Abstract
Rheumatoid arthritis (RA) is a systemic autoimmune disease whose main extra-articular organ affected is the lung, sometimes in the form of diffuse interstitial lung disease (ILD) and conditions the prognosis. A multicenter, observational, descriptive and cross-sectional study of consecutive patients diagnosed with RA-ILD. Demographic, analytical, respiratory functional and evolution characteristics were analyzed to evaluate the predictors of progression and mortality. 106 patients were included. The multivariate analysis showed that the diagnostic delay was an independent predictor of mortality (HR 1.11, CI 1.01–1.23, p = 0.035). Also, age (HR 1.33, 95% CI 1.09–1.62, p = 0.0045), DLCO (%) (HR 0.85, 95% CI 0.73–0.98, p = 0.0246), and final SatO2 (%) in the 6MWT (HR 0.62, 95% CI 0.39–0.99, p = 0.0465) were independent predictor variables of mortality, as well as GAP index (HR 4.65, 95% CI 1.59–13.54, p = 0.0051) and CPI index (HR 1.12, 95% CI 1.03–1.22, p = 0.0092). The withdrawal of MTX or LFN after ILD diagnosis was associated with disease progression in the COX analysis (HR 2.18, 95% CI 1.14–4.18, p = 0.019). This is the first study that highlights the diagnostic delay in RA-ILD is associated with an increased mortality just like happens in IPF.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is characterized by inflammation of synovial tissues and joint destruction that may involve other organs, including the lung1. Interstitial lung disease (ILD) is a common manifestation of RA and may precede the joint inflammation2. ILD associated to RA (RA-ILD) presents clinical and radiological features similar to idiopathic ILDs3. The prevalence of RA-ILD varies depending on the method used to diagnose the disease and the population under study4. Several studies based on reference cohorts have estimated a prevalence ranging between 1 and 58%5,6. According to Bongartz et al. approximately 1 in 10 patients with RA will be diagnosed of ILD during the course of their disease6.
ILD is one of the main causes of mortality in RA4. Predicting the evolution and outcome of RA-ILD patients is difficult because the evidence is based on small population and short-term follow-up studies. The most frequent radiological and histological pattern in RA-ILD is usual interstitial pneumonia (UIP)7. Furthermore, this pattern is associated with higher mortality compared to others such as non-specific interstitial pneumonia (NSIP). In fact, the survival of RA-ILD patients with a UIP pattern is closer to that patients diagnosed of idiopathic pulmonary fibrosis (IPF)8.
Oher factors associated with a higher mortality in RA-ILD are age, male sex, worse pulmonary function at diagnosis, duration of RA, disease RA activity, extensive lung involvement on chest HRCT and elevated serum levels of Krebs von den Lungen 6 (KL-6) biomarker9,10,11,12,13. Although these findings are all based on small series of cases, they have been reproduced consistently in different patient cohorts. Furthermore, predictive factors of disease progression are the presence of UIP pattern, high titers of anti-citrullinated cyclic peptide (ACPA) antibodies, baseline deterioration of DLCO, a %FVC decline ≥ 10% during the follow-up, and elevated serum levels of interleukin 6 (IL-6) and KL-6. However, there is no clear definition of progression specifically for RA-ILD6,14,15,16,17.
Other reasons that make difficult to predict survival of RA-ILD patients is the heterogeneity in their evolution and the absence of an individual variable that is sufficiently accurate to predict mortality. In this regard, the use of multidimensional risk scales can be useful for physicians. The main ones are the GAP index that combines age, sex and pulmonary physiologic values and the composite physiologic index (CPI) that only includes pulmonary physiologic characteristics18,19. These models were originally developed for IPF but recent studies have assessed the utility of these predictive models in RA-ILD20. A better predictive prognostic model could help in setting the appropriate timing for lung transplantation or optimizing treatments.
Finally, the potential harmful effect of some common medications for RA remains controversial. There is not enough evidence to recommend a specific treatment for RA-ILD, consequently the optimal treatment for RA-ILD has not yet been established21. Current treatment regimens usually include corticosteroid therapy, methotrexate -MTX- , leflunomide -LEF-, and biologic disease-modifying anti-rheumatic drugs (e.g. rituximab, abatacept), immunosuppressant agents (cyclophosphamide and Mycophenolate Mofetil), and antifibrotic treatment (nintedanib)22. In counterpoint frequently used treatments, as MTX and LEF, have also been implicated in the development of pneumonitis or exacerbation of an existing ILD23, although the use of MTX has been recently associated with a better outcome24,25. In addition, some studies have shown an increased risk of infections associated to the immunosuppressive treatments26.
