Abstract
Pulmonary Arterial Hypertension (PAH) is a rare and progressive disease with low incidence and prevalence, and elevated mortality. PAH is characterized by increased mean pulmonary artery pressure. The aim of this study was to analyse patients with combined mutations in BMPR2, ACVRL1, ENG and KCNA5 genes and to establish a genotype-phenotype correlation. Major genes were analysed by polymerase chain reaction (PCR) and direct sequencing. Genotype-phenotype correlation was performed. Fifty-seven (28 idiopathic PAH, 29 associated PAH group I) were included. Several mutations in different genes, classified as pathogenic by in silico analysis, were present in 26% of PAH patients. The most commonly involved gene was BMPR2 (12 patients) followed by ENG gene (9 patients). ACVRL1 and KCNA5 genes showed very low incidence of mutations (5 and 1 patients, respectively). Genotype-phenotype correlation showed statistically significant differences for gender (p = 0.045), age at diagnosis (p = 0.035), pulmonary vascular resistance (p = 0.030), cardiac index (p = 0.035) and absence of response to treatment (p = 0.011). PAH is consequence of a heterogeneous constellation of genetic arrangements. Patients with several pathogenic mutations seem to display a more severe phenotype.
Similar content being viewed by others
Introduction
Pulmonary Arterial Hypertension (PAH; OMIM #178600, ORPHA 422) is a progressive, poorly characterized disease with low incidence and prevalence in the general population1, and a poor prognosis in terms of quality of life, morbidity and mortality2. Pulmonary circulation in PAH is characterized by an increased mean pulmonary arterial pressure at rest ≥25 mmHg2,3. The aetiology is quite diverse, resulting in a large variability at both clinical and genetic levels4 which further complicates the patient management, a systematic diagnostic evaluation and finally, lead to a premature heart failure and death5.
In the V World Symposium on Pulmonary Hypertension in Nice3, PAH was classified as idiopathic (IPAH) when the origin is unknown, heritable (HPAH) when the disease is inherited within an autosomal dominant pattern with incomplete penetrance, or associated when other conditions co-occur (APAH)3. The global incidence is 2–5 cases per million per year4. However, in countries as USA or France this value is lower, 1–2 and 2.4 cases per million per year, respectively6,7, whereas Scotland shows a higher incidence with 7.6 cases per million per year8. Finally, in Spain this value is 3.3 cases per million per year1,9. There is a female predominance in patients with PAH, with a gender ratio of 1.7:1 female to male10,11, as it has been widely reported.
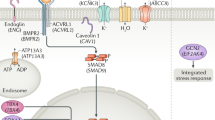
The bone morphogenetic protein type 2 receptor gene (BMPR2; MIM #600799), a member of the transforming growth factor (TGF-β) superfamily, was the first causal gene identified in PAH and is mutated in approximately 10 to 40% of IPAH patients and 80% of patients with HPAH. This gene is located on chromosome 2q3310,12,13,14,15. Other genes have been associated with the disease, including Activin A type II receptor like kinase 1 (ALK1/ACVRL1; MIM #601284), located on chromosome 12q1316,17, Endoglin (ENG; MIM #601284)10, located on chromosome 9q33-3417,18, and Potassium voltage-gated channel, shakerrelated subfamily, member 5 (KCNA5; MIM #176267), located on chromosome 12p1319,20. Mutations in ACVRL1, ENG and KCNA5 genes are less frequent than mutations in BMPR2 gene in patients with PAH12,18. Patients with a pathogenic mutation in these genes develop a more severe phenotype and have an earlier age at diagnosis12,13,18. Recently, new genes related to PAH have been described as Potassium Channel Subfamily K, Member 3 (KCNK3; MIM #603220)21, Caveolin-1 (CAV1; MIM #601047)22, Cerebellin 2 Precursor (CBLN2; MIM #600433)23 or T-box 4 (TBX4; MIM #601719)24. Besides, there are genetic modifiers that affect PAH pathogenicity in combination with mutations in those genes already described25,26,27.
Recent findings point out that the penetrance and expressivity of PAH are likely to be directed by the mutational load of all genes involved in the disease. Thus, we aimed to analyse here the implication of harbouring a range of pathogenic mutations in PAH. In addition, we tried to establish a genotype-phenotype correlation between clinical and hemodynamic features of patients with several pathogenic mutations.
