted Zeiss LSM880 laser scanning confocal microscope. For quantification of AstA, Akh, and CaLexA staining intensities, stacks were Z-projected, and the signals of AstA in each midgut cell and Akh or CaLexA (GFP) in the whole CC were quantified using Integrated Density in ImageJ. The background was subtracted to give the net signal.
Cell culture and glucagon secretion
αTC1 cells were cultured in DMEM (PM150211, ProCell, China) supplemented with 1 g/L glucose, 10% FBS and antibiotics. After incubation with 100 nM galanin with or without 1 nM M35 for 4 h, αTC1 cells were washed and cultured with DMEM containing different doses of glucose for 4 h. The supernatants were collected and centrifuged at 5000× g for 5 min at 4 °C to remove cells and debris. Glucagon contents in the supernatants were detected using an ELISA kit (DGCG0, R&D Systems).
qPCR
Fifteen brains, 15 midguts, or five adult flies from each genotype and αTC1 cells were lysed to extract total RNA using Trizol (Invitrogen). cDNA synthesis was performed using HiScript II reverse transcriptase supermix (R223-01, Vazyme). Quantitative real-time PCR was performed using ChamQ SYBR master mix (Q311-02, Vazyme) on a CFX384 Real-Time System/C1000 Thermal Cycler (BioRad). Fly and mouse gene expressions were normalized to RpL32 and β-actin, respectively. qPCR primers used in this study are listed in Supplementary Table S4.
TG and glycogen measurement
Five flies that were fed or starved from each group were homogenized with 0.5 mL PBS containing 0.2% Triton X-100 and heated at 70 °C for 5 min. The supernatant was collected after centrifugation at 12,000 rpm for 10 min at 4 °C. Ten microliters of supernatant was used for protein quantification using Bradford Reagent (E111-01, Vazyme). Whole-body glycogen levels were measured from 10 μL of supernatant treated with or without 0.4 μL Amyloglucosidase (2 mg/mL, A7420, Sigma) to degrade glycogen into glucose at 37 °C for 30 min using glucose assay reagent (K-GLUC, Megazyme) following the manufacturer’s protocol. We subtracted the amount of free glucose from the measurement and then normalized the subtracted values to protein levels in the supernatant. To measure whole-body triglycerides, we processed 10 μL of supernatant using a Serum Triglyceride Determination kit (TR0100, Sigma). We subtracted the amount of free glycerol in the supernatant from the measurement and then normalized the subtracted values to protein levels in the supernatant.
Dot-blot analysis of hemolymph Akh
Forty fed or 60 starved flies were decapitated and centrifuged at 1000× g for 15 min at 4 °C in the 0.6 mL Eppendorf tube embedding in a 1.5 mL Eppendorf tube. About 0.1 μL hemolymph was diluted into 10 μL PBS and loaded onto 0.2 μM nitrocellulose membrane (GE Healthcare) and air-dried for 20 min. The membrane was subsequently boiled in PBS for 3 min, fixed with 4% PFA for 20 min, and recovered in PBS for 5 min at room temperature. After Ponceau Red staining as loading control, the membrane was blocked with 5% BSA in PBS for 1 h and incubated with purified anti-Akh antibodies (1:1000, Abclonal, A22867) in 5% BSA at 4 °C overnight, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies in 5% BSA in PBS for 1 h at room temperature. The membrane was developed using X-ray film in the darkroom.
Mortality under starvation
Adult flies of each genotype were aged for 5–6 days at 25 °C then transferred into 1% agar food (just agar and H2O). Dead flies were counted every 12 h.
Statistical analysis
Data were representative of observations made in at least two independent experiments. Data were presented as the means ± SEM. Unpaired Student’s t-test and one-way ANOVA followed by post hoc test were performed to assess differences. A P value of < 0.05 was considered statistically significant.
References
Bringmann, H. Genetic sleep deprivation: using sleep mutants to study sleep functions. EMBO Rep. 20, e46807 (2019).
Mavanji, V., Billington, C. J., Kotz, C. M. & Teske, J. A. Sleep and obesity: a focus on animal models. Neurosci. Biobehav. Rev. 36, 1015–1029 (2012).
Novati, A., Hulshof, H. J., Granic, I. & Meerlo, P. Chronic partial sleep deprivation reduces brain sensitivity to glutamate N-methyl-D-aspartate receptor-mediated neurotoxicity. J. Sleep Res. 21, 3–9 (2012).
Koban, M. & Swinson, K. L. Chronic REM-sleep deprivation of rats elevates metabolic rate and increases UCP1 gene expression in brown adipose tissue. Am. J. Physiol. Endocrinol. Metab. 289, E68–E74 (2005).
Zager, A., Andersen, M. L., Ruiz, F. S., Antunes, I. B. & Tufik, S. Effects of acute and chronic sleep loss on immune modulation of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R504–R509 (2007).
Killgore, W. D. et al. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 9, 517–526 (2008).
Villafuerte, G. et al. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid. Med. Cell Longev. 2015, 234952 (2015).
