ABSTRACT
Transgenic mice ubiquitously overexpressing murine r-aminobutyric acid transporter subtype I were created. Unexpectedly, these mice markedly exhibited heritable obesity, which features significantly increased body weight and fat deposition. Behavioral examination revealed that transgenic mice have slightly reduced spontaneous locomotive capacity and altered feeding pattern. This preliminary finding indicates that the inappropriate level of g-aminobutyric acid transporters may be directly or indirectly involved in the pathogenic mechanism underlying certain types of obesity.
Similar content being viewed by others
INTRODUCTION
γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the vertebrate central nervous system where it activates a variety of GABA receptor types. The neurotransmission of GABA is thought to be terminated by it's rapid re-uptake via GABA transporters into presynaptic neurons and surrounding glial cells1,2. Besides functioning in the termination of synaptic transmission, GABA transporters play a critical role in the regulation of the magnitude and duration of GABA's action and may also mediate the release of GABA into the extracellular space in a Ca2+-independent manner3. Therefore, GABA transporters are speculated to possess a spectrum of physiological functions. For example, GABA transporters were reported to be involved in the maturation of GABAergic inhibitory system in the brain4. The alteration of the level of GABA transporters is correlated with the temporal lobe epilepsy5. To further explore the role of GABA transporter in vivo, we created the transgenic mice that ubiquitously overexpress GABA transporter subtype I (GAT1), which is considered to be the predominant GABA transporter in the rodent brain6. Beyond expectation, a number of transgenic founders and their progeny exhibited remarkably obese phenotype though displaying grossly normal development. The following study preliminarily investigated this phenomenon.
MATERIALS AND METHODS
Animals
Transgenic mice carrying the murine GAT1 (mGAT1) cDNA have been previously produced and identified (data submitted in a separate manuscript). A full length cDNA coding for mGAT1 that screened from the λ phage murine brain cDNA library was cloned into the EcoR I and Apa I site of pcDNA3, under the control of human cytomegalovirus (HCMV) promoter/enhancer. This construct, linearized with Nru I and Tth111 I and subsequently gel-purified, was microinjected into the pronuclei of fertilized eggs of (C57BL/6J) F1 hybrid mice (Jackson Laboratory). Polymerase chain reaction (PCR) and Southern-blot analysis was carried out with tail DNA to verify the integration of variable copy numbers of transgene into the genomes of founder mice and their progeny. Semi-quantitative reverse transcription-PCR (RT-PCR) and Northern-blot analysis of whole RNA samples, extracted from a variety of tissues, characterized the expression pattern of the transgene. Age-matched wild-type mice with the same genetic background were used as control in the present study. All animals were housed in groups (2-5 mice per cage) in temperature- and humidity- controlled environment with a 12 h light /12 h dark rhythm, and allowed free access to food and water.
Behavioral experiments
All behavioral experiments were performed with experimenter blind to the genotype of each subject. Mean value and significant difference between transgenic mice and wild-type control were analyzed by variance analysis with student's t-test.
Body weight, retroperitoneal fat pad weight and food intake measurements
Body weight was measured with electronic balance at the indicated month of age (Fig 1). 6 month old mice were sacrificed by cervical dislocation and dissected to thoroughly remove the retroperitoneal fat pads for weight measurement. Fat depots in other adipose tissue regions were also examined by macrography. For food intake analysis, the mice were housed individually on a standard chow diet for 10 d before measurement, then the cumulative food intake was monitored with a 12 h daytime /12 h nighttime interval over a 1 w period.
Body weight gained with growth of the F3 transgenic mice from two independent founders (Tg 1 and Tg 3), and wild-type control (Wt). Data expressed as mean ± s.e.m. (The amount of mice employed for each group in experiment: Wt, n = 14; Tg 1, n = 14; Tg 3, n = 8) Asterisk denotes significance at P<0.01; Student's t-test.
Open field test
3-4 month old mice were individually placed in an open box (50 × 50 × 30 cm high) with bottom divided into 10 cm × 10 cm squares. During the test session of 5 min, the motion path of the mouse was continuously traced manually. The number of squares crossed was counted for evaluation. Test was carried out within 3 consecutive days (One test session per day).
