Abstract
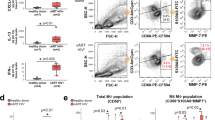
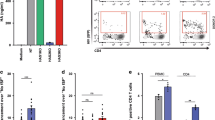
Human immunodeficiency virus type 1 (HIV-1) eradication is prevented by the establishment on infection of cellular HIV-1 reservoirs that are not fully characterized, especially in genital mucosal tissues (the main HIV-1 entry portal on sexual transmission). Here, we show, using penile tissues from HIV-1-infected individuals under suppressive combination antiretroviral therapy, that urethral macrophages contain integrated HIV-1 DNA, RNA, proteins and intact virions in virus-containing compartment-like structures, whereas viral components remain undetectable in urethral T cells. Moreover, urethral cells specifically release replication-competent infectious HIV-1 following reactivation with the macrophage activator lipopolysaccharide, while the T-cell activator phytohaemagglutinin is ineffective. HIV-1 urethral reservoirs localize preferentially in a subset of polarized macrophages that highly expresses the interleukin-1 receptor, CD206 and interleukin-4 receptor, but not CD163. To our knowledge, these results are the first evidence that human urethral tissue macrophages constitute a principal HIV-1 reservoir. Such findings are determinant for therapeutic strategies aimed at HIV-1 eradication.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request.
References
Sengupta, S. & Siliciano, R. F. Targeting the latent reservoir for HIV-1. Immunity 48, 872–895 (2018).
Gaudin, R. et al. Dynamics of HIV-containing compartments in macrophages reveal sequestration of virions and transient surface connections. PLoS ONE 8, e69450 (2013).
Castellano, P., Prevedel, L. & Eugenin, E. A. HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci. Rep. 7, 12866 (2017).
Swingler, S., Mann, A. M., Zhou, J., Swingler, C. & Stevenson, M. Apoptotic killing of HIV-1-infected macrophages is subverted by the viral envelope glycoprotein. PLoS Pathog. 3, 1281–1290 (2007).
Clayton, K. L. et al. Resistance of HIV-infected macrophages to CD8+ T lymphocyte-mediated killing drives activation of the immune system. Nat. Immunol. 19, 475–486 (2018).
Hashimoto, D. et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Murray, P. J. Macrophage polarization. Annu. Rev. Physiol. 79, 541–566 (2017).
Cassol, E., Cassetta, L., Alfano, M. & Poli, G. Macrophage polarization and HIV-1 infection. J. Leukoc. Biol. 87, 599–608 (2010).
Bailey, J. R. et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 80, 6441–6457 (2006).
Perelson, A. S. et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387, 188–191 (1997).
Rasmussen, T. A. et al. Comparison of HDAC inhibitors in clinical development: effect on HIV production in latently infected cells and T-cell activation. Hum. Vaccin. Immunother. 9, 993–1001 (2013).
Honeycutt, J. B. et al. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Invest. 126, 1353–1366 (2016).
Honeycutt, J. B. et al. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat. Med. 23, 638–643 (2017).
Sullivan, P. S., Salazar, L., Buchbinder, S. & Sanchez, T. H. Estimating the proportion of HIV transmissions from main sex partners among men who have sex with men in five US cities. AIDS 23, 1153–1162 (2009).
Ganor, Y. et al. The adult penile urethra is a novel entry site for HIV-1 that preferentially targets resident urethral macrophages. Mucosal Immunol. 6, 776–786 (2013).
Ganor, Y. et al. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans–T cell conjugates. Mucosal Immunol. 3, 506–522 (2010).
Zhou, Z. et al. HIV-1 efficient entry in inner foreskin is mediated by elevated CCL5/RANTES that recruits T cells and fuels conjugate formation with Langerhans cells. PLoS Pathog. 7, e1002100 (2011).
Jensen, M. A. et al. Improved coreceptor usage prediction and genotypic monitoring of R5-to-X4 transition by motif analysis of human immunodeficiency virus type 1 env V3 loop sequences. J. Virol. 77, 13376–13388 (2003).
Sasaki, Y., Ohsawa, K., Kanazawa, H., Kohsaka, S. & Imai, Y. Iba1 is an actin-cross-linking protein in macrophages/microglia. Biochem. Biophys. Res. Commun. 286, 292–297 (2001).
Prevedel, L. et al. Identification, localization, and quantification of HIV reservoirs using microscopy. Curr. Protoc. Cell Biol. https://doi.org/10.1002/cpcb.64 (2018).
