Abstract
The antitumour activity of a medicinal mushroom Phellinus linteus (PL), through the stimulation of immune system or the induction of apoptosis, has been recently described. However, the molecular mechanisms responsible for the inhibition of invasive behaviour of cancer cells remain to be addressed. In the present study, we demonstrate that PL inhibits proliferation (anchorage-dependent growth) as well as colony formation (anchorage-independent growth) of highly invasive human breast cancer cells. The growth inhibition of MDA-MB-231 cells is mediated by the cell cycle arrest at S phase through the upregulation of p27Kip1 expression. Phellinus linteus also suppressed invasive behaviour of MDA-MB-231 cells by the inhibition of cell adhesion, cell migration and cell invasion through the suppression of secretion of urokinase-plasminogen activator from breast cancer cells. In addition, PL markedly inhibited the early event in angiogenesis, capillary morphogenesis of the human aortic endothelial cells, through the downregulation of secretion of vascular endothelial growth factor from MDA-MB-231 cells. These effects are mediated by the inhibition of serine-threonine kinase AKT signalling, because PL suppressed phosphorylation of AKT at Thr308 and Ser473 in breast cancer cells. Taken together, our study suggests potential therapeutic effect of PL against invasive breast cancer.
Similar content being viewed by others
Main
Despite the fact that breast cancer mortality of breast cancer decreased by 22.9% from 1991 to 2003, breast cancer remains the leading cause of cancer death among 20- to 59-year-old US women with estimated 40 460 new death in 2007 (Jemal et al, 2007). One of the reasons for such a high mortality is the invasive behaviour of cancer cells, which results in the metastasis of breast cancer. Cancer metastasis consists of several interdependent processes including uncontrolled cell proliferation, invasion through surrounding tissues, migration to the distant sites of the human body, and adhesion, invasion and colonisation of other organs and tissues (Price et al, 1997). Tumour growth and metastasis also require angiogenesis, the formation of blood vessels by capillaries sprouting from pre-existing vessels (Folkman, 1995). Therefore, inhibition of growth, invasive behaviour and cancer cell-mediated angiogenesis will lead to the suppression of cancer metastasis and would further increase survival of breast cancer patients.
Phellinus linteus (PL) is a basidiomycete fungus, located mainly in tropical America, Africa and Asia, where it gained significant recognition as medicinal mushroom in the traditional Oriental medicine (Dai and Xu, 1998). The biologically active compounds isolated from PL are polysaccharides (Song et al, 1995), acidic proteo-heteroglycans with mixed α- and β-linkages, and a (1 → 6)-branched type (1 → 3)-glycan (Kim et al, 2003b). These complex polysaccharides have been detected in a variety of different mushroom species and linked to the immunostimulatory and antitumour activities (Wasser, 2002). Therefore, PL stimulated proliferation of T lymphocytes and activated B cells (Kim et al, 1996, 2003c), and induced maturation of bone marrow-derived dendritic cells (Park et al, 2003) and the macrophage response (Kim et al, 2003a). On the other hand, PL demonstrated anti-inflammatory effects in lipopolysaccharide-stimulated macrophages (Kim et al, 2006). The direct anticancer effect of PL has been demonstrated by the inhibition of invasive melanoma B16BL6 cells through the downregulation of mRNA level of urokinase-plasminogen activator (uPA), and by the inhibition of pulmonary metastasis in mice (Lee et al, 2005). Phellinus linteus suppressed proliferation by the inhibition of cyclin-dependent kinases cdk2, 4 and 6, and induced apoptosis through the activation of caspase 3 in lung cancer cells (Guo et al, 2007) and apoptosis of prostate cancer cells (Collins et al, 2006; Zhu et al, 2007).
In the present study, we evaluated the effect of PL on the highly invasive and metastatic human breast cancer cells. Here, we show that PL inhibits proliferation (anchorage-dependent growth) as well as colony formation (anchorage-independent growth) of highly invasive human breast cancer cells. In addition, PL also suppresses invasive behaviour of MDA-MB-231 cells by the inhibition of cell adhesion, cell migration and cell invasion. Finally, PL suppressed breast cancer cell-mediated angiogenesis of endothelial cells in vitro. Collectively, our study suggests the mechanism(s) employed for the inhibition of proliferation, invasive behaviour and angiogenesis of invasive breast cancer cells by an extract from medicinal mushroom PL.
Materials and methods
Cell culture and reagents
Human breast cancer cells (MCF-7, MDA-MB-231) and human prostate cancer cells (PC-3, LNCaP) were obtained from ATCC (Manassas, VA, USA). MCF-7 and MDA-MB-231 cells were maintained in DMEM medium, PC-3 cells were maintained in F-12 medium and LNCaP cells were maintained in RPMI 1640 medium. All media contained penicillin (50 U ml−1), streptomycin (50 U ml−1), and 10% fetal bovine serum (FBS). Media and supplements came from GIBCO BRL (Grand Island, NY, USA). Fetal bovine serum was obtained from Hyclone (Logan, UT, USA). Aqueus extract of PL was supplied by the Maitake Products Inc. (Paramus, NJ, USA). Stock solution was prepared by dissolving PL in sterile water at a concentration 50 mg ml−1 and stored at 4°C. AKT inhibitors LY294002 and AKT inhibitor III were obtained from Calbiochem (San Diego, CA, USA).
Cell proliferation assay
Cell proliferation was determined by the tetrazolium salt method, according to the manufacturer's instructions (Promega, Madison, WI, USA). Briefly, cancer cells were cultured in a 96-well plate and treated at indicated times with PL (0–1.0 mg ml−1). At the end of the incubation period, the cells were harvested and absorption was determined with an ELISA plate reader at 570 nm, as described (Jiang et al, 2004b). Data points represent mean±s.d. in the representative experiment of triplicate determinations. Similar results were obtained in two independent experiments.
