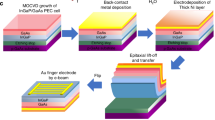
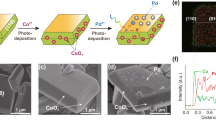
Abstract
Harvesting solar energy to convert CO2 into chemical fuels is a promising technology to curtail the growing atmospheric CO2 levels and alleviate the global dependence on fossil fuels; however, the assembly of efficient and robust systems for the selective photoconversion of CO2 without sacrificial reagents and external bias remains a challenge. Here we present a photocatalyst sheet that converts CO2 and H2O into formate and O2 as a potentially scalable technology for CO2 utilization. This technology integrates lanthanum- and rhodium-doped SrTiO3 (SrTiO3:La,Rh) and molybdenum-doped BiVO4 (BiVO4:Mo) light absorbers modified by phosphonated Co(ii) bis(terpyridine) and RuO2 catalysts onto a gold layer. The monolithic device provides a solar-to-formate conversion efficiency of 0.08 ± 0.01% with a selectivity for formate of 97 ± 3%. As the device operates wirelessly and uses water as an electron donor, it offers a versatile strategy toward scalable and sustainable CO2 reduction using molecular-based hybrid photocatalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of the study are available in the paper and its supplementary materials. Source data for the main figures (Figs. 2–5) are provided with the paper. Other source data supporting the findings of this study are available from the Cambridge data repository (https://doi.org/10.17863/CAM.54840).
References
Costentin, C., Robert, M. & Savéant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 42, 2423–2436 (2013).
Francke, R., Schille, B. & Roemelt, M. Homogeneously catalyzed electroreduction of carbon dioxide—methods, mechanisms, and catalysts. Chem. Rev. 118, 4631–4701 (2018).
Sato, S., Morikawa, T., Saeki, S., Kajino, T. & Motohiro, T. Visible-light-induced selective CO2 reduction utilizing a ruthenium complex electrocatalyst linked to a p-type nitrogen-doped Ta2O5 semiconductor. Angew. Chem. Int. Ed. 49, 5101–5105 (2010).
Wang, W.-H., Himeda, Y., Muckerman, J. T., Manbeck, G. F. & Fujita, E. CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction. Chem. Rev. 115, 12936–12973 (2015).
Kuriki, R. et al. Nature-inspired, highly durable CO2 reduction system consisting of a binuclear ruthenium(ii) complex and an organic semiconductor using visible light. J. Am. Chem. Soc. 138, 5159–5170 (2016).
Liu, X., Inagaki, S. & Gong, J. Heterogeneous molecular systems for photocatalytic CO2 reduction with water oxidation. Angew. Chem. Int. Ed. 55, 14924–14950 (2016).
Kuriki, R. et al. Robust binding between carbon nitride nanosheets and a binuclear ruthenium(ii) complex enabling durable, selective CO2 reduction under visible light in aqueous solution. Angew. Chem. Int. Ed. 56, 4867–4871 (2017).
Dalle, K. E. et al. Electro- and solar-driven fuel synthesis with first row transition metal complexes. Chem. Rev. 119, 2752–2875 (2019).
Tu, W., Zhou, Y. & Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: state-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 26, 4607–4626 (2014).
Sato, S. et al. Selective CO2 conversion to formate conjugated with H2O oxidation utilizing semiconductor/complex hybrid photocatalysts. J. Am. Chem. Soc. 133, 15240–15243 (2011).
Arai, T., Sato, S., Kajino, T. & Morikawa, T. Solar CO2 reduction using H2O by a semiconductor/metal-complex hybrid photocatalyst: enhanced efficiency and demonstration of a wireless system using SrTiO3 photoanodes. Energy Environ. Sci. 6, 1274–1282 (2013).
Sahara, G. et al. Photoelectrochemical reduction of CO2 coupled to water oxidation using a photocathode with a Ru(ii)–Re(i) complex photocatalyst and a CoOx/TaON photoanode. J. Am. Chem. Soc. 138, 14152–14158 (2016).
Kumagai, H. et al. Hybrid photocathode consisting of a CuGaO2 p-type semiconductor and a Ru(ii)–Re(i) supramolecular photocatalyst: Non-biased visible-light-driven CO2 reduction with water oxidation. Chem. Sci. 8, 4242–4249 (2017).
Nakada, A. et al. Solar water oxidation by a visible-light-responsive tantalum/nitrogen-codoped rutile titania anode for photoelectrochemical water splitting and carbon dioxide fixation. ChemPhotoChem 3, 37–45 (2019).