Results
Description of the cohort
Fifty-seven unrelated, Caucasian PAH patients (28 idiopathic, 20 associated to connective tissue disease, 4 related to HIV and 5 porto-pulmonary hypertension) (Fig. 1) were included. At the time of diagnosis 8 patients were in functional class (FC) I, 20 patients in FC II, 25 patients in FC III and 4 in FC IV (Table 1). This cohort has been partially characterized in previous studies12,25,28. We have recruited patients during the last years and we performed several genetic analyses with them. The clinical description is so similar for the cohort, but for the genotype-phenotype correlation, we select only those patients of interest.
Graphical representation of the patients included in this study and their clinical features.
Age displayed is the age at diagnosis. PAH: Pulmonary Arterial Hypertension; IPAH: Idiopathic Pulmonary Arterial Hypertension; APAH: Associated Pulmonary Arterial Hypertension; CTD: connective tissue disease; HIV: Human Immunodeficiency virus; P-P: Porto-pulmonary hypertension.
Mutational study of BMPR2, ACVRL1, ENG and KCNA5 genes
After mutational screening of BMPR2, ACVRL1, ENG and KCNA5 genes, we identified pathogenic mutations in 72% (40) patients. BMPR2 was the gene with a greater number of pathogenic mutations (44% of patients with mutations), followed by ENG (29%), ACVRL1 (17%) and, finally KCNA5 (10%) gene (Fig. 2). These results have been partially reported in Pousada et al.12 and Pousada et al.28.
During the mutational analysis, we found a high percentage of patients, 26% (15 patients), with several mutations classified as pathogenic after in silico analysis. Among them, some patients had several mutations in the same gene whereas others harboured several mutations in different genes (Table 2). Besides, 12 of these patients were carriers of at least one mutation in BMPR2 gene. ENG gene was the second most important gene involved, with 9 patients showing a mutation in this gene. However, ACVRL1 and KCNA5 genes were less represented, since they were mutated only in 5 and 1 patients, respectively (Fig. 3). None of these mutations were detected in 55 control samples. The variants were analyzed with an exhaustive in silico analysis with bioinformatics tools (Tables 3 and 4).
Focusing on patients with several mutations, all the mutations identified here were located in coding region for BMPR2, ACVRL1 and KCNA5 genes, and also in intronic junctions for ENG gene. Moreover, missense changes accounted for 86% of total, whereas nonsense mutations were only identified in 13% of patients. Synonymous changes and intronic variants were detected in 40% and 26% of these patients, respectively. These results are shown in Fig. 4.
We found several mutations in BMPR2 gene in a 20% of patients with several mutations included in this study. In addition, we detected only one patient that showed two pathogenic mutations in ENG gene.
Genotype correlation with clinical and hemodynamic parameters
Clinical and hemodynamic parameters were compared between patients with several mutations and patients with only one pathogenic mutation. We also performed genotype-phenotype correlation between patients with several pathogenic mutations and patients without mutations. The statistical variables considered here were gender, age at diagnosis, mean pulmonary arterial pressure (mPaP), systolic pulmonary arterial pressure (sPaP), pulmonary vascular resistance (PVR), cardiac index (CI), 6 minute walking text (6MWT), PAH type (IPAH vs APAH) and response to treatment. Patients who did not respond were treated with Phosphodiesterase 5 Inhibitors. Variables were categorized according to the best cut off point by ROC curve.
Regarding to the correlation between patients with several mutations and patients with a single mutation, we found statistically significant differences for gender (p = 0.045), with a greater number of women with several mutations, the age at diagnosis (p = 0.035), showing disease symptoms 11 years earlier, and a significantly higher PVR (p = 0.030) than patients with single mutation. Furthermore, patients with several mutations showed significant differences regarding CI (p = 0.035) and no response to therapy (p = 0.011) (Table 5). When comparing patients with several mutations and patients with no mutations, the results are quite similar to the abovementioned (Table 5). We did not find statistically significant differences according to PAH type (p = 0.401).