Cell viability
Cell viability of MCF-7 and MDA-MB-231 cells was determined after incubation with PL (0–1.0 mg ml−1) for 24, 48 and 72 h by staining with Trypan Blue as described (Sliva et al, 2002a).
Anchorage-independent growth
MDA-MB-231 cells were harvested and seeded in six-well plates coated with 1% agarose. Anchorage-independent growth was assessed after incubation for 10–14 days with culture media with or without PL (0–1.0 mg ml−1), which were replaced every 4 days. Plates were stained with 0.005% crystal violet, and the colonies were counted manually under a microscope and photographed (Slivova et al, 2004).
Cell cycle analysis
MDA-MB-231 cells (0.75 × 106) were seeded and after 24 h treated with PL (0.5 mg ml−1) for the indicated period of time (0–48 h). After incubation, the cells were harvested by trypsinisation, washed with Dulbecco's phosphate-buffered saline containing 2% FBS, and resuspended in propidium iodine (50 μg ml−1). Cell cycle analysis was performed on a FACStarPLUS flow cytometer (Becton-Dickinson, San Jose, CA, USA), as previously described (Sliva et al, 2001). Data are the mean±s.d. from three independent experiments.
Cell adhesion, migration and invasion assays
Cell adhesion was performed with Cytomatrix Adhesion Strips coated with human vitronectin (Chemicon International, Temecula, CA, USA). Briefly, MDA-MB-231 cells were treated with PL (0–0.5 mg ml−1) for 24 h, harvested and counted. Cell adhesion was determined after 1.5 h of incubation at 37°C (Lloyd et al, 2003). Cell migration of MDA-MB-231 cells treated with PL (0–1.0 mg ml−1) was assessed in Transwell chambers in the DMEM medium containing 10% FBS (Slivova et al, 2004). Invasion of MDA-MB-231 cells treated with PL (0–1.0 mg ml−1) was assessed in Transwell chambers coated with 100 μl of Matrigel™ (BD Biosciences, Bedford, MA, USA) diluted 1:4 with DMEM, after 72 h of incubation (Slivova et al, 2004).
In vitro endothelial cell morphogenesis assay (capillary morphogenesis)
Human aortic endothelial cell (HAEC) differentiation into ‘capillary-like’ structures was observed using a two-dimensional Matrigel-based assay as we described previously (Harvey et al, 2002). Initially, 200 μl of ice-cold growth factor-reduced Matrigel (Becton Dickinson Labware, Bedford, MA, USA), an extracellular matrix preparation derived from the Engelbreth–Holm–Swarm tumour, was placed into each well of a 24-well tissue culture-treated plate. Human aortic endothelial cells were harvested, resuspended in serum-free EBM media and plated at 3.5 × 104 cells per well-coated with Matrigel. Human aortic endothelial cells were further incubated with PL (0–0.5 mg ml−1) or with conditioned media from MDA-MB-231 cells, which were prepared by the incubation of MDA-MB-231 cells in the presence of PL (0–0.5 mg ml−1) for 24 h. Endothelial HAE cells differentiated into capillary-like structures within 16 h of incubation at 37°C in the presence of 5% CO2. These structures were examined microscopically ( × 40) using an inverted Olympus CK40 microscope. To facilitate analysis of the structures, non-adherent cells incorporated in excess medium were removed from each well prior to quantitative analysis. Photomicrographs were taken to assess the extent of capillary-like structural formation. Quantification of the capillary-like structures was performed counting the number of nodes per field, where a node is defined as an intersection of at least three cells. Each sample was assayed in triplicate and reproduced in at least two additional experiments.
Western blot analysis
MDA-MB-231 cells were treated with PL (0–1.0 mg ml−1) for 24–72 h as indicated in the text and whole-cell extracts prepared as described previously (Sliva et al, 2002a, 2002b). Equal amounts of proteins (20 μg per lane) were separated on NuPAGE 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA, USA) and transferred to a PVDF membrane (Millipore, Bedford, MA, USA). The protein expression was detected with the corresponding primary antibodies: anti-cyclin-D1, anti-cyclin-E, anti-cyclin-A, anti-cdk2, anti-cdk4, anti-p21, anti-p27, anti-β-actin, (Santa, Cruz Biotechnology, Santa Cruz, CA, USA), anti-AKT, anti-phospho-AKT (Thr308) and anti-phospho-AKT (Ser473) (Cell Signaling, Beverly, MA, USA), respectively. Protein expression was visualised using the ECL Western Blotting Detection System (Amersham Biosciences, Buckinghamshire, UK).
Urokinase-plasminogen activator secretion
DMEM media from MDA-MB-231 cells treated with PL (0–1.0 mg ml−1) for 24 h were collected and concentrated, and the secretion of uPA was detected by western blot analysis with anti-uPA antibody (Oncogene Research Products, Cambridge, MA, USA), as described (Sliva et al, 2002b).
Densitometric analysis
Autoradiograms of the western blots were scanned with HP scanjet 5470c scanner. The optical densities of p27, phospho-AKT (Thr308), phospho-AKT (Ser473), AKT, β-actin and uPA proteins on the films were quantified and analysed with the UN-SCAN-IT software (Silk Scientific, Orem, UT, USA). The ratios of p27/β-actin and phosphoAkt/Akt were calculated by standardising the ratios of each control to the unit value.
Vascular endothelial growth factor secretion
MDA-MB-231 cells were treated with PL (0–0.5 mg ml−1) for 24 h, cell media collected and secretion of vascular endothelial growth factor (VEGF) was determined using a respective Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Statistical analysis
Data are presented as means±s.d. Statistical comparison between the control group (0 μg ml−1 of PL) and groups with different PL doses was carried out using two-sided Student's t-tests. The value of P<0.05 was considered to be significant.