The aims of this study are: (a) to evaluate the predictive factors of mortality, (b) to investigate the potential associated factors to ILD progression.
Methods
Patient population
This is a multicenter, observational, descriptive and cross-sectional study of consecutive patients with diagnosis of RA-ILD from nine Spanish hospitals. Clinical records were reviewed between 2013 and 2018. The diagnosis of RA was done fulfilling ACR/EULAR 2010 criteria27 and the CTD-ILD classification according to the 2003 Respiratory Spanish Society (SEPAR) consensus3. The ILD pattern was identified and defined by HRCT. Patients with diagnosis of different CTD or other respiratory diseases were excluded. Only patients with ≥ 3 pulmonary function test measurements during the follow-up were included.
The Regional Research Ethics Committee of Galicia approved the study protocol (Registration Code 2016/176). Each participating hospital obtained the ethic approval from the local Human Research Ethics Committee. Informed consent was obtained from each patient.
Procedures were in accordance with guidelines established in the Declaration of Helsinki, and with the principles of Good Clinical Practices (GCPs).
Collected data and definitions
Demographic, epidemiological, radiological and treatment data were collected. Pulmonary disease progression was considered if the patient presented any of the following criteria: forced vital capacity (FVC) decrease ≥ 10%, diffusion capacity for carbon monoxide (DLCO) decrease ≥ 15%, radiological increase of lung fibrotic signs or death. Respiratory infectious events were defined as episodes of respiratory deterioration with or without respiratory failure that requires antibiotic treatment and may have required hospitalization. Heart failure or other non-respiratory reasons of hospitalization were excluded.
Two independent ILD expert radiologists evaluated the HRCT images over time and considered worsening of fibrotic radiological signs when an increase of more than 5% was identified.
Time to diagnosis of ILD from the onset of symptoms was classified as follows; < 12 months, 12–24 months, > 24 months (the last two groups were considered diagnostic delay).
The GAP index was calculated using the continuous predictor variables sex, age, and the pulmonary functional values, through the online model (www.acponline.org/journals/annals/extras/gap)18. Composite physiologic index (CPI) was calculated using the formula: CPI = 91-(0.65xDLCO% pred.)–(0.53xFVC% pred.) + (0.34 × FEV1% pred.)19.
Statistical analysis
The distribution of the continuous variables was verified with the Shapiro–Wilk test. The Student T-test was used to compare continuous variables if there was a normal distribution. If it did not have a normal distribution the Mann–Whitney U-test was used. The chi-squared test or Fisher test was used for comparison of categorical variables. Logistic regression analysis was used to identify significant variables capable of predicting respiratory infectious events.
Survival and progression differences between groups were evaluated by Kaplan–Meier analysis. Survival curves were compared with the log-rank test. A univariate Cox regression was performed to calculate the hazard ratio (HR) with a 95% confidence interval (CI) of the independent variables. A multivariate Cox regression model was carried out adjusting for all confounding variables that in univariate analysis had a p value < 0.2. Time to death was obtained from medical records and the censoring time was defined as the last medical visit or the end of the study at December 31, 2018.
It was considered appropriate to determine a cut-off level of CRP, ESR and RF by maximizing the Youden index from ROC curves for analysis of progression instead of using continuous data because they did not satisfy the assumption that the log hazard increased linearly with the covariate28. Since including variables with substantial missing data can introduce significant bias, anti-CCP antibodies and DAS28 score, which had a substantial number of missing values, were not included in the models of survival and progression.
The results were reported as mean ± standard deviation (SD). P < 0.05 was considered statistically significant. MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium) was used to perform all the analysis.
Results
Population
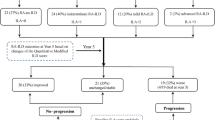
A total of 106 patients with RA-ILD were included, 61 women (57.5%). The mean age was 70.21 ± 9.8 years. RA was diagnosed 126.66 ± 117.28 months before the diagnosis of ILD. In 10 patients (9.43%) the ILD was present at the onset of disease. 40.6% of patients (n = 43) were never smokers. A confident UIP pattern in the HRCT was present in 51.9% of the patients. At the moment of ILD diagnosis 10 patients (9.43%) presented severe oxygen desaturation on effort (defined as SatO2 < 88% in the 6MWT). Therefore, a delay in the ILD identification was observed in a high proportion of patients. An abnormally high levels of anti-CCP was observed in 63 cases (66.8%). The rest of the characteristics are described in Table 1.