Laird, G. M., Rosenbloom, D. I., Lai, J., Siliciano, R. F. & Siliciano, J. D. Measuring the frequency of latent HIV-1 in resting CD4+ T cells using a limiting dilution coculture assay. Methods Mol. Biol. 1354, 239–253 (2016).
Fun, A., Mok, H. P., Wills, M. R. & Lever, A. M. A highly reproducible quantitative viral outgrowth assay for the measurement of the replication-competent latent HIV-1 reservoir. Sci. Rep. 7, 43231 (2017).
Sanyal, A. et al. Novel assay reveals a large, inducible, replication-competent HIV-1 reservoir in resting CD4+ T cells. Nat. Med. 23, 885–889 (2017).
Cassol, E., Cassetta, L., Rizzi, C., Alfano, M. & Poli, G. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J. Immunol. 182, 6237–6246 (2009).
Sharova, N., Swingler, C., Sharkey, M. & Stevenson, M. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 24, 2481–2489 (2005).
Zanin-Zhorov, A. et al. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J. Immunol. 179, 41–44 (2007).
Tough, D. F., Sun, S. & Sprent, J. T cell stimulation in vivo by lipopolysaccharide (LPS). J. Exp. Med. 185, 2089–2094 (1997).
Pudney, J. & Anderson, D. J. Expression of toll-like receptors in genital tract tissues from normal and HIV-infected men. Am. J. Reprod. Immunol. 65, 28–43 (2011).
Orenstein, J. M. Replication of HIV-1 in vivo and in vitro. Ultrastruct. Pathol. 31, 151–167 (2007).
Orenstein, J. M., Meltzer, M. S., Phipps, T. & Gendelman, H. E. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J. Virol. 62, 2578–2586 (1988).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004).
Baxter, A. E. et al. Macrophage infection via selective capture of HIV-1-infected CD4+ T cells. Cell Host Microbe 16, 711–721 (2014).
Calantone, N. et al. Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity 41, 493–502 (2014).
DiNapoli, S. R. et al. Tissue-resident macrophages can contain replication-competent virus in antiretroviral-naive, SIV-infected Asian macaques. JCI Insight 2, e91214 (2017).
Chomont, N. et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15, 893–900 (2009).
Wiegand, A. et al. Single-cell analysis of HIV-1 transcriptional activity reveals expression of proviruses in expanded clones during ART. Proc. Natl Acad. Sci. USA 114, E3659–E3668 (2017).
Avalos, C. R. et al. Brain macrophages in simian immunodeficiency virus-infected, antiretroviral-suppressed macaques: a functional latent reservoir. MBio 8, e01186-17 (2017).
Kandathil, A. J. et al. No recovery of replication-competent HIV-1 from human liver macrophages. J. Clin. Invest. 128, 4501–4509 (2018).
Cassetta, L. et al. M1 polarization of human monocyte-derived macrophages restricts pre and postintegration steps of HIV-1 replication. AIDS 27, 1847–1856 (2013).
Bruner, K. M. et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 22, 1043–1049 (2016).
Ho, Y. C. et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551 (2013).
Real, F., Sennepin, A., Ganor, Y., Schmitt, A. & Bomsel, M. Live imaging of HIV-1 transfer across T cell virological synapse to epithelial cells that promotes stromal macrophage infection. Cell Rep. 23, 1794–1805 (2018).
Catalfamo, M., Le Saout, C. & Lane, H. C. The role of cytokines in the pathogenesis and treatment of HIV infection. Cytokine Growth Factor Rev. 23, 207–214 (2012).
Clerici, M. & Shearer, G. M. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol. Today 14, 107–111 (1993).
Houzet, L., Matusali, G. & Dejucq-Rainsford, N. Origins of HIV-infected leukocytes and virions in semen. J. Infect. Dis. 210, S622–S630 (2014).
Galvin, S. R. & Cohen, M. S. The role of sexually transmitted diseases in HIV transmission. Nat. Rev. Microbiol. 2, 33–42 (2004).
Matusali, G. et al. Detection of simian immunodeficiency virus in semen, urethra, and male reproductive organs during efficient highly active antiretroviral therapy. J. Virol. 89, 5772–5787 (2015).
Dumaurier, M. J., Gratton, S., Wain-Hobson, S. & Cheynier, R. The majority of human immunodeficiency virus type 1 particles present within splenic germinal centres are produced locally. J. Gen. Virol. 86, 3369–3373 (2005).