Results
Phellinus linteus suppresses proliferation and colony formation of highly invasive breast cancer cells
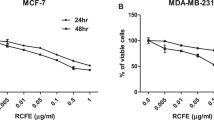
Invasive behaviour of cancer cells is directly linked to their metastatic potential resulting in the high cancer mortality. Therefore, we evaluated if PL inhibits growth of highly invasive (MDA-MB-231) and poorly invasive (MCF-7) breast cancer cells. As seen in Figure 1, increased concentration of PL (0–1.0 mg ml−1) markedly suppressed proliferation of MDA-MB-231 as well as MCF-7 cells in a dose- and time-dependent manner. Nevertheless, the effect of PL on poorly invasive cells was more pronounced, because the concentration 0.25, 0.5 and 1.0 mg ml−1 of PL suppressed proliferation of MCF-7 cells by 60.2, 70.1 and 78.0%, respectively (Figure 1B), whereas the same concentration suppressed proliferation of MDA-MB-231 cells by 15.5, 21.5 and 43.1%, respectively (Figure 1A), after 24 h of incubation. The same sensitivity of MCF-7 cells was evident also after additional 48 and 72 h of incubation, where only the highest concentration of PL (1.0 mg ml−1) suppressed proliferation of poorly invasive and highly invasive breast cancer cells with the same potency (Figure 1). To determine if the effect of PL on cancer cells is cytotoxic or cytostatic, we evaluated the cell viability after 24, 48 and 72 h of PL treatment. Although PL decreased the viability of MDA-MB-231 and MCF-7 cells, the strongest inhibition of cell viability at the highest used concentration of PL (1.0 mg ml−1) after 72 h was only 13.5% for MDA-MB-231 cells (Figure 1C) and 10.6% for MCF-7 cells (Figure 1D), whereas the same concentration suppressed proliferation of MDA-MB-231 cells by 86.6% (Figure 1A) and MCF-7 cells by 90.6% (Figure 1B). Therefore, these data suggest that the PL inhibits growth of breast cancer cells predominantly through its cytostatic effect. Interestingly, PL also suppressed proliferation of poorly invasive prostate (LNCaP) and highly invasive prostate (PC-3) cancer cells in a dose- and time-dependent manner, and LNCaP cells were more sensitive to the PL treatment (not shown).
Effect of PL on proliferation of breast cancer cells. Proliferation: (A) MDA-MB-231, (B) MCF-7 cells were treated with PL (0–1.0 mg ml−1) for 24, 48 and 72 h. Cell proliferation was determined as described in Materials and Methods. Data are the means±s.d. of triplicate determinations. Similar results were obtained in at least two additional experiments. *P<0.05. Viability: (C) MDA-MB-231, (D) MCF-7 cells were treated with PL (0–1.0 mg ml−1) for 24, 48 and 72 h. Cell viability was determined by Trypan blue staining as described in Materials and Methods. Data are the means±s.d. of triplicate determinations. Similar results were obtained in at least one additional experiment. *P<0.05.
In addition to cell proliferation (anchorage-dependent growth), colony formation (anchorage-independent growth) is one of the typical characteristic of the metastatic potential of cancer cells in vitro and strongly correlates with tumorigenesis in vivo (Freedman and Shin, 1974). To determine whether PL suppresses colony formation of highly invasive breast cancer cells, we evaluated the anchorage-independent growth of MDA-MB-231 cells. As seen in Figure 2, MDA-MB-231 cells formed colonies on agar after 14 days of incubation, and the presence of increased concentration of PL (0–1.0 mg ml−1) resulted in the significant suppression of number of colonies (Figure 2E). Therefore, PL inhibits anchorage-dependent as well as anchorage-independent growth of highly aggressive breast cancer cells.
Effect of PL on colony formation of MDA-MB-231 cells. Anchorage-independent growth (colony formation) of MDA-MB-231 cells was assessed on 1% agarose after incubation for 14 days with culture media containing: (A) 0 mg ml−1 PL, (B) 0.25 mg ml−1 PL, (C) 0.5 mg ml−1 PL, (D) 1.0 mg ml−1 as described in Materials and Methods. (E) The number of colonies was determined as described in Materials and Methods. The data are the means±s.d. from three experiments. *P<0.05.
Phellinus linteus induces cell cycle arrest at S phase
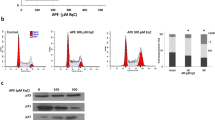
To determine whether the inhibition of cell proliferation is associated with cell cycle arrest, MDA-MB-231 cells were treated for 24 and 48 h with PL (0.5 mg ml−1) and analysed by flow cytometry. Cell cycle analysis demonstrated that PL causes cell cycle arrest at S phase of cell cycle (Table 1), where the amount of cells in S phase significantly increased from 27% (control – 0 h) to 34% (24 h) and 44% (48 h). In addition, PL treatment did not induce the amount of cells in sub-G0/G1 phase, further suggesting the cytostatic effect of PL on MDA-MB-231 cells (Table 1). To examine the mechanism responsible for the cell cycle arrest at S phase, we evaluated the expression of cell cycle regulatory proteins (cyclins) and cdks involved in the progression from G1 phase to S phase (Sherr and Roberts, 1999; Ekholm and Reed, 2000). Therefore, MDA-MB-231 cells were treated with PL (0–1.0 mg ml−1) for 24 h and the expression of cyclin D1, E, A, cdk4, cdk2, p21 and p27 in cell extracts was evaluated by western blot analysis with respective antibody. Neither of the G1 regulatory proteins cyclin-D1, cdk4 or p21, G1-late phase or G1/S-phase regulatory proteins cyclin E, A and cdk2 demonstrated changes in their expression after the treatment with PL (Figure 3A). Nevertheless, PL (0.5 and 1.0 mg ml−1) markedly induced expression of a cyclin-dependent kinase inhibitor p27 (Figure 3B). Therefore, PL inhibits proliferation of breast cancer cells by the upregulation of p27 resulting in S-phase cell cycle arrest.