Predictors of disease progression
After defining the pre-specified variables of ILD progression, 53 patients (50%) presented disease progression. Of those progressors, 35 patients presented a FVC decline > 10%, 25 presented a decrease > 15% of DLCO and 24 an increase of the lung fibrotic signs in the HRCT. The respiratory functional impairment (FVC% and DLco%) is represented differentiating between the groups of progressive and non-progressive patients in the Fig. 1a, b.
After multivariate COX analysis, withdrawal of MTX or LFN after ILD diagnosis was associated to ILD progression in the first 5 years (HR 2.18, 95% CI 1.14–4.18, p = 0.019) (Table 2). From 88 patients that were treated with MTX or LFN previous ILD diagnosis , the medication was changed in 27 cases after the ILD identification due to the potential drug-induced ILD effect of MTX (n = 22) or the existence of extensive pulmonary fibrosis (n = 5). Patients in whom MTX and/or LFN were suspended had initially similar radiological pattern (p = 0.874) and respiratory function test values, FVC and DLCO, compared to those who were not discontinued (Supplementary data, Table S1). Disease progression free-survival curves over time are displayed in Fig. 2.
Survival predictors
Of the 106 subjects reviewed, 18 subjects died (17%). All of them presented fibrotic ILD. Causes of death were mostly related to ILD: 10 patients died of ILD progression, 3 of acute pneumonia and 4 of acute exacerbation of ILD. A missing data about the cause of death was present in one patient and no patient of the cohort underwent lung transplantation. Respiratory functional impairment in deceased patients is represented in Fig. 3a, b.
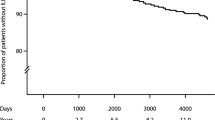
Patients had a median survival of 41.16 ± 27.94 months. Age (HR 1.33, 95% CI 1.09–1.62, p = 0.0045), DLCO predicted (%) (HR 0.85, 95% CI 0.73–0.98, p = 0.0246) and final oxygen saturation in the 6MWT (HR 0.62, 95% CI 0.39–0.99, p = 0.0465) were independent predictors of mortality in a multivariate model that included all those variables determined in the univariate analysis as a potentially influential predictors (Table 3).
The median time to ILD diagnosis after appearance of respiratory symptoms in RA patients was 14.36 ± 15.3 months. This information was available for 101 subjects. Also, diagnostic delay was an independent predictor of survival (HR 1.11, CI 1.01–1.23, p = 0.035) in the multivariate analysis, with no significant differences depending on received treatments or initial pulmonary functional severity (Supplementary data, Tables S2 and S3). The representation of survival by Kaplan–Meier curves, dividing the diagnostic delay in less than 12 months, 12–24 months and more than 24 months from the beginning of the respiratory symptomatology is displayed in Fig. 4.
Both, CPI and GAP indexes were predictors of mortality in the univariate Cox analysis. After adjusting for radiological pattern on HRCT, RPC > 23 mg/L, ESR > 33 mm/H, ILD diagnostic delay and final oxygen saturation in the 6MWT, GAP index remained as a significant predictor of mortality (HR 4.65, 95% CI 1.59–13.54, p = 0.0051). Furthermore, CPI index was a significant predictor of mortality in the multivariate analysis adjusted by age, radiological pattern on HRCT, ESR > 33 mm/H, RPC > 23 mg/L, ILD diagnostic delay and final oxygen saturation in the 6MWT (HR 1.12, 95% CI 1.03–1.22, p = 0.0092) (Table 3).
Among the deceased subjects, the median survival was 76.66 ± 46.23 months (n = 3) in the GAP stage I, 38.03 ± 17.35 months (n = 12) in the stage II and only 18 ± 9,54 months (n = 3) in the stage III. The best cut-off point of CPI index for predicting mortality was 50.58. The median survival was 51 ± 34.31 months (n = 9) for patients with CPI < 50 points. Nevertheless if the CPI score was > 50 points, the median survival was 31.33 ± 16.26 months (n = 9) (Fig. 5A, B) . The predictive value of CPI and GAP stages was assessed by ROC curve analysis (Fig. 6A, B).