Chun, T. W. et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl Acad. Sci. USA 94, 13193–13197 (1997).
Liszewski, M. K., Yu, J. J. & O’Doherty, U. Detecting HIV-1 integration by repetitive-sampling Alu-gag PCR. Methods 47, 254–260 (2009).
Folks, T. M., Justement, J., Kinter, A., Dinarello, C. A. & Fauci, A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 238, 800–802 (1987).
Spina, C. A. et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 9, e1003834 (2013).
Bagasra, O., Wright, S. D., Seshamma, T., Oakes, J. W. & Pomerantz, R. J. CD14 is involved in control of human immunodeficiency virus type 1 expression in latently infected cells by lipopolysaccharide. Proc. Natl Acad. Sci. USA 89, 6285–6289 (1992).
Fujihara, M. et al. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol. Ther. 100, 171–194 (2003).
Pomerantz, R. J., Feinberg, M. B., Trono, D. & Baltimore, D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J. Exp. Med. 172, 253–261 (1990).
Hirsch, V. M. et al. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J. Virol. 69, 955–967 (1995).
Salmon, H. et al. Ex vivo imaging of T cells in murine lymph node slices with widefield and confocal microscopes. J. Vis. Exp. 15, e3054 (2011).
Cromey, D. W. Avoiding twisted pixels: ethical guidelines for the appropriate use and manipulation of scientific digital images. Sci. Eng. Ethics 16, 639–667 (2010).
Dutertre, C. A. et al. Pivotal role of M-DC8+ monocytes from viremic HIV-infected patients in TNFα overproduction in response to microbial products. Blood 120, 2259–2268 (2012).
Sennepin, A. et al. NKp44L expression on CD4+ T cells is associated with impaired immunological recovery in HIV-infected patients under highly active antiretroviral therapy. AIDS 27, 1857–1866 (2013).
Sennepin, A. et al. The human penis is a genuine immunological effector site. Front. Immunol. 8, 1732 (2017).
Acknowledgements
The authors thank J. P. Wolf (Reproductive Biology, Hôpital Cochin, AP-HP, Paris, France) for helpful discussion. This study was supported by grants from l’Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) to M.B. (ANRS-2014AO21038) and A.H. (ANRS-2016AO11023), and from the French Government’s Investissement d’Avenir programme, Laboratoire d’Excellence ‘Integrative Biology of Emerging Infectious Diseases’ (ANR-10-LABX-62-IBEID) to A.H. C.-A.D., F.R. and J.-P.J. were supported by ANRS. F.R. was supported by SIDACTION. L.X. was supported by the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
Y.G. and M.B. conceived the study, designed the experiments and wrote the paper. Y.G. performed the majority of the experiments. A.S., F.R., C.-A.D., J.-P.J. and Z.Z. performed the flow cytometry experiments, which were designed by A.H. F.R. and S.M. performed the p24 staining and confocal microscopy experiments. A.S., L.X., D.T., B.C. and A.C.-C. performed the PCR experiments, which were designed by R.C. L.P. and E.A.E. performed and designed the DNA FISH experiments. A.-R.Z. performed the immunohistochemistry experiments. M.R. and S.C. provided the penile tissues. A.S. performed the electron microscopy experiments. C.C. provided T cells from HIV-1-infected patients under suppressive cART.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–7 and Supplementary Tables 1–2.
Supplementary Data
High resolution original images obtained by microscopy used to build panels.
Rights and permissions
About this article
Cite this article
Ganor, Y., Real, F., Sennepin, A. et al. HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat Microbiol 4, 633–644 (2019). https://doi.org/10.1038/s41564-018-0335-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0335-z
This article is cited by
-
A macrophage-cell model of HIV latency reveals the unusual importance of the bromodomain axis
Virology Journal (2024)
-
Immune targeting of HIV-1 reservoir cells: a path to elimination strategies and cure
Nature Reviews Microbiology (2024)
-
Epigenetic modulation of myeloid cell functions in HIV and SARS-CoV-2 infection
Molecular Biology Reports (2024)
-
HIV-1 Myeloid Reservoirs — Contributors to Viral Persistence and Pathogenesis
Current HIV/AIDS Reports (2024)
-
Viruses, bacteria and parasites: infection of the male genital tract and fertility
Basic and Clinical Andrology (2023)