Effect of PL on the expression of cell cycle regulatory proteins. MDA-MB-231 cells were treated with PL (0–1.0 mg ml−1) for (A) 24 h, or (B) 24–48 h and whole-cell extracts were subjected to Western blot analysis. The expression of cyclin D1, cyclin E, cyclin A, cdk2, cdk4, p21 and p27 was evaluated by western blot analysis with their respective antibodies. The equal protein loading was verified with anti-β-actin antibody. The results are representative of three separate experiments. The expression level of p27 (ratio p27/β-actin) was quantified by densitometry as described in Materials and Methods.
Phellinus linteus suppresses invasive behaviour of breast cancer cells
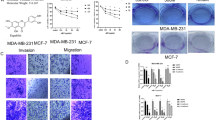
The ability of cancers to metastasise is directly associated to cell adhesion, migration and invasion. Integrin receptor αVβ3 is involved in adhesion of breast cancer cells through its interaction with extracellular matrix (ECM) protein vitronectin (Wong et al, 1998). To investigate if PL affects adhesion of invasive breast cancer cells, MDA-MB-231 cells were pretreated with PL (0–0.5 mg ml−1) for 24 h and their adhesion to vitronectin was determined. As seen in Figure 4A, adhesion of MDA-MB-231 cells was markedly suppressed by the PL treatment. Next, we evaluated if PL also inhibits cell migration. MDA-MB-231 cells were pretreated with PL (0–1.0 mg ml−1) for 1 h and cell migration was determined after additional 5 h of incubation. As seen in Figure 4B, PL also markedly suppressed migration of breast cancer cells in a dose-dependent manner. Finally, the effect of PL on cell invasiveness was evaluated. MDA-MB-231 cells were plated on the Matrigel-coated filters in the presence of PL (0–1.0 mg ml−1) and the amount of cells invaded through Matrigel counted after 48 h of incubation. As seen in Figure 4C, PL inhibits invasion of MDA-MB-231 cells in a dose–response manner. Because cancer metastasis and invasiveness are associated with the uPA–uPA receptor (uPAR) system, we evaluated whether PL affects secretion of uPA from cancer cells. MDA-MB-231 cells were treated with PL (0–1.0 mg ml−1) for 24 h and uPA secretion evaluated by western blot analysis in conditioned media. In agreement with the data demonstrating inhibition of cell adhesion, migration and invasion PL markedly decreased secretion of uPA from MDA-MB-231 cells (Figure 4D).
Effect of PL on invasive behaviour of MDA-MB-231 cells. (A) Cell adhesion. MDA-MB-231 cells were treated with PL (0–0.5 mg ml−1) for 24 h and cell adhesion to vitronectin determined as described in Materials and Methods. Each bar represents the mean±s.d. of three experiments. *P<0.05. (B) Cell migration. Cell was determined after 5 h of incubation in the presence of PL (0–1.0 mg ml−1) in Boyden Chambers as described in Materials and Methods. Each bar represents the mean±s.d. of three experiments. *P<0.05. (C) Cell invasion. Cell invasion was determined after 24 h of incubation in the presence of PL (0–1.0 mg ml−1) in Boyden Chambers coated with Matrigel as described in Materials and Methods. Each bar represents the mean±s.d. of three experiments. *P<0.05. (D) uPA secretion. MDA-MB-231 cells were treated with PL (0–1.0 mg ml−1) for 24 h, and the expression of uPA detected in conditioned media with anti-uPA antibody by western blot analysis as described in Materials and Methods. The secretion of uPA was quantified by densitometry as described in Materials and Methods. The results are representative of three separate experiments.
Phellinus linteus inhibits capillary morphogenesis of endothelial cells through the suppression of VEGF secretion
Capillary morphogenesis (tube formation) of human endothelial cells is one of the first important steps in angiogenesis associated with the cancer progression and metastasis. Because cancer microenvironment contains a variety of cells, we also evaluated if the inhibition of capillary morphogenesis of endothelial cells can be mediated through breast cancer cells. Therefore, we determined if PL itself or conditioned media from breast cancer cells exposed to PL suppress capillary morphogenesis of HAECs. Human aortic endothelial cells grown on Matrigel were treated directly with PL (0–0.5 mg ml−1) or with the conditioned media from MDA-MB-231 cells treated with PL (0–0.5 mg ml−1, PL-CM) for 24 h, and tube formation were evaluated. As seen in Figure 5A, PL significantly suppressed capillary morphogenesis of HAECs. Moreover, conditioned media from MDA-MB-231 cells without PL induced capillary morphogenesis of HAECs (PL vs PL-CM at 0 PL), and conditioned media from cells exposed to PL (PL-CM) also suppressed capillary morphogenesis of HAECs in a dose–response manner (Figure 5A–C). Because MDA-MB-231 cells express pro-angiogenic VEGF (Basu et al, 2005), we hypothesised that secreted VEGF from these cells induces capillary morphogenesis of endothelial cells, which can be inhibited by PL. Therefore, we evaluated whether PL inhibits secretion of VEGF from MDA-MB-231 cells treated with PL (0–0.5 mg ml−1). As seen in Figure 5D, secretion of VEGF from MDA-MB-231 cells was markedly decreased by PL in a dose–response manner. Collectively, our data suggest that PL inhibits capillary morphogenesis of endothelial cells directly as well as indirectly through the suppression of secretion of VEGF from breast cancer cells.