(A) ROC curve for CPI and GAP indexes. For CPI, the AUC was 0.742 and the best cut-off point for predicting survival was 50.58 (sensitivity of 50% and specificity of 96.2%). The AUC for GAP ROC curve was 0.857 with a best cut-off point of 3 (sensitivity 83.3% and specificity 85.2%). (B) Receiver operator characteristic (ROC) curves for GAP stage (I, II and III) and CPI (with a cut-off point of 50) to predict mortality in AR-ILD patients.
Risk of respiratory infection in ILD-RA treatments
Of the 106 RA-ILD subjects, 21 (19.8%) suffered respiratory infectious events. Ten patients (9.4%) were being treated with corticosteroids plus azathioprine and 18 (17%) with biological agents. Logistic regression model, as reflected in Table 4, showed that the association of azathioprine plus corticosteroids was related with an increased risk of respiratory infection (OR 20.86, 95% CI 3.5–124.28, p = 0.0008).
Discussion
This is the first study that has identified diagnostic delay as a variable associated with an increased mortality in RA-ILD patients, independently of received treatment. The suspension of MTX and/or LFN was more frequently present among those cases that presented ILD progression. RA-ILD is the most frequent extra-articular manifestation in RA and it's considered the second cause of mortality after cardiovascular comorbidities in RA4,6.
The patient characteristics of this cohort are consistent with the previous published data29,30, highlighting the high prevalence of patients with tobacco exposure as well as the predominant UIP radiological pattern at diagnosis. Furthermore, in line with previous work6,31, age and pulmonary function were identified as predictive mortality variables.
Interestingly, this study demonstrates that the ILD diagnostic delay is a predictive factor of mortality in RA-ILD. After multivariate analysis, disease severity, radiologic pattern, sex and age did not influence in this result. IPF studies had suggested that the diagnostic delay increases mortality, due in part to the late evaluation by a multidisciplinary committee and the potential access to specialized and comprehensive treatment management32,33,34.
Regarding other predictors of mortality, age, DLCO at diagnosis and significant desaturation to effort in 6MWT were independent predictive variables in this cohort. In our study, survival was 41 months on average, similar to IPF8,9,17,35. There are several studies that have evaluated predictors of mortality in RA-ILD, including the age8,9,17,35,36. The main limitations of these studies have always been the methodology and sample size. Other variables as the disease severity assessed by DLCO have also been confirmed by Zamora et al.11. We also suggest desaturation during effort as a prognostic marker in RA-ILD, which is already known as poor prognostic factor in IPF but it had not yet been suggested in RA-ILD population37.
No significant differences in mortality were found according to the HRCT pattern of presentation, although all of them showed lung fibrotic features. Previous studies have suggested a different survival in RA-ILD with UIP pattern vs NSIP pattern38. However, this has not been a universal finding15, with a recent study by Zamora-Legoff et al. proving no association between different patterns and mortality16. The discrepancies in the previous reported cohorts could be due to the heterogeneity of NSIP and the broad spectrum of progression depending on the presence of fibrotic changes. Similar to IPF, the clue would be the presence of fibrotic radiological findings that are associated with disease progression; honeycombing or traction bronchiectasis39. Therefore, a RA-ILD patient with a UIP or a fibrotic NSIP pattern would have higher probability to death or disease progression than non-fibrotic NSIP or other forms of RA-ILDs.
The existence of this large number of prognostic variables of survival suggests the possibility of using multidimensional models in RA-ILD. Several multidimensional risk scales have been created and validated in ILD including gender, age and lung function (GAP index) for IPF18 and subsequently ILD-GAP index for non-IPF ILD40. In the Zamora-Legoff study, the GAP model demonstrated good discriminative and predictive value for RA-ILD in contrast to the ILD-GAP model which did not perform well16. This partially coincides with our results where the GAP index is a good marker of mortality in the multivariate analysis when adjusted for all those variables that can be predictive and are not included in the index itself. As we have shown, Numri et al. demonstrated in a multivariate analysis adjusted for age that the CPI index was also a predictor of mortality together with DLCO20.