Three out of 57 patients in our cohort died during the mean follow up period (14 months). The first deceased patient had APAH (connective tissue disease) and he was carrier of c.251G > T (p.C84F) and c.981T > C (p.P327P) BMPR2 mutations. The second one had IPAH and showed c.229A > T (p.I77L) and c.633A > G (p.R211R) mutations in BMPR2 gene and c.1272 + 6A > T mutation in ENG gene. Finally, the last deceased patient was classified as APAH (porto-pulmonary hypertension) and harboured c.1021G > A (p.V341M) mutation in BMPR2 gene and c.498G > A (p.Q166Q) mutation in ENG gene.
Discussion
In this study, we have identified and characterized 15 out of 57 PAH patients carrying more than one pathogenic mutation in several genes related to PAH, such as BMPR2, ENG, ACVRL1 and KCNA5. Twelve of these patients harboured at least one mutation in BMPR2, reinforcing the role of this gene in the development of PAH. On the other hand, nine patients were carriers of mutations in the ENG gene, representing the second gene most frequently involved in our cohort of PAH patients with several mutations. Remarkably, eight patients showed mutations in both genes. However, only five and one patients had mutations in ACVRL1 and KCNA5 genes, respectively. As a whole, it is difficult to elucidate the role that each of the different mutations could have had in the development of disease. Thus, the molecular pathogenic mechanism of PAH is not fully understood; in fact multiple genetic and environmental factors have been related to the disease. Many of the involved genes are part of the TGF-β signalling pathway, so several mutations in one or more genes in the same pathway could explain the reduced penetrance for PAH.
The characterization of putative missense mutations was performed by in silico analysis, selecting only those identified as pathogenic by at least three software tools, whereas synonymous and intronic mutations were classified as pathogenic if two bioinformatic programs that analyse splice sites gave positive results. Thus, we consider this approach is stringent enough to make an accurate classification at this level. However, it is important to note that this is only a bioinformatic prediction to characterize the nature of the change, the variants do not appear in public databases, nor detected in general population so those are pieces of evidence for the pathogenic nature of the change29, although functional analyses should be performed in order to identify them as clearly pathogenic.
Recently, Mallet et al.30 performed functional analysis for several ENG mutations. They detected 10 patients with Hereditary Hemorrhagic Telangiectasia (HHT; OMIM #187300) with more than one mutation in ENG or with one mutation in ENG and another one in ACVLR1 gene, but after functional analysis there were not differences compared to wild-type, considering these ENG missense mutations as rare benign variants30. We detected not only missense mutations in ENG, but also mutations affecting the splicing process, and interestingly a high proportion of patients with ENG mutations harbouring an additional mutation in BMPR2 gene. ENG inhibits the TGF-β pathway in endothelial cells by down-regulating the ALK5/Smad3 pathway but enhanced ALK1 signalling. As it have been described in other oligogenic diseases with a specific major gene involved in their development, mutations in other genes within the same pathway should be considered31. Rodríguez-Viales et al.32 published a study of two PAH families in which index patients showed one mutation in the 5′UTR region of BMPR2 gene described by Wang et al.33 in an IPAH patient, along with another mutation in the coding region of BMPR2 or in the ENG gene, respectively. They suggested that the mutation in the promoter region could explain the variable penetrance of the disease32 as it has been related to a decrease in the expression of BMPR2.
Total mutational load has been described for other pathologies, involving mutations in several genes that codify for proteins belonging to the same or related pathways, in the same individual34,35. Taking this into account, we could not discard an oligogenic inheritance model for PAH as described for others diseases, with a major gene being BMPR2. Approaches like next generation sequencing (NGS) analysis could give us genetic information that will help in the understanding of the molecular basis of PAH. The oligogenic inheritance might increase the risk of developing the disease and perhaps a more severe phenotype, as occurs in other diseases, like Bardet-Biedl Syndrome or Autosomal Dominant Retinitis Pigmentosa34,35,36.
Thirteen of our patients were carriers of a mutation in BMPR2 gene, and four of them showed two mutations in this gene. All these changes were predicted to alter the splicing process or the conservation of the protein, producing a shorter transcript or a misfolding protein susceptible of degradation, which could prevent achieving a minimum protein translation and therefore, the development of the disease29,37. Two of these patients were carriers of a third mutation, previously described, in ENG gene. These mutations are located in the first exons and were predicted to affect the splicing process. Thus, mutations in ENG gene could prevent the correct anchoring of ENG protein in the cell membrane, impairing TGF-β/ALK1 signalling responses33.