Vaccaro, A. et al. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell 181, 1307–1328.e15 (2020).
Gribble, F. M. & Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 15, 226–237 (2019).
Miguel-Aliaga, I., Jasper, H. & Lemaitre, B. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics 210, 357–396 (2018).
Zhou, X. et al. Physiological and pathological regulation of peripheral metabolism by gut-peptide hormones in Drosophila. Front. Physiol. 11, 577717 (2020).
Kwon, Y. et al. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev. Cell 33, 36–46 (2015).
Song, W. et al. Tumor-Derived Ligands Trigger Tumor Growth and Host Wasting via Differential MEK Activation. Dev. Cell 48, 277–286.e6 (2019).
Ding, G. et al. Coordination of tumor growth and host wasting by tumor-derived Upd3. Cell Rep. 36, 109553 (2021).
Chen, Y. et al. Renal NF-kappaB activation impairs uric acid homeostasis to promote tumor-associated mortality independent of wasting. Immunity 55, 1594–1608.e6 (2022).
Hung, R. J. et al. A cell atlas of the adult Drosophila midgut. Proc. Natl. Acad. Sci USA 117, 1514–1523 (2020).
Guo, X. et al. The cellular diversity and transcription factor code of Drosophila enteroendocrine cells. Cell Rep. 29, 4172–4185.e5 (2019).
Reiher, W. et al. Peptidomics and peptide hormone processing in the Drosophila midgut. J. Proteome Res. 10, 1881–1892 (2011).
Song, W., Veenstra, J. A. & Perrimon, N. Control of lipid metabolism by tachykinin in Drosophila. Cell Rep. 9, 40–47 (2014).
Ameku, T. et al. Midgut-derived neuropeptide F controls germline stem cell proliferation in a mating-dependent manner. PLoS Biol. 16, e2005004 (2018).
Scopelliti, A. et al. A neuronal relay mediates a autrient responsive gut/fat body axis regulating energy homeostasis in adult Drosophila. Cell Metab. 29, 269–284 e210 (2019).
Song, W. et al. Midgut-derived activin regulates glucagon-like action in the fat body and glycemic control. Cell Metab. 25, 386–399 (2017).
Chen, J., Kim, S. M. & Kwon, J. Y. A systematic analysis of Drosophila regulatory peptide expression in enteroendocrine cells. Mol. Cells 39, 358–366 (2016).
Chen, J. et al. Allatostatin A signalling in Drosophila regulates feeding and sleep and is modulated by PDF. PLoS Genet. 12, e1006346 (2016).
Hentze, J. L., Carlsson, M. A., Kondo, S., Nassel, D. R. & Rewitz, K. F. The neuropeptide allatostatin A regulates metabolism and feeding decisions in Drosophila. Sci Rep. 5, 11680 (2015).
Hergarden, A. C., Tayler, T. D. & Anderson, D. J. Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc. Natl. Acad. Sci. USA 109, 3967–3972 (2012).
Deveci, D., Martin, F. A., Leopold, P. & Romero, N. M. AstA signaling functions as an evolutionary conserved mechanism timing juvenile to adult transition. Curr. Biol. 29, 813–822.e4 (2019).
Thimgan, M. S., Suzuki, Y., Seugnet, L., Gottschalk, L. & Shaw, P. J. The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. PLoS Biol. 8, e1000466 (2010).
Erion, R., DiAngelo, J. R., Crocker, A. & Sehgal, A. Interaction between sleep and metabolism in Drosophila with altered octopamine signaling. J. Biol. Chem. 287, 32406–32414 (2012).
Thimgan, M. S., Seugnet, L., Turk, J. & Shaw, P. J. Identification of genes associated with resilience/vulnerability to sleep deprivation and starvation in Drosophila. Sleep 38, 801–814 (2015).
Cirelli, C. et al. Reduced sleep in Drosophila shaker mutants. Nature 434, 1087–1092 (2005).
Stavropoulos, N. & Young, M. W. insomniac and Cullin-3 regulate sleep and wakefulness in Drosophila. Neuron 72, 964–976 (2011).
Ueno, T. et al. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat. Neurosci. 15, 1516–1523 (2012).
Kubrak, O. et al. The gut hormone allatostatin C/somatostatin regulates food intake and metabolic homeostasis under nutrient stress. Nat. Commun. 13, 692 (2022).
Lee, G. H. & Park, J. H. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167, 311–323 (2004).
Kim, S. K. & Rulifson, E. J. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431, 316–320 (2004).
Bharucha, K. N., Tarr, P. & Zipursky, S. L. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J. Exp. Biol. 211, 3103–3110 (2008).
Baumbach, J., Xu, Y., Hehlert, P. & Kuhnlein, R. P. Galphaq, Ggamma1 and Plc21C control Drosophila body fat storage. J. Genet. Genomics 41, 283–292 (2014).
Galikova, M. et al. Energy homeostasis control in Drosophila adipokinetic hormone mutants. Genetics 201, 665–683 (2015).