Phellinus linteus inhibits capillary morphogenesis of aortic endothelial cells. (A) HAECs were seeded onto Matrigel, and the cells were treated with PL (0–0.5 mg ml−1) (black bars) or with the conditioned media from MDA-MB-231 cells treated with PL (0–0.5 mg ml−1, PL-CM) (shaded bars) for 24 h and capillary morphogenesis was determined as described in Materials and Methods. Capillary morphogenesis at 0 mg ml−1 PL-CM (B) and at 0.5 mg ml−1 PL-CM (C). The number of nodules was quantified by counting from the three fields per data point and each bar represents the mean±s.d. of three experiments. Statistical analysis: black line P<0.05 PL (black bars) vs PL-CM (shaded bars) at the same concentration of PL, #P<0.05 PL (black bars) at the PL concentration (0–0.5 mg ml−1), *P<0.05 PL-CM (shaded bars) at the PL concentration (0–0.5 mg ml−1). (D) MDA-MB-231 cells were treated with PL (0–0.5 mg ml−1) for 24 h, media collected and secretion of VEGF determined as described in Materials and Methods. Each bar represents the mean±s.d. (pg per ml of secreted VEGF) of minimum three experiments repeated twice. *P<0.05.
Phellinus linteus inhibits activity of AKT kinase
AKT serine-threonine kinase (protein kinase B) regulates a variety of cellular processes through the phosphorylation of a wide spectrum of downstream substrates finally resulting in the expression of proteins involved in cell proliferation, invasiveness and angiogenesis among others (Woodgett, 2005; Dillon et al, 2007). To determine if PL modulates AKT activity in breast cancer cells, MDA-MB-231 cells were treated with PL (0–1.0 mg ml−1) for 24 h and the phosphorylation status of AKT evaluated in whole-cell extracts by western blot analysis. As seen in Figure 6A, PL inhibits phosphorylation of AKT at Thr308 in a dose–response manner. In addition, PL treatment also markedly decreased phosphorylation of AKT at Ser473 (Figure 6B).
Effect of PL on AKT activity. MDA-MB-231 cells were treated with PL (0–1.0 mg ml−1) for 24 h and whole-cell extracts were subjected to western blot analysis with (A) anti-p-AKT-Thr308 or (B) anti-p-AKT-Ser473 antibodies. The equal protein loading was verified with anti-AKT antibody. The level of pAKT (ratio pAKT/AKT) was quantified by densitometry as described in Materials and Methods. The results are representative of three separate experiments.
To evaluate if the suppression of AKT activity PL is directly responsible for the inhibition of capillary morphogenesis through the downregulation of expression of VEGF in MDA-MB-231 cells, we used a pharmacological approach with specific AKT inhibitors. Therefore, MDA-MB-231 cells were treated with LY294002 (10 μ M) or AKT inhibitor III (10 μ M) for 24 h, and secretion of VEGF was evaluated. As seen in Figure 7A, AKT inhibitors markedly suppressed secretion of VEGF from breast cancer cells. Moreover, conditioned media from MDA-MB-231 cells treated with LY294002 and AKT inhibitor III also inhibited capillary morphogenesis of endothelial cells (Figure 7B). Therefore, these data suggest that the inhibition of angiogenesis in vitro by PL is mediated, to some extent, through the suppression of AKT activity, which results in the suppression of secretion of VEGF from breast cancer cells.
AKT inhibition suppresses VEGF secretion and capillary morphogenesis. MDA-MB-231 cells were treated with vehicle, LY294002 (10 μ M) or AKT inhibitor III (10 μ M) for 24 h and conditioned media collected. (A) Vascular endothelial growth factor secretion from MDA-MB-231 cells and (B) capillary morphogenesis of HEAC was determined as described in Materials and Methods. Each bar represents the mean±s.d. of minimum three experiments repeated twice. *P<0.05.
Discussion
Regardless of different theories of biology of breast cancer as (i) a local disease that spreads over time to develop distant metastases; (ii) a systemic disease from the outset, with distant metastases present well before diagnosis (iii) or the combination of both as a heterogeneous disease, cancer metastasis is one of the major medical problems in breast cancer patients (Punglia et al, 2007). While several chemotherapeutic agents or their combinations (e.g. taxanes, trastumazab, gemcitabine or capecitabine) demonstrated activity in the metastatic breast cancer setting (Tripathy, 2007), there is a paucity of natural antiproliferative and anti-metastatic nontoxic agents.
As demonstrated practically four decades ago, polysaccharide extracts from basidiomycete fungus PL suppressed tumour growth in vivo (Chihara et al, 1969). In addition, PL also reduced tumour growth and the frequency of pulmonary metastasis without toxic effects (Han et al, 1999). Recent studies elucidated some of the molecular mechanism(s) responsible for the inhibition of growth through cell cycle arrest and induction of apoptosis in lung and prostate cancer cells (Collins et al, 2006; Guo et al, 2007; Zhu et al, 2007). Nevertheless, the molecular mechanism(s) responsible for the inhibition of invasive behaviour and angiogenesis was not fully addressed.