Wang, Q. et al. Particulate photocatalyst sheets based on carbon conductor layer for efficient Z-scheme pure-water splitting at ambient pressure. J. Am. Chem. Soc. 139, 1675–1683 (2017).
Pinaud, B. A. et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 6, 1983–2002 (2013).
Lian, S., Kodaimati, M. S., Dolzhnikov, D. S., Calzada, R. & Weiss, E. A. Powering a CO2 reduction catalyst with visible light through multiple sub-picosecond electron transfers from a quantum dot. J. Am. Chem. Soc. 139, 8931–8938 (2017).
Rao, H., Schmidt, L. C., Bonin, J. & Robert, M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature 548, 74–77 (2017).
Cometto, C. et al. A carbon nitride/Fe quaterpyridine catalytic system for photostimulated CO2-to-CO conversion with visible light. J. Am. Chem. Soc. 140, 7437–7440 (2018).
Li, P. et al. All-solid-state Z-scheme system arrays of Fe2V4O13/RGO/CdS for visible light-driving photocatalytic CO2 reduction into renewable hydrocarbon fuel. Chem. Commun. 51, 800–803 (2015).
Iwase, A. et al. Water splitting and CO2 reduction under visible light irradiation using Z-scheme systems consisting of metal sulfides, CoOx-loaded BiVO4, and a reduced graphene oxide electron mediator. J. Am. Chem. Soc. 138, 10260–10264 (2016).
Suzuki, T. M. et al. Z-schematic and visible-light-driven CO2 reduction using H2O as an electron donor by a particulate mixture of a Ru-complex/(CuGa)1−xZn2xS2 hybrid catalyst, BiVO4 and an electron mediator. Chem. Commun. 54, 10199–10202 (2018).
Li, X., Yu, J., Jaroniec, M. & Chen, X. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem. Rev. 119, 3962–4179 (2019).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016).
Minegishi, T., Nishimura, N., Kubota, J. & Domen, K. Photoelectrochemical properties of LaTiO2N electrodes prepared by particle transfer for sunlight-driven water splitting. Chem. Sci. 4, 1120–1124 (2013).
Lin, F. et al. Photocatalytic oxidation of thiophene on BiVO4 with dual co-catalysts Pt and RuO2 under visible light irradiation using molecular oxygen as oxidant. Energy Environ. Sci. 5, 6400–6406 (2012).
Ma, X. et al. Surface photovoltage spectroscopy observes sub-band-gap defects in hydrothermally synthesized SrTiO3 nanocrystals. J. Phys. Chem. C 123, 25081–25090 (2019).
Leung, J. J. et al. Solar-driven reduction of aqueous CO2 with a cobalt bis(terpyridine)-based photocathode. Nat. Catal. 2, 354–365 (2019).
Kudo, A., Omori, K. & Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 121, 11459–11467 (1999).
Chang, X. et al. Enhanced surface reaction kinetics and charge separation of p−n heterojunction Co3O4/BiVO4 photoanodes. J. Am. Chem. Soc. 137, 8356–8359 (2015).
Bassegoda, A., Madden, C., Wakerley, D. W., Reisner, E. & Hirst., J. Reversible interconversion of CO2 and formate by a molybdenum-containing formate dehydrogenase. J. Am. Chem. Soc. 136, 15473–15476 (2014).
Wakerley, D. W. & Reisner, E. Oxygen-tolerant proton reduction catalysis: much O2 about nothing? Energy Environ. Sci. 8, 2283–2295 (2015).
Ryu, J., T. N. Andersen, T. N. & Eyring, H. The electrode reduction kinetics of carbon dioxide in aqueous solution. J. Phys. Chem. 76, 3278–3286 (1972).
Akira, M. & Yoshio, H. Product selectivity affected by cationic species in electrochemical reduction of CO2 and CO at a Cu electrode. Bull. Chem. Soc. Jpn. 64, 123–127 (1991).
Pérez-Gallent, E., Marcandalli, G., Figueiredo, M. C., Calle-Vallejo, F. & Koper, M. T. M. Structure- and potential-dependent cation effects on CO reduction at copper single-crystal electrodes. J. Am. Chem. Soc. 139, 16412–16419 (2017).
Feng, S. et al. Enriched surface oxygen vacancies of photoanodes by photoetching with enhanced charge separation. Angew. Chem. Int. Ed. 59, 2044–2048 (2020).
Hykaway, N., Sears, W. M., Morisaki, H. & Morrison, S. R. Current-doubling reactions on titanium dioxide photoanodes. J. Phys. Chem. 90, 6663–6667 (1986).