Determination of the plasma concentrations of triglyceride (TG), total cholesterol (CHO), low density lipoprotein (LDL) and high density lipoprotein (HDL)
Blood samples were obtained by retro-orbital sinus bleeding without anesthesia. The concentrations of TG, CHO, LDL and HDL were subsequently assayed with clinical kits according to the protocols detailed by the manufacturer (Shanghai Kehua-Dongling Diagnostic, China)
Immunofluorescent detection
4 mon old transgenic and wild-type mice were anesthetized with sodium pentobarbital, and consequently perfused intracardially with phosphate-buffered saline (PBS) and then chilled 95% ethanol. After perfusion, tissues were removed and subsequently immersed in 30% sucrose in PBS and frozen in OCT. 20 μm-thick frozen sections were cut and then rinsed in 0.01 M PBS. In staining procedure, cryostat sections were first blocked with 10% goat serum plus 0.1% Triton ×−100 in PBS and incubated with affinity-purified polyclonal rabbit anti-mGAT1(GAT11-50) antibody7 at 4°C overnight. Subsequently, the sections were exposed to biotin conjugated goat anti-rabbit IgG (Sigma) for 45 min at room temperature, and then to TRITC-Extravidin (Sigma) for 45 min at room temperature, finally coverslipped with PBS-buffered glycerol. Between each step, slides were intensively rinsed three times and each time for 10 min in PBS buffer. Slides were examined and photographed under Olympus fluorescence microscope.
RESULTS
Change of body weight with growth
The body weights of the transgenic F3 progeny from two independent lines, fed with a standard chow diet, were measured at various developmental stage (Fig 1). Within the 3 mon of postnatal growth, transgenic mice developed normally and insignificantly differed with the pair-fed and age-matched wild-type mice in body weight, but thereafter, both transgenic lines became conspicuously obese relative to control. At 9 mon of age, transgenic mice gained weight approximately 50 % more than control. Besides evidently increased body weight, this characteristic phenotype also includes expanded body size. No significant difference in body weight at various postnatal growth stages was observed between two transgenic lines, therefore, the obese phenotype should be independent of the transgene integration locus.
Macroscopic inspection of the fat deposition
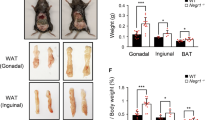
Necropsy examination revealed that transgenic mice formed normal adipose tissues at birth, but greatly developed fat deposition during postnatal development. In 6 mon old transgenic mice, the apparently accumulated fat depots were found in either interscapular, subcutaneous, epididymal, mesentric and retroperitoneal (Fig 2a) and other adipose tissue regions. Especially, the weight of the retroperitoneal fat pads of transgenic mouse is astonishingly increased compared with control (Fig 2c). This increase was also significantly different when expressed as a percentage of body weight (Fig 2d). In addition, this abnormal fat accumulation seems became progressively severe.
a.Representative appearance of the 6 mon old obese transgenic mouse (Tg 3, Right) and the wild-type control (Left). Note the apparently increased body size and the massive retroperitoneal fat pad (Arrowhead) in transgenic mouse. b. The average individual body weight of transgenic (Tg 1 and Tg 3) and wild-type mice (Wt), which were sacrificed for the following various experiments ( b, c, d, e, f). c. The weights of the retroperitoneal fat pads. d. The percentage of the retroperitoneal fat pads weight to the body weight. e. Plasma concentrations of triglyceride (TG), total cholesterol (CHO), low density lipoprotein (LDL) and high density lipoprotein (HDL). f. The concentration ratio of HDL to LDL. Data expressed as mean ±s.e.m. (The amount of mice employed for each group in experiment: Wt, n = 11; Tg 1, n = 12; Tg 3, n = 7) Single asterisk and double asterisk indicate significantly difference between transgenic mice and control with P<0.05 and P<0.01, respectively; Student's t-test.
Plasma concentrations of triglyceride (TG), total cholesterol (CHO), low density lipoprotein (LDL) and high density lipoprotein (HDL)
To characterize whether the obesity observed in transgenic mice is related with the modifications in lipid metabolism, biochemical analysis available were performed to determine the TG, CHO, LDL and HDL concentrations in the plasma of 6 mon old mice. This assay revealed that the plasma concentrations of TG, CHO, LDL and HDL are significantly elevated by 84 %, 44 %, 61 %, and 24 %, respectively, in transgenic mice compared with that of control (Fig 2e). In addition, transgenic mice exhibit considerably decreased concentration of HDL relative to that of LDL (Fig 2f). Above results indicated that the obesity is possibly associated with the alteration of the lipid metabolism.