In the present study, we demonstrate that PL inhibits cell proliferation (anchorage-dependent growth) as well as colony formation (anchorage-independent growth) of highly invasive breast cancer cells through the S-phase cell cycle arrest mediated by the upregulation of expression of p27. Cell growth, which is the reflection of the progression of cell cycle, is aberrantly regulated in the majority of cancers. The cell cycle is regulated by a series of checkpoints employing cyclins, cdks and cdk inhibitors (Sherr, 1966). p27 is one of the cdk inhibitors, which binds to S-phase cyclin–cdk complexes and inhibits their cell cycle stimulatory activities. Because a loss of p27 expression has been linked to the aggressive behaviour in a variety of human epithelial tumours including breast cancer, the induction of p27 expression could lead to cell cycle arrest and inhibition of tumour growth (Tan et al, 1997; Lloyd et al, 1999; Macri and Loda, 1999). Our data are in agreement with recent papers also demonstrating the inhibition of growth of prostate cancer and leukaemia cells through the S-phase cell cycle arrest by the upregulation of expression of p27 (Zhang et al, 2006; Shishodia et al, 2007). Recently, Guo et al (2007) demonstrated that PL suppresses growth of lung cancer cells through G1-phase cell cycle arrest mediated by the inhibition of cdk2, 4 and 6 activities. Our results show cell cycle arrest at S phase in breast cancer cells through the upregulation of p27. Nevertheless, our data are not in a disagreement with Guo et al because the passage through G1 phase into S phase is regulated by the activities of cdk2, 4 and 6, which are controlled by cdk inhibitor p27 (Slingerlan and Pagano, 2000). Moreover, cell cycle arrest at S phase can also be interpreted as the arrest at G1-S, because the majority of cells are at G0/G1 and S but not G2 phases (Table 1). Most importantly, PL suppresses growth of cancer cells by the cell cycle arrest.
Here, we show that PL inhibits adhesion, migration and invasion through the suppression of secretion of uPA from highly invasive breast cancer cells. Our data are in agreement with the study by Lee et al (2005) demonstrating the inhibition of adhesion, invasion and expression of uPA in mouse melanoma cells. Furthermore, our data suggest the mechanism of inhibition of invasiveness by PL. Therefore, secreted uPA from breast cancer cells interacts with uPAR and converts plasminogen to plasmin (Blasi and Carmeliet, 2002). Plasmin degrades ECM components and stimulates other proteolytic enzymes (MMPs), which through the degradation of ECM contribute to cell invasion (Blasi and Carmeliet, 2002). Secreted uPA can bind to uPAR and forms a complex with integrin receptor αVβ3, which through its interaction with vitronectin is involved in adhesion and migration of breast cancer cells (Slivova et al, 2005). Inhibition of uPA secretion will reduce the formation of uPA–uPAR–αVβ3–vitronectin complex, with the consequent suppression of adhesion and migration of invasive breast cancer cells. Alternatively, PL can also modulate activities of other proteins involved in the invasive behaviour of breast cancer cells (e.g. matrix metalloproteinases, β1 and β4 integrins, epidermal growth factor receptors and others (Carraway and Sweeney, 2006; Bissell, 2007)). Nevertheless, in the present study, we propose that PL suppresses invasiveness through the inhibition of uPA secretion. Finally, we and others have previously demonstrated that inhibition of uPA suppressed invasiveness of breast cancer cells (Sliva et al, 2002b; Das et al, 2003: Mi et al, 2006).
Recently, Song et al (2003) demonstrated anti-angiogenic activity of PL in chorioallantoic membrane (CAM) chick embryo assay. However, the mechanism of the inhibition of angiogenesis by PL, related to cancer, was not previously addressed. In the present study, we demonstrate that PL inhibits one of the first steps in angiogenesis – tube formation of endothelial cells. While PL directly suppressed capillary morphogenesis of endothelial cells, our data further suggest that this effect can be mediated by the inhibition of secretion of VEGF from breast cancer cells. Although in our experimental conditions it was impossible to remove PL from the conditioned media from breast cancer cells (PL-CM), and therefore their inhibitory effect on capillary morphogenesis of HEACs could be considered as a direct effect of PL on HEACs, our data suggest that both (direct and indirect) effects are involved. Thus, (i) conditioned media from breast cancer cells without PL significantly increased capillary morphogenesis of endothelial cells, suggesting that proangiogenic factor is released from breast cancer cells, (ii) PL itself as well as conditioned media containing PL suppressed capillary morphogenesis and (iii) the secretion of VEGF from breast cancer cells was inhibited by PL. In agreement with our observation, suppression of endothelial capillary morphogenesis through the inhibition of secreted VEGF from a variety of cancer cells was described recently (Fukumoto et al, 2005; Stanley et al, 2005; Jang et al, 2007; Kong et al, 2007). Therefore, inhibition of specific pro-angiogenic protein within cancer cells will affect the whole cancer microenvironment (containing different cells) and will finally result in the suppression of tumour angiogenesis.
One of the suitable molecular cancer targets is AKT kinase, which inhibition in breast cancer cells resulted in cell cycle arrest, inhibition of growth and colony formation, inhibition of migration, invasion and suppression of angiogenesis (Das et al, 2003; Yacoub et al, 2003; Jiang et al, 2004a; Basu et al, 2005; Fukumoto et al, 2005; Jallal et al, 2007). Our data clearly demonstrate that PL suppresses AKT activity through the inhibition of AKT phosphorylation at Thr308 and at Ser473 in MDA-MB-231 cells, which demonstrate high levels of constitutively active AKT. Furthermore, inhibition of AKT with LY294002 and more specific AKT inhibitor III suppressed secretion of VEGF from breast cancer cells resulting in the decrease of capillary morphogenesis of endothelial cells. Our observation is in agreement with Xia et al (2006) who demonstrated, by using siRNA against AKT, the downregulation of VEGF expression in ovarian cancer cells, and the inhibition of angiogenesis in CAM chick embryo assay.