On the other hand, eight patients were double heterozygotes for BMPR2 and ENG mutations (one of them showed a third mutation in ACVRL1 gene), one patient had two mutations in ENG gene and the remaining patients showed different combination of mutated genes: one patient was double heterozygote for ENG and ACVRL1, another two for BMPR2 and ACVRL1genes and finally, one patient showed a combination of ACVRL1 with KCNA5 genes.
In the last years a second hit hypothesis have been proposed that two mutations, one major and other as modulator, in the same gene or different gene take place32,33. It has been described that after BMPR2, ACVRL1 is the gene most frequently mutated in PAH patients. However, we show that ENG was the second gene most frequent in our cohort. All of these genes have been described to be involved in the development of the disease with or without HHT, being BMPR2 the major causal gene and the others genetic modifiers modulating the penetrance of the disease32,36,38.
Although almost all mutations described in BMPR2 gene have been established as pathogenic, others remains indeterminate, as mutations in the cytoplasmic tail that still retain capacity for downstream signalling. The pathogenic impact of others genes in the disruption of the TGF-β pathway directly or by modulating related pathways, is still unknown39,40,41,42.
None of our patients had relatives with PAH, so we could not perform segregation analysis; but none of the mutations described here were detected in 110 control chromosomes. As most of the mutations identified in PAH are private, and due to the confluence of two or more mutations in several genes, performing genotype-phenotype correlations revealed as a hard task. For this reason, the genotype-phenotype correlation has been performed grouping mutations identified on the same gene, comparing the clinical and hemodynamic parameters with patients carrying only one pathogenic mutation and also with the group of patients without pathogenic mutations.
The co-occurrence of several pathogenic mutations was more prevalent in women, which is in agreement with the higher prevalence of PAH in women10,11,38. However, Liu et al.43 postulated that the pathogenic mutations are more severe and prevalent in men for BMPR2 gene, suggesting hormonal implication. Our study did not corroborate such hypothesis, but it seems that the molecular basis of this disease could be more complex in women than men. The age of diagnosis was 11 years younger in patients with several mutations as previously described by Rodríguez-Viales et al.32 and Wang et al.33. These studies reported that patients carrying one or more pathogenic mutations exhibit an early age at diagnosis than patients without mutations. PVR were also significantly higher in patients with several mutations whereas the CI was lower. Furthermore, these patients had a worse response to treatment, compared with patients with a single or none mutation. This suggests that patients with several mutations need a more specifically treatment, in some cases directed to more than one cellular pathway. Accordingly, these patients seem to have a more severe illness and a worse prognosis. These results agree with those obtained by Rodríguez-Viales et al.32, who reported patients with several pathogenic mutations with a more severe phenotype. Also, in a previous study made by our group12, we pointed out that patients with several pathogenic mutations may show a greater predisposition to develop the disease.
Three patients died after the follow-up period. They had an early age at diagnosis and were carriers of several pathogenic mutations. In addition, these patients did not respond to treatment, achieving a gradual increase of the characteristic phenotype of PAH leading to a premature death. These patients, as well as all cases with various pathogenic mutations, had a more severe phenotype and a higher functional class at diagnosis than patients without pathogenic mutations or with only a single one, but this small number does not allow us to perform statistical analysis.
Our results are consistent with those obtained by other authors, but the small number of patients can be considered a limitation. However, the extensive genetic study and monitoring of our patients add extra values to our results.
In summary, we report a series of IPAH and APAH patients with a high percentage of them carrying more than one pathogenic mutation in several genes. Moreover, BMPR2 was the more frequently affected gene, followed by ENG, ACVRL1 and KCNA5 genes. Some mutations had not been previously described. We cannot rule out that patients with a single pathogenic mutation have other mutations in genes not included in this study. There is no doubt that other genes could be involved in PAH and it will be important to understand their role in the development of the disease. Patients with several pathogenic mutations seem to show a more severe phenotype. We wonder whether these additional mutations act as a second event in the development of the disease, increasing the penetrance or simply modifying the phenotype of patients.