MTX and LFN are first-line drugs in the treatment of RA to which other drugs are added during the course of the disease41. The association between MTX and acute pulmonary toxicity is well known. Sathi et al. estimated the incidence of MTX-induced pneumonitis at 1 per 192 patient-years and usually occurs during the first year of treatment42. Since MTX can exacerbate a preexisting lung disease the American College of Rheumatology recommends not using it in patients with symptomatic RA-ILD43. However, the association between MTX and appearance of a chronic ILD other than a hypersensitivity reaction is not so clear. Recent studies do not support this association44. On the other hand, LFN has been less frequently associated with ILD exacerbation or development of new ILD45. In our cohort, discontinuation of MTX or LFN after the diagnosis of fibrotic ILD in RA patients was associated with disease progression46. Our observation, together with recent data, suggests that withdrawing MTX in fibrotic ILD patients doesn’t avoid disease progression and also it points the fact that prospective clinical trials are required for evaluating the exact role of MTX in fibrotic RA-ILD43. Thus, we consider that it may be necessary to monitor lung function and perform an HRCT before starting MTX or other drugs that may present pulmonary toxicity.
Regardless of the role of MTX and LFN in RA-ILD disease, we have to consider adding a treatment focused on lung involvement, such as biological agents (e.g. rituximab, abatacept) or antifibrotic treatment such as nintedanib after the recent clinical trials in progressive pulmonary fibrosis. We must individualize the treatment, differentiating the radiological pattern, the severity and progression of lung involvement to better target the proper treatment from a multidisciplinary approach39,40,41.
Respiratory infection or community-acquired pneumonia are the most common type of infections seen in patients with RA47. A retrospective study defined serious infection as the requirement of antimicrobial therapy and hospitalization. The authors observed that the highest infection rate was in those patients with a daily prednisone use > 10 mg per day48. The use of corticosteroids concurrently with another immunosuppressant such as azathioprine increased mortality, hospitalization rate and serious adverse events in IPF mainly due to pulmonary infection and the presence of telomere shortening49. Similar results were obtained in the PANTHER study where increased risks of death and hospitalization were observed in patients with IPF who were treated with a combination of prednisone, azathioprine, and acetylcysteine, as compared with placebo50. In our study it seems that the use of prednisone at a dose greater than 10 mg together with azathioprine is associated with a higher occurrence of respiratory infections in a multivariate logistic model corrected by pneumococcal vaccination, flu vaccination and antibiotic prophylaxis. No data on telomere length was available since it is not a current standardized evaluation in these patients.
Our study has several limitations. The major one is related to the retrospective analysis design despite being consecutive cases collected in a multicenter study.
Incomplete clinical information or loss of variables are a constant in this type of studies, hence the need to eliminate some variables such as DAS28 or anti-CCP antibodies to avoid biasing the results in the survival and progression models. Due to the retrospective design of the study and the limited number of patients for analyzing by subgroups (depending on all received therapies), analyzing the impact of other treatments on progression and mortality was not feasible. The majority of patients were assessed in the pulmonology department due to respiratory symptoms. Thus, it is possible that asymptomatic patients and therefore milder cases were not included. Finally, the evaluation of radiological patterns in the HRCT was not centralized, although in all cases it was done in a specialized ILD consultation and after the evaluation in a multidisciplinary discussion with expert ILD radiologists.
In conclusion, the delay in identifying fibrotic ILD in RA patients increases the probability of death or disease progression. The existence of several prognostic variables in RA-ILD makes multidimensional predictive models also useful. Early referral to expert ILD consultation would improve the prognostic and treatment approach, including the proper referral to lung transplant.
Data availability
The dataset generated and analysed during the current study is available from the corresponding on reasonable request.
Abbreviations
- 6MWT:
-
Six minutes walking test
- ACR/EULAR:
-
American College of Rheumatology/European League Against Rheumatism
- CCP:
-
Anticyclic citrullinated peptide
- CPI:
-
Composite physiologic index
- CTD-ILD:
-
Connective tissue disease associated interstitial lung disease
- DAS28:
-
Disease activity score 28
- DLCO:
-
Diffusion capacity of the lung for carbon monoxide
- ESR:
-
Erythrocyte sedimentation rate
- FVC:
-
Forced vital capacity
- GAP index:
-
Gender, age and physiology variables index
- GCPs:
-
Good clinical practices
- HRCT:
-
High-resolution computed tomography
- IL-6:
-
Interleukin 6
- ILD:
-
Interstitial lung disease
- IPF:
-
Idiopathic pulmonary fibrosis
- KL-6:
-
Krebs von den Lungen 6
- LEF:
-
Leflunomide
- MTX:
-
Methotrexate
- NSIP:
-
Non-specific interstitial pneumonia
- RA:
-
Rheumatoid arthritis
- RA-ILD:
-
Rheumatoid arthritis associated interstitial lung disease
- RPC:
-
Reactive protein C
- SatO2:
-
Oxygen saturation
- SD:
-
Standard deviation
- SEPAR:
-
Respiratory Spanish Society
- UIP:
-
Usual interstitial pneumonia
References
Solomon, J. J. & Brown, K. K. Rheumatoid arthritis-associated interstitial lung disease. Open Access Rheumatol Res Rev 4, 21–31 (2012).