In conclusion, our study suggests PL as a natural compound possessing antiproliferative, antimetastatic and anti-angiogenic effects, which could be considered for the therapy of invasive breast cancers. However, further studies are necessary to confirm and evaluate these anticancer effects in vivo.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Basu GD, Pathangey LB, Tinder TL, Gendler SJ, Mukherjee P (2005) Mechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cells. Breast Cancer Res 7: R422–R435
Bissell MJ (2007) Modeling molecular mechanisms of breast cancer and invasion: lessons from the normal gland. Biochem Soc Trans 35: 18–22
Blasi F, Carmeliet P (2002) uPAR: a versatile signaling orchestrator. Nat Rev Mol Cell Biol 3: 932–943
Carraway III KL, Sweeney C (2006) Co-opted integrin signaling in ErbB2-induced mammary tumor progression. Cancer Cell 10: 93–95
Chihara G, Maeda Y, Hamuro J, Sasaki T, Fukuoka F (1969) Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk.) sing. Nature 222: 687–688
Collins L, Zhu T, Guo J, Xiao ZJ, Chen CY (2006) Phellinus linteus sensitises apoptosis induced by doxorubicin in prostate cancer. Br J Cancer 95: 282–288
Dai YC, Xu MQ (1998) Studies on the medicinal polypore, Phellinus baumii, and its kin, P. linteus. Mycotaxon 67: 191–200
Das R, Mahabeleshwar GH, Kundu GC (2003) Osteopontin stimulates cell motility and nuclear factor kappaB-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-kinase/Akt signaling pathways in breast cancer cells. J Biol Chem 278: 28593–28606
Dillon RL, White DE, Muller WJ (2007) The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene 26: 1338–1345
Ekholm SV, Reed SI (2000) Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol 12: 676–684
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1: 27–31
Freedman VH, Shin SI (1974) Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell 3: 355–359
Fukumoto S, Morifuji M, Katakura Y, Ohishi M, Nakamura S (2005) Endostatin inhibits lymph node metastasis by a down-regulation of the vascular endothelial growth factor C expression in tumor cells. Clin Exp Metastasis 22: 31–38
Guo J, Zhu T, Collins L, Xiao ZX, Kim SH, Chen CY (2007) Modulation of lung cancer growth arrest and apoptosis by Phellinus Linteus. Mol Carcinog 46: 144–154
Han SB, Lee CW, Jeon YJ, Hong ND, Yoo ID, Yang KH, Kim HM (1999) The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology 41: 157–164
Harvey K, Siddiqui RA, Sliva D, Garcia JGN, English D (2002) Serum factors involved in human microvascular endothelial cell morphogenesis. J Lab Clin Med 140: 188–198
Jallal H, Valentino ML, Chen G, Boschelli F, Ali S, Rabbani SA (2007) A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res 67: 1580–1588
Jang YJ, Kim DS, Jeon OH, Kim DS (2007) Saxatilin suppresses tumor-induced angiogenesis by regulating VEGF expression in NCI-H460 human lung cancer cells. J Biochem Mol Biol 40: 439–443
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57: 43–66
Jiang J, Slivova V, Harvey K, Valachovicova T, Sliva D (2004a) Ganoderma lucidum suppresses growth of breast cancer cells through the inhibition of Akt/NF-kappaB signaling. Nutr Cancer 49: 209–416
Jiang J, Slivova V, Valachovicova T, Harvey K, Sliva D (2004b) Ganoderma lucidum inhibits proliferation and induces apoptosis in human prostate cancer cells PC-3. Int J Oncol 24: 1093–1099
Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH (2007) Inhibition of angiogenesis and invasion by 3,3′-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res 67: 3310–3319
Kim BC, Choi JW, Hong HY, Lee SA, Hong S, Park EH, Kim SJ, Lim CJ (2006) Heme oxygenase-1 mediates the anti-inflammatory effect of mushroom Phellinus linteus in LPS-stimulated RAW264.7 macrophages. J Ethnopharmacol 106: 364–371
Kim HM, Han SB, Oh GT, Kim YH, Hong DH, Hong ND, Yoo ID (1996) Stimulation of humoral and cell mediated immunity by polysaccharide from mushroom Phellinus linteus. Int J Immunopharmac 18: 295–303
Kim GY, Oh YH, Park YM (2003a) Acidic polysaccharide isolated from Phellinus linteus induces nitric oxide-mediated tumoricidal activity of macrophages through protein tyrosine kinase and protein kinase C. Biochem Biophys Res Commun 309: 399–407
Kim GY, Park HS, Nam BH, Lee SJ, Lee JD (2003b) Purification and characterization of acidic proteo-heteroglycan from the fruiting body of Phellinus linteus (Berk. & M.A. Curtis) Teng. Bioresour Technol 89: 81–87
Kim GY, Park SK, Lee MK, Lee SH, Oh YH, Kwak JY, Yoon S, Lee JD, Park YM (2003c) Proteoglycan isolated from Phellinus linteus activates murine B lymphocytes via protein kinase C and protein tyrosine kinase. Int Immunopharmacol 3: 1281–1292
Lee HJ, Lee HJ, Lim ES, Ahn KS, Shim BS, Kim HM, Gong SJ, Kim DK, Kim SH (2005) Cambodian Phellinus linteus inhibits experimental metastasis of melanoma cells in mice via regulation of urokinase type plasminogen activator. Biol Pharm Bull 28: 27–31
Lloyd Jr FP, Slivova V, Valachovicova T, Sliva D (2003) Aspirin inhibits highly invasive prostate cancer cells. Int J Oncol 23: 1277–1283
Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW (1999) p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 154: 313–323
Macri E, Loda M (1999) Role of p27 in prostate carcinogenesis. Cancer Metastasis Rev 17: 337–344
Mi Z, Guo H, Wai PY, Gao C, Kuo PC (2006) Integrin-linked kinase regulates osteopontin-dependent MMP-2 and uPA expression to convey metastatic function in murine mammary epithelial cancer cells. Carcinogenesis 27: 1134–1145