Masuyama, K., Zhang, Y., Rao, Y. & Wang, J. W. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J. Neurogenet. 26, 89–102 (2012).
Ahmad, M., He, L. & Perrimon, N. Regulation of insulin and adipokinetic hormone/glucagon production in flies. Wiley Interdiscip. Rev. Dev. Biol. 9, e360 (2020).
Webling, K. E., Runesson, J., Bartfai, T. & Langel, U. Galanin receptors and ligands. Front. Endocrinol. 3, 146 (2012).
Tatemoto, K., Rokaeus, A., Jornvall, H., McDonald, T. J. & Mutt, V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 164, 124–128 (1983).
Kask, K. et al. Binding and agonist/antagonist actions of M35, galanin(1-13)-bradykinin(2-9)amide chimeric peptide, in Rin m 5F insulinoma cells. Regul. Pept. 59, 341–348 (1995).
Furman, B. L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 70, 5.47.1–5.47.20 (2015).
Kang, W. S., Park, H. J., Chung, J. H. & Kim, J. W. REM sleep deprivation increases the expression of interleukin genes in mice hypothalamus. Neurosci. Lett. 556, 73–78 (2013).
Du, E. J. et al. TrpA1 regulates defecation of food-borne pathogens under the control of the Duox pathway. PLoS Genet. 12, e1005773 (2016).
Gu, P. et al. Polymodal nociception in Drosophila requires alternative splicing of TrpA1. Curr. Biol. 29, 3961–3973.e6 (2019).
Xu, C., Luo, J., He, L., Montell, C. & Perrimon, N. Oxidative stress induces stem cell proliferation via TRPA1/RyR-mediated Ca(2+) signaling in the Drosophila midgut. Elife 6, e22441 (2017).
Amcheslavsky, A. et al. Enteroendocrine cells support intestinal stem-cell-mediated homeostasis in Drosophila. Cell Rep. 9, 32–39 (2014).
Yoshinari, Y. et al. The sugar-responsive enteroendocrine neuropeptide F regulates lipid metabolism through glucagon-like and insulin-like hormones in Drosophila melanogaster. Nat. Commun. 12, 4818 (2021).
Deng, H., Gerencser, A. A. & Jasper, H. Signal integration by Ca(2+) regulates intestinal stem-cell activity. Nature 528, 212–217 (2015).
Tang, G. et al. Go2 G protein mediates galanin inhibitory effects on insulin release from pancreatic beta cells. Proc. Natl. Acad. Sci. USA 109, 2636–2641 (2012).
Legakis, I., Mantzouridis, T. & Mountokalakis, T. Positive correlation of galanin with glucose in type 2 diabetes. Diabetes Care 28, 759–760 (2005).
Celi, F. et al. Circulating acylated and total ghrelin and galanin in children with insulin-treated type 1 diabetes: relationship to insulin therapy, metabolic control and pubertal development. Clin. Endocrinol. 63, 139–145 (2005).
Lee, Y. H., Wang, M. Y., Yu, X. X. & Unger, R. H. Glucagon is the key factor in the development of diabetes. Diabetologia 59, 1372–1375 (2016).
Li, Z., Cardoso, J. C. R., Peng, M., Inacio, J. P. S. & Power, D. M. Evolution and potential function in molluscs of neuropeptide and receptor homologues of the insect allatostatins. Front. Endocrinol. 12, 725022 (2021).
Veenstra, J. A., Agricola, H. J. & Sellami, A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 334, 499–516 (2008).
Acknowledgements
We thank the BDSC, VDRC, and NIG for providing fly stocks; the Core Facility of Drosophila Resource and Technology, Center for Excellence in Molecular Cell Science, and Chinese Academy of Sciences for providing fly stocks; Dr. Ronald Kühnlein for AkhSAP and AkhA flies; Dr. Chang Liu for sssΔ40, DATfmn, and wiso31 flies; Dr. Yufeng Pan for LexAop-TrpA1 flies; Drs. Yang Xiang and Pengyu Gu for T2A-GAL4 lines for different TrpA1 isoforms. Work in the Song lab was supported by the Chinese National Natural Science Foundation (91957118, 31800999, and 31971079), the Ministry of Science and Technology (the National Key R&D Program of China 2021YFC2700704), and the Fundamental Research Funds for the Central Universities (2042022dx0003).
Author information
Authors and Affiliations
Contributions
Y.L. and X.Z. performed fly and mouse experiments. C.C. and K.T. performed mouse experiments. G.D. and P.Z. identified anti-Akh antibodies and performed dot-blot analysis. L.C. and L.Z. performed fly sleep analysis. N.P. and J.A.V. generated AstA33-GAL4 flies. W.S. analyzed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Zhou, X., Cheng, C. et al. Gut AstA mediates sleep deprivation-induced energy wasting in Drosophila. Cell Discov 9, 49 (2023). https://doi.org/10.1038/s41421-023-00541-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41421-023-00541-3