Cottin, V. Idiopathic interstitial pneumonias with connective tissue diseases features: a review. Respirology 21, 245–258 (2016).
Xaubet A, Ancochea J, Blanquer R, Montero C, Morell F, Becerra ER, et al. [Diagnosis and treatment of diffuse interstitial lung diseases]. 2003.
Olson, A. L. et al. Rheumatoid arthritis-interstitial lung disease–associated mortality. Am J Resp Crit Care 183, 372–378 (2011).
Koduri, G. et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology (Oxford) 49, 1483–1489 (2010).
Bongartz, T. et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 62, 1583–1591 (2010).
Tanaka, N. et al. Rheumatoid arthritis-related lung diseases: CT findings. Radiology 232, 81–91 (2004).
Kim, E. J. et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur. Respir. J. 35, 1322–1328 (2010).
Assayag, D. et al. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology 19, 493–500 (2014).
Lee, Y. S. et al. The Value of biomarkers as predictors of outcome in patients with rheumatoid arthritis-associated usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis Official J Wasog 33, 216–223 (2016).
Zamora-Legoff, J. A., Krause, M. L., Crowson, C. S., Ryu, J. H. & Matteson, E. L. Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 69, 542–549 (2017).
Dawson, J. K., Fewins, H. E., Desmond, J., Lynch, M. P. & Graham, D. R. Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann Rheum Dis 61, 517 (2002).
Jacob, J. et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur. Respir. J. 53, 1800869 (2019).
England BR, Sayles H, Michaud K, Thiele GM, Poole JA, Caplan L, et al. Chronic lung disease in U.S. Veterans with rheumatoid arthritis and the impact on survival. Clin Rheumatol 2018;37:2907–15.
Singh N, Varghese J, England BR, Solomon JJ, Michaud K, Mikuls TR, et al. Impact of the pattern of interstitial lung disease on mortality in rheumatoid arthritis: a systematic literature review and meta-analysis. Seminars in Arthritis and Rheumatism 2019.
Zamora-Legoff, J. A., Krause, M. L., Crowson, C. S., Ryu, J. H. & Matteson, E. L. Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology (Oxford) 56, 344–350 (2017).
Solomon, J. J. et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur. Respir. J. 47, 588–596 (2016).
Ley, B. et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 156, 684–691 (2012).
Wells, A. U. et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Resp Crit Care 167, 962–969 (2003).
Nurmi, H. M. et al. Are risk predicting models useful for estimating survival of patients with rheumatoid arthritis-associated interstitial lung disease?. BMC Pulm. Med. 17, 16 (2017).
Iqbal, K. & Kelly, C. Treatment of rheumatoid arthritis-associated interstitial lung disease: a perspective review. Ther Adv Musculoskeletal Dis 7, 247–267 (2015).
Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. New Engl J Med 2019.
Chen, J. et al. Biologics-induced interstitial lung diseases in rheumatic patients: facts and controversies. Expert Opin. Biol. Ther. 17, 265–283 (2017).
Rojas-Serrano, J. et al. Rheumatoid arthritis-related interstitial lung disease (RA-ILD): methotrexate and the severity of lung disease are associated to prognosis. Clin. Rheumatol. 36, 1493–1500 (2017).
Wasko, M. C. M., Dasgupta, A., Hubert, H., Fries, J. F. & Ward, M. M. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 65, 334–342 (2013).
Galloway, J. B. et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 50, 124–131 (2011).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 (2010).
Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5.
Zhu, J., Zhou, Y., Chen, X. & Li, J. A metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J Rheumatology 41, 1282–1289 (2014).
Li, L. et al. A retrospective study on the predictive implications of clinical characteristics and therapeutic management in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol 39, 1457–1470 (2020).
Mochizuki, T., Ikari, K., Yano, K., Sato, M. & Okazaki, K. Long-term deterioration of interstitial lung disease in patients with rheumatoid arthritis treated with abatacept. Mod. Rheumatol. 29, 413–417 (2019).