Sekizawa, K., Sato, S., Arai, T. & Morikawa, T. Solar-driven photocatalytic CO2 reduction in water utilizing a ruthenium complex catalyst on p-type Fe2O3 with a multiheterojunction. ACS Catal. 8, 1405–1416 (2018).
Leung, J. J., Vigil, J. A., Warnan, J., Edwardes Moore, E. & Reisner, E. Rational design of polymers for selective CO2 reduction catalysis. Angew. Chem. Int. Ed. 58, 7697–7701 (2019).
Bélanger, D. & Jean Pinson, J. Electrografting: a powerful method for surface modification. Chem. Soc. Rev. 40, 3995–4048 (2011).
Wang, Q. et al. Z-scheme water splitting using particulate semiconductors immobilized onto metal layers for efficient electron relay. J. Catal. 328, 308–315 (2015).
Iwase, A., Ng, Y. H., Ishiguro, Y., Kudo, A. & Amal, R. Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light. J. Am. Chem. Soc. 133, 11054–11057 (2011).
Reisner, E. et al. Data Set for Molecularly Engineered Photocatalyst Sheet for Scalable Solar Formate Production from Carbon Dioxide and Water (Cambridge Data Repository, 2020); https://doi.org/10.17863/CAM.54840
Sasaki, Y., Iwase, A., Katoa, H. & Kudo, A. The effect of co-catalyst for Z-scheme photocatalysis systems with an Fe3+/Fe2+ electron mediator on overall water splitting under visible light irradiation. J. Catal. 259, 133–137 (2008).
Acknowledgements
We thank C. Sahm and A. Eisenschmidt at the University of Cambridge for assisting with the isotopic labelling experiment, S. Roy and M. Rahaman at the University of Cambridge for helpful discussions, S. Young at the University of Cambridge for performing ICP–OES analysis, and M. Isaacs at HarwellXPS for carrying out XPS. This work was supported by EU Marie Sklodowska-Curie individual Fellowships (GAN 793996 to Q.W. and GAN 744317 to S.K.), European Commission Future and Emerging Technologies (FET) Open programme project SoFiA (GAN 828838 to S.R.-J. and E.R.), Christian Doppler Research Association (Austrian Federal Ministry for Digital and Economic Affairs and the National Foundation for Research, Technology and Development) and the OMV Group (to J.W. and E.R.). V.A. is grateful for the financial support from the Cambridge Trusts (Vice-Chancellor’s Award) and the Winton Programme for the Physics of Sustainability. J.J.L. is supported by the Woolf Fisher Trust in New Zealand. The XPS data collection was performed at the EPSRC National Facility for XPS (HarwellXPS)—operated by Cardiff University and University College London—under contract no. PR16195.
Author information
Authors and Affiliations
Contributions
Q.W. and E.R. conceived the idea. E.R. supervised the project. J.W., J.J.L. and S.R.-J. synthesised and characterized CotpyP. Q.W. prepared the photocatalyst sheet and photoelectrodes and conducted physical characterizations and photo(electro)chemical experiments. S.K. carried out NMR spectroscopy, V.A. assisted with O2 quantification and scalability tests. All authors analysed the data, discussed the results and assisted with manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Tables 1–4 and refs. 1–10.
Source data
Source Data Fig. 2
Numerical data used to generate graphs.
Source Data Fig. 3
Numerical data used to generate graphs.
Source Data Fig. 4
Numerical data used to generate graphs and statistical Source Data.
Source Data Fig. 5
Numerical data used to generate graphs.
Rights and permissions
About this article
Cite this article
Wang, Q., Warnan, J., Rodríguez-Jiménez, S. et al. Molecularly engineered photocatalyst sheet for scalable solar formate production from carbon dioxide and water. Nat Energy 5, 703–710 (2020). https://doi.org/10.1038/s41560-020-0678-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-0678-6
This article is cited by
-
Solar-driven sugar production directly from CO2 via a customizable electrocatalytic–biocatalytic flow system
Nature Communications (2024)
-
Multiscale structural engineering of cobalt single-atom catalyst for highly efficient photocatalytic CO2 reduction
Science China Materials (2024)
-
Agave sisalana: towards distributed manufacturing of absorbent media for menstrual pads in semi-arid regions
Communications Engineering (2023)
-
Light-driven synthesis of C2H6 from CO2 and H2O on a bimetallic AuIr composite supported on InGaN nanowires
Nature Catalysis (2023)
-
Photocatalytic CO2 reduction
Nature Reviews Methods Primers (2023)