Material and Methods
Patients and samples
Fifty-seven patients with idiopathic or associated PAH (group 1 of the new classification of Nice)6 followed in our Pulmonary Arterial Hypertension Unit were enrolled. This cohort has been described previously by our group12,25. Fifty-five healthy individuals of Spanish origin without a familial history of PAH were also included to determine their mutational frequencies, kindly provided by Complexo Hospitalario Universitario de Vigo (Vigo, Spain). All patients are included in the CHUVI DNA Biobank (Biobanco del Complejo Hospitalario Universitario de Vigo). Patients signed an informed consent and the Regional Ethics Committee approved the study (Galician Ethical Committee for Clinical Research; Comité Autonómico de Ética da Investigación de Galicia - CAEI de Galicia), following the clinical-ethical guidelines of the Spanish Government and the Helsinki Declaration.
Cardiac catheterization was performed using the latest consensus diagnostic criteria of the ERS-ESC (European Respiratory Society-European Society of Cardiology)44. PAH was considered idiopathic after exclusion of the possible causes associated with the disease. Clinical data included use of drugs, especially appetite suppressants, and screening for connective tissue diseases and hepatic disease. The study also included serology for HIV, autoimmunity, thoracic CT scan, echocardiography, right catheterization and 6 minute walking test (6MWT). Patients with PAH that could be related to chronic lung disease were excluded12,25. The criteria of good response to treatment after 6 months were: decrease of at least one functional class, increase the distance walked in the 6MWT at least 10%, no hospital admissions and no episodes of right heart failure.
Identification of mutations in BMPR2, ACVRL1, ENG and KCNA5 genes
Genomic DNA was extracted from leukocytes isolated from venous blood using the FlexiGene DNA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. We used primers described by Deng et al.45 for BMPR2 gene, by Berg et al.46 for ACVRL1 gene, by Gallione et al.47, with minor modifications, for ENG gene, and by Yang et al.48 for KCNA5 gene. Amplification of exons and intronic junctions was performed with 50 ng of genomic DNA using GoTaq® Green Master Mix (Promega Corporation, Madison, Wisconsin, USA), according to the manufacturer’s protocol. GoTaq® Green Master Mix contained MgCl2, dNTPs, reaction buffer and Taq DNA polymerase. PCR was performed in a GeneAmp PCR System 2700 (Applied Biosystems, Carlsbad, California, USA).
PCR products were confirmed by electrophoresis through 2% agarose gels with SYBR® Safe DNA Gel Stain (Invitrogene, San Diego, California, USA) in a Sub-Cell GT (Bio-Rad, Hercules, California, USA). HyperLadder V was used as molecular weight marker (New England Biolabs, Ipswich, Massachusetts, USA). The PCR product was purified using the Nucleic Acid and Protein Purification NucleoSpin Extract II kit (Macherey-Nagel, Düren, Germany) or ExoSAP-IT kit (USB Corporation, Cleveland, Ohio, USA). Purified PCR products were sequenced for both forward and reverse strands with BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, California, USA). The sequencing reactions were precipitated with Agencourt CleanSEQ - Dye Terminator Removal (Beckman coulter, Brea, California, USA) and analyzed in an ABI PRISM 3100 genetic analyzer (Applied Biosystems, Carlsbad, California, USA). All results were confirmed by a second independent PCR.
Analysis of mutations
Sequence data were aligned with the reference Ensembl cDNA sequence [ENST00000374580] for BMPR2 gene, [ENST00000388922] for ACVRL1 gene, [ENST00000344849] for ENG gene and [ENST00000252321] for KCNA5 gene, and examined for sequence variations. We use the Basic Local Alignment Search Tool (BLAST) software to align sequences and compare them with different organisms. Rare missense variants were analyzed to predict their potential pathogenicity, used combined computer algorithms: Polyphen-249, Pmut50, Sort Intolerant from Tolerant (SIFT)51 and MutationTaster2 software52. Other combined computer algorithms were used to predict whether that change could affect donor/acceptor splice sites: HSF Human53, NetGene254, Splice View54 and NNSplice54. Fifty-five control samples were checked in order to established genetic frequencies for all mutations detected.