Lamas, D. J. et al. Delayed access and survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 184, 842–847 (2011).
Oldham, J. M. & Noth, I. Idiopathic pulmonary fibrosis: early detection and referral. Respir. Med. 108, 819–829 (2014).
Solomon, J. J. et al. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Respir. Med. 107, 1247–1252 (2013).
Wolfe, F., Michaud, K., Gefeller, O. & Choi, H. K. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 48, 1530–1542 (2003).
Kelly CA, Nisar M, Arthanari S, Carty S, Woodhead FA, Price-Forbes A, et al. Rheumatoid arthritis related interstitial lung disease – improving outcomes over 25 years: a large ulticenter UK study. Rheumatology 2020.
Raghu, G. et al. An OFFICIAL ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183, 788–824 (2011).
Fu, Q. et al. Risk factors for progression and prognosis of rheumatoid arthritis–associated interstitial lung disease: single center study with a large sample of Chinese population. Clin. Rheumatol. 38, 1109–1116 (2019).
Wells, A. U. & Hirani, N. Society on behalf of the BILDGG a subgroup of the British Thoracic Society Standards of Care Committee, in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic, Society on behalf of the BILDGG a subgroup of the British Thoracic Society Standards of Care Committee, in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Interstitial lung disease guideline. Thorax 63, v1-58 (2008).
Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, et al. Predicting survival across chronic interstitial lung disease: The ILD-GAP model. Chest 2013.
Kiely, P. et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open 9, e028466 (2019).
Sathi, N., Chikura, B., Kaushik, V. V., Wiswell, R. & Dawson, J. K. How common is methotrexate pneumonitis? A large prospective study investigates. Clin. Rheumatol. 31, 79–83 (2012).
Saag, K. G. et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Care Res. 59, 762–784 (2008).
Conway, R., Low, C., Coughlan, R. J., O’Donnell, M. J. & Carey, J. J. Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheumatol 66, 803–812 (2014).
Conway, R., Low, C., Coughlan, R. J., O’Donnell, M. J. & Carey, J. J. Leflunomide use and risk of lung disease in rheumatoid arthritis: a systematic literature review and metaanalysis of randomized controlled trials. J. Rheumatol. 43, 855–860 (2016).
Zhang, Y., Li, H., Wu, N., Dong, X. & Zheng, Y. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin. Rheumatol. 36, 817–823 (2017).
Mhuircheartaigh, O. M. N., Matteson, E. L., Green, A. B. & Crowson, C. S. Trends in serious infections in rheumatoid arthritis. J. Rheumatol. 40, 611–616 (2013).
Zamora-Legoff, J. A., Krause, M. L., Crowson, C. S., Ryu, J. H. & Matteson, E. L. Risk of serious infection in patients with rheumatoid arthritis-associated interstitial lung disease. Clin. Rheumatol. 35, 2585–2589 (2016).
Newton, C. A. et al. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur Respir J 53, 1801641 (2019).
Network IPFCR, Raghu G, Anstrom KJ, King TE, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012;366:1968–77.
Acknowledgements
Thanks to the Emerging Group (GEEPID) of ILD from SEPAR (Respiratory Spanish Society), which has participated in conducting this study.
Funding
This investigation has not received specific support from public sector agencies, the commercial sector or non-profit entities.
Author information
Authors and Affiliations
Contributions
E.C.J. design study, data analysis and drafting article. T.V.R. design study. I.M.R., D.C.V., J.J.G., E.B.M., A.R.P., M.F.G., C.M.R., S.H.R., G.B. and M.G.M. data collection, J.A.R.P. and J.S.T. critical revision of the article. J.N.G: critical revision of the article. M.M: critical revision and final approval of the version to be published. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cano-Jiménez, E., Vázquez Rodríguez, T., Martín-Robles, I. et al. Diagnostic delay of associated interstitial lung disease increases mortality in rheumatoid arthritis. Sci Rep 11, 9184 (2021). https://doi.org/10.1038/s41598-021-88734-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88734-2
This article is cited by
-
Epidemiology and clinical characteristics of interstitial lung disease in patients with rheumatoid arthritis from the JointMan database
Scientific Reports (2023)
-
Ist JAK-Hemmung eine Option in der Behandlung der interstitiellen Lungenerkrankung bei einer rheumatoiden Arthritis?
Zeitschrift für Rheumatologie (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.