Assessment of the spontaneous locomotive activity
The spontaneous locomotive activity of the 3-4 mon old mice was evaluated by open field test. The total number of squares crossed by the transgenic mice and control during the observation on 3 consecutive d is depicted in Fig 3. Two independent transgenic lines developed approximately 80 % and 85 % the locomotive capacity of wild-type mice. This was not due to any overt neurological impairment such as pareses of fore- or hindlimbs or signs of ataxia. Transgenic mice, however, discontinued their locomotion more frequently with longer periods of time. The slightly decreased locomotive activity indicated that the energy expenditure may be reduced in transgenic mice.
Reduced spontaneous locomotive activity in transgenic mice. The difference between wild-type mice (Wt) and both transgenic lines (Tg 1 and Tg 2) is statistically significant with P$<$0.05 for all values (The amount of mice employed for each group in experiment: Wt, n = 18; Tg 1, n = 19; Tg 2, n = 9); Student's t-test.
Behavioral alteration on food intake
To explore whether transgenic mice exhibit alterations in feeding behavior when they begin to gain more body weights than control, food intake was monitored in 3-4 mon old transgenic and wild-type mice. Consecutive observation revealed that transgenic mice showed no significant difference in total daily food intake (Fig 4b), but displayed altered feeding pattern (Fig 4a). Either transgenic line ate 90% more in daytime and 10% less in nighttime daily than did wild-type mice (Fig 4b).
Food intake of 3-4 mon old transgenic mice from two independent lines (Tg 1 and Tg 3) and age-matched wild-type control (Wt). a. Food intake observed within 4 consecutive days. D and N denote day-time (A.M. 7:00 -- P.M. 7:00) and night-time (P.M.7:00 -- A.M.7:00), respectively. b. The average food intake per day-time, night-time and whole day were statistically calculated, respectively, on the observation within 1 w. Data expressed as mean ± s.e.m. (The amount of mice employed for each group in experiment: Wt, n = 10; Tg 1, n = 9; Tg 3, n = 7). Single asterisk indicates P<0.05; Double asterisk indicates P<0.01; Student's-test.
DISCUSSION
Transgenic mice ubiquitously overexpressing GAT1 were generated in our laboratory (data submitted in a separate manuscript). Unexpectedly, the founder mice developed conspicuously obesity characterized by greatly increased body weight and fat deposition. Systematical measurement of the body weight gained with growth of F3 transgenic mice confirmed that the obesity phenotype is heritable and relatively early in onset (Fig 1). In addition, the abnormally accumulated fat deposition associated with significantly elevated level of plasma triglyceride indicates that the energy homeostasis of the transgenic mice may be altered, since the adipose tissue itself serves as the site of triglyceride storage and free fatty acids/glycerol release in response to changing energy demands8. It is noteworthy that mice raised from all independent founders developed obesity. Because there is hardly any possibility for the transgene introduced into the genome of various lines to possess the identical integration pattern, the phenotype should not be resulted from an insertional mutation.
In the present study, no statistically significant differences in gaining body weight (Fig 1) and feeding behavior (Fig 4) between the two independent lines (Tg 1 and Tg 3) were observed. It is reasonable since they displayed similar (not identical) level and distribution of GAT1 (data submitted in a separate manuscript). However, the retroperitoneal fat accumulation in Tg1 and Tg 3 mice appeared apparently different (Fig 2, c, d), which perhaps implied that the fat deposition is highly sensitive to the slight alteration in the expression of GAT1. It potentially suggested that the obesity phenotype might be correlated with expression pattern of GAT1.