We classified a missense variant as a mutation when is considered pathogenic by at least three software tools. In addition, synonymous and intronic variants were classified as pathogenic if at least two out of four bioinformatics tools used to predict alterations in mRNA processing showed a new donor/acceptor splice site or if the prediction change dramatically.
Statistical analysis
We used statistical package SPSS v19 for Microsoft. A non-parametric test (U Mann-Whitney) was used for comparisons between patients and controls, but this approach was only exploratory. To compare the different genotypes with clinical and hemodynamic variables we used the Chi-square test. Values were expressed as mean ± SD (standard deviation). P-values < 0.05 were considered statistically significant.
Additional Information
How to cite this article: Pousada, G. et al. Complex inheritance in Pulmonary Arterial Hypertension patients with several mutations. Sci. Rep. 6, 33570; doi: 10.1038/srep33570 (2016).
References
McGoon, M. D. et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 62 (25 Suppl), D51–9 (2013).
Galiè, N. et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 34, 1219–1263 (2009).
Simonneau, G. et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 62 (25 Suppl), D34–41 (2013).
Peacock, A. J., Murphy, N. F., McMurray, J. J. V., Caballero, L. & Stewart, S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 30, 104–109 (2007).
Yang, X., Long, L., Reynolds, P. N. & Morrell, N. W. Expression of mutant BMPR-II in pulmonary endothelial cells promotes apoptosis and a release of factors that stimulate proliferation of pulmonary arterial smooth muscle cells. Pulm Circ. 1(1), 103–111 (2010).
Taichman, D. B. & Mandel, J. Epidemiology of pulmonary arterial hypertension. Clin Chest Med. 34(4), 619–37 (2013).
Humbert, M. et al. Pulmonary Arterial Hypertension in France. Am J Respir Critical Care Medicine. 173, 1023–30 (2006).
Sztrymf, B. et al. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J 35, 1286–1293 (2010).
del Cerro Marín, M. J. et al. Assessing pulmonary hypertensive vascular disease in childhood. Data from the Spanish registry. Am J Respir Crit Care Med. 190(12), 1421–9 (2014).
Machado, R. D. et al. Genetics and Genomics of Pulmonary Arterial Hypertension. J Am Coll Cardiol 54(Suppl S), 1 (2009).
Sanchez, O., Marié, E., Lerolle, U., Wermert, D., Israël-Biel, D. & Meyer, G. Pulmonary arterial hypertension in women. Rev Mal Respir. 27, e79–e87 (2010).
Pousada, G., Baloira, A., Vilariño, C., Cifrian, J. M. & Valverde D. Novel mutations in BMPR2, ACVRL1 and KCNA5 genes and hemodynamic parameters in patients with pulmonary arterial hypertension. PLoS One. 9(6), e100261 (2014).
Pfarr, N. et al. Hemodynamic and clinical onset in patients with hereditary pulmonary arterial hypertension and BMPR2 mutations. Respir Res. 12, 99 (2011).
Davies, R. J. & Morrell, N. W. Molecular mechanisms of Pulmonary Arterial Hypertension: Morphogenetic protein type II receptor. Chest. 134, 1271–1277 (2008).
Girerd, B. et al. Absence of influence of gender and BMPR2 mutation type on clinical phenotypes of pulmonary arterial hypertension. Respir Res. 1, 73 (2010).
O’Callaghan, A. S., Balada, E., Serrano-Acedo, S., Simeon-Aznar, C. P. & Ordi-Ros, J. Mutations of activin-receptor-like kinase 1 (ALK-1) are not found in patients with pulmonary hypertension and underlying connective tissue disease. Clin Rheumatol. 26, 947–949 (2007).
Sadick, H. et al. Mutation analysis of “Endoglin” and “Activin receptor-like kinase” genes in German patients with hereditary hemorrhagic telangiectasia and the value of rapid genotyping using an allele-specific PCR-technique. BMC Med Genet. 10, 53 (2009).
Pfarr, N. et al. Hemodynamic and genetic analysis in children with idiopathic, heritable, and congenital heart disease associated pulmonary arterial hypertension. Respir Res. 14, 3 (2013).