Park SK, Kim GY, Lim JY, Kwak JY, Bae YS, Lee JD, Oh YH, Ahn SC, Park YM (2003) Acidic polysaccharides isolated from Phellinus linteus induce phenotypic and functional maturation of murine dendritic cells. Biochem Biophys Res Commun 312: 449–458
Price JT, Bonovich MT, Kohn EC (1997) The biochemistry of cancer dissemination. Crit Rev Biochem Mol Biol 32: 175–253
Punglia RS, Morrow M, Winer EP, Harris JR (2007) Local therapy and survival in breast cancer. N Engl J Med 356: 2399–2405
Sherr CJ (1966) Cancer cell cycles. Science 274: 1672–1677
Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512
Shishodia S, Sethi G, Ahn KS, Aggarwal BB (2007) Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem Pharmacol 74: 118–130
Slingerlan J, Pagano M (2000) Regulation of the Cdk inhibitor and its deregulation in cancer. J Cell Physiol 183: 10–17
Sliva D, Harvey K, Mason R, Lloyd Jr F, English D (2001) Effect of phosphatidic acid on human breast cancer cells exposed to doxorubicin. Cancer Invest 19: 781–788
Sliva D, Labarrere C, Slivova V, Sedlak M, Lloyd Jr FP, Ho NWY (2002a) Ganoderma lucidum suppresses motility of highly invasive breast and prostate cancer cells. Biochem Biophys Res Commun 298: 603–612
Sliva D, Rizzo MT, English D (2002b) Phosphatidylinositol 3-kinase and NF-κB regulate motility of invasive MDA-MB-231 human breast cancer cells by the secretion of urokinase-type plasminogen activator (uPA). J Biol Chem 277: 3150–3157
Slivova V, Valachovicova T, Jiang J, Sliva D (2004) Ganoderma lucidum inhibits invasiveness of breast cancer cell. J Cancer Integr Med 2: 25–30
Slivova V, Zaloga G, DeMichele SJ, Mukerji P, Huang YS, Siddiqui R, Harvey K, Valachovicova T, Sliva D (2005) Green tea polyphenols modulate secretion of urokinase plasminogen activator (uPA) and inhibit invasive behavior of breast cancer cells. Nutr Cancer 52: 65–72
Song KS, Cho SM, Lee JH, Kim HM, Han SB, Ko KS, Yoo ID (1995) B-lymphocyte-stimulating polysaccharide from mushroom Phellinus linteus. Chem Pharm Bull (Tokyo) 43: 2105–2108
Song YS, Kim SH, Sa JH, Jin C, Lim CJ, Park EH (2003) Anti-angiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus linteus. J Ethnopharmacol 88: 113–116
Stanley G, Harvey K, Slivova V, Jiang J, Sliva D (2005) Ganoderma lucidum suppresses angiogenesis through the inhibition of secretion of VEGF and TGF-beta1 from prostate cancer cells. Biochem Biophys Res Commun 330: 46–52
Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, Lavin P, Draetta G, Pagano M, Loda M (1997) The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res 57: 1259–1263
Tripathy D (2007) Capecitabine in combination with novel targeted agents in the management of metastatic breast cancer: underlying rationale and results of clinical trials. Oncologist 12: 375–389
Wasser SP (2002) Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol 60: 258–274
Wong NC, Mueller BM, Barbas CF, Ruminski P, Quaranta V, Lin EC, Smith JW (1998) Alphav integrins mediate adhesion and migration of breast carcinoma cell lines. Clin Exp Metastasis 16: 50–61
Woodgett JR (2005) Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol 17: 150–157
Yacoub A, Han SI, Caron R, Gilfor D, Mooberry S, Grant S, Dent P (2003) Sequence dependent exposure of mammary carcinoma cells to Taxotere and the MEK1/2 inhibitor U0126 causes enhanced cell killing in vitro. Cancer Biol Ther 2: 670–676
Xia C, Meng Q, Cao Z, Shi X, Jiang BH (2006) Regulation of angiogenesis and tumor growth by p110 alpha and AKT1 via VEGF expression. J Cell Physiol 209: 56–66
Zhang Y, Wang Z, Ahmed F, Banerjee S, Li Y, Sarkar FH (2006) Down-regulation of Jagged-1 induces cell growth inhibition and S phase arrest in prostate cancer cells. Int J Cancer 119: 2071–2077
Zhu T, Guo J, Collins L, Kelly J, Xiao ZJ, Kim SH, Chen CY (2007) Phellinus linteus activates different pathways to induce apoptosis in prostate cancer cells. Br J Cancer 96: 583–590
Acknowledgements
This work was supported by the Methodist Health Foundation, by the Department of the Army Medical Research and Materiel Command, Breast Cancer Research Training Program and by Maitake Products Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Sliva, D., Jedinak, A., Kawasaki, J. et al. Phellinus linteus suppresses growth, angiogenesis and invasive behaviour of breast cancer cells through the inhibition of AKT signalling. Br J Cancer 98, 1348–1356 (2008). https://doi.org/10.1038/sj.bjc.6604319
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604319
Keywords
This article is cited by
-
Effect of Smilax spp. and Phellinus linteus combination on cytotoxicity and cell proliferation of breast cancer cells
BMC Complementary Medicine and Therapies (2023)
-
Systematic evaluation of the anti-tumor effect of Phellinus linteus polysaccharide in thyroid carcinoma in vitro
Molecular Biology Reports (2022)
-
In silico identification of potential inhibitors of MPS1 from edible mushroom (Pleurotus ostreatus) to prevent aneuploidy and tumorigenesis
Journal of Proteins and Proteomics (2022)
-
Nutrient profiles, functional compositions, and antioxidant activities of seven types of grain fermented with Sanghuangporus sanghuang fungus
Journal of Food Science and Technology (2021)
-
The high-efficient production of phelligridin LA by Inonotus baumii with an integrated fermentation-separation process
Bioprocess and Biosystems Engineering (2020)