Previous studies indicated that body-weight regulation occurred through a complex net work involving many brain areas, among which the hypothalamic region is thought to be a pivotal target9. This region is closely associated with the actions of two important regulators of food intake and energy balance, leptin10 and neuropeptide Y11,12. GABA and glutamate decarboxylase (GAD) which produce the GABA from glutamic acid, and GABAA receptors are densely distributed in the hypothalamus13,14. GABA mediated transmission in hypothalamus is involved in the regulation of appetite15. Furthermore, GABAergic transmission may be correlated with the actions of glucocorticoids, which play an important role in the development of genetic obesity16. In our transgenic mice, immunofluorescent detection revealed that the GAT1 was highly overexpressed in the hypothalamus in contrast with that of normal mice (Fig 5). Since GAT1 is critical to GABAergic transmission, it is possible that the inappropriate overproduction of GAT1 may affect the functions mediated by GABA system, and further disturb the normal signaling pathways in hypothalamus and in the control of obesity with unknown mechanism. This opinion is potentially supported by the fact that the feeding behavior has been altered in transgenic mice though its exact cause and implication in obesity are not clear.
In summary, we demonstrated that transgenic mice overexpressing GAT1 can develop obesity, but its underlying mechanism remains to be elucidated. To our knowledge, no reports so far published suggest that neurotransmitter transporter is involved in the control of obesity. The present finding would provide a clue to evaluate such possibility.
References
Clark JA, Amara SG . Amino acid neurotransmitter transporters: structure, function, and molecular diversity. BioEssays 1993; 15:323–32.
Schloss P, Puschel AW, Betz H . Neurotransmitter transporters: new members of known families. Curr Opin Cell Biol 1994; 6:595–9.
Levi G, Raiteri M . Carrier-mediated release of neurotransmitters. Trends Neurosci 1993; 16:415–8.
Jursky F, Nelson N . Developmental expression of GABA transporters GAT1 and GAT4 suggests involvement in brain maturation. J. Neurochem 1996; 67:857–67.
During MJ, Ryder KM, Spencer DD . Hippocampal GABA transporter function in temporal-lobe epilepsy. Nature 1995; 376:174–7.
Radian R, Ohersen OP, Mathisen-storm J, Castel M, Kanner BI . Immunocytochemical localization of the GABA transporter in rat brain. J Neurosci 1990; 10:1319–30.
Cai GQ, Fei J, Xu YP, Guo LH . Nuclear proteins from liver and kidney bind a 37 bp sequence in the 5′ upstream region of the mGAT1 gene. Neuroreport 1998; 9:4059–62.
Spiegelman BM, Flier JS . Adipogenesis and obesity: rounding out the big picture. Cell 1996; 87:377–89.
Levine AS, Billington CJ . Obesity: progress through genetic manipulation. Curr Biol 1998; 8:251–2.
Huang L, Li C . Leptin: a multifunctional hormone. Cell Res 2000; 10:81–92.
De Quidt ME, Emson PC . Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system-II. Immunohistochemical analysis. Neuroscience 1986; 18:545–618.
Kristensen P, Judge ME, Thim L et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 1998; 393:72–6.
Sakaue M, Saito N, Taniguchi H, Baba S, Tanaka C . Immunohistochemical localization of r-aminobutyric acid in the rat pituitary gland and related hypothalamic regions. Brain Res 1988; 446:343–53.
Vincent SR, Hokfelt T, Wu JY . GABA neuron systems in hypothalamus and the pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology 1982; 34:117–25.
Cooper SJ . GABA and endorphin mechanisms in relation to the effects of benzodiazepines on feeding and drinking. Prog Neuro-Psychopharcol Biol Psychiat 1983; 7:495–503.
Tsujii S, Bray GA . GABA-related feeding control in genetically obese rats. Brain Res 1991; 540:48–54.
Acknowledgements
We thank Prof. Da WANG and Dr. Jingwei ZHAO for invaluable assistance with histological techniques, Dr. Guoqiang CAI for kindly providing anti-mGAT1 antibody, Dr. Chunxiao ZHOU for biochemical analysis, Mr. Maohu ZHANG for help on laboratory affairs.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
MA, Y., HU, J., ZHOU, X. et al. Transgenic mice overexpressing γ-aminobutyric acid transporter subtype I develop obesity. Cell Res 10, 303–310 (2000). https://doi.org/10.1038/sj.cr.7290057
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290057
Keywords
This article is cited by
-
The Human Obesity Gene Map: The 2004 Update
Obesity Research (2005)
-
The Human Obesity Gene Map: The 2002 Update
Obesity Research (2003)
-
Norepinephrine transporter (NET) is expressed in cardiac sympathetic ganglia of adult rat
Cell Research (2001)