Wipff, J. et al. Association of a KCNA5 gene polymorphism with Systemic Sclerosis–Associated Pulmonary Arterial Hypertension in the European Caucasian Population. Arthritis & Rheumatism. 62, 3093–3100 (2010).
Burg, E. D., Remillard, C. V. & Yuan, J. X. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: pharmacotherapeutic implications. Br J Pharmacol. 153, S99–S111 (2008).
Ma, L. et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 369(4), 351–61 (2013).
Austin, E. D. et al. Whole Exome Sequencing to Identify a Novel Gene (Caveolin-1) Associated with Human Pulmonary Arterial Hypertension. Circ Cardiovasc Genet. 5(3), 336–343 (2012).
Germain, M. et al. Genome-wide association analysis identifies a susceptibility locus for pulmonary arterial hypertension. Nat Genet. 45(5), 518–21 (2013).
Kerstjens-Frederikse, W. S. et al. TBX4 mutations (small patella syndrome) are associated with childhood-onset pulmonary arterial hypertension. J Med Genet. 50(8), 500–6 (2013).
Pousada, G., Baloira, A. & Valverde, D. Molecular and clinical analysis of TRPC6 and AGTR1 genes in patients with pulmonary arterial hypertension. Orphanet J Rare Dis. 10(1), 1 (2015).
Yu, J., Taylor, L., Wilson, J., Comhair, S., Erzurum, S. & Polgar, P. Altered expression and signal transduction of endothelin-1 receptors in heritable and idiopathic pulmonary arterial hypertension. J Cell Physiol. 228, 322–9 (2013).
Baloira-Villar, A., Pousada-Fernández, G., Vilariño-Pombo, C., Núñez-Fernández, M., Cifrián-Martínez, J. & Valverde-Pérez, D. CCTTT pentanucleotide repeats in inducible nitric oxide synthase gene expression in patients with pulmonary arterial hypertension. Arch Bronconeumol. 50, 141–5 (2014).
Pousada, G., Baloira, A., Fontán, D., Núñez, M. & Valverde, D. Mutational and clinical analysis of the ENG gene in patients with pulmonary arterial hypertension. BMC Genetics. 17, 72 (2016).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 17(5), 405–24 (2015).
Mallet, C. et al. Functional analysis of endoglin mutations from hereditary hemorrhagic telangiectasia type 1 patients reveals different mechanisms for endoglin loss of function. Hum Mol Genet. 144(6), 261–264 (2015).
Daniels, A. B., Sandberg, M. A., Chen, J., Weigel-DiFranco, C., Fielding Hejtmancic, J. & Berson, E. L. Genotype-phenotype correlations in Bardet-Biedl syndrome. Arch Ophtthalmol. 130(7), 901–7 (2012).
Rodríguez-Viales, R. et al. Mutation in BMPR2 Promoter: A ‘Second Hit’ for Manifestation of Pulmonary Arterial Hypertension? PLoS One. 10(7), e0133042 (2015).
Wang, G. et al. Early onset severe pulmonary arterial hypertension with ‘two-hit’ digenic mutations in both BMPR2 and KCNA5 genes. Int J Cardiol. 177(3), e167–9 (2014).
Hamid, R. et al. Penetrance of Pulmonary Arterial Hypertension is modulated by the expression of normal BMPR2 allele. Hum Mutat. 30(4), 649–654 (2009).
Zaghloul, N. A. et al. Functional analyses of variants reveal a significant role for dominant negative and common alleles in oligogenic Bardet-Biedl syndrome. Proc Natl Acad Sci USA 107(23), 10602–7 (2010).
Kousi, M. & Katsanis, N. Genetic modifiers and oligogenic inheritance. Cold Spring Harb Perspect Med. 5(6), 017145 (2015).
Hunt, R. C., Simhadri, V. L., Iandoli, M., Sauna, Z. E. & Kimchi-Sarfaty, C. Exposing synonymous mutations. Trends Genet. 30(7), 308–21 (2014).
Machado, R. D. et al. Pulmonary Arterial Hypertension: A Current Perspective on Established and Emerging Molecular Genetic Defects. Hum Mutat. 36(12), 1113–27 (2015).
Machado, R. D. et al. Mutations of the TGF-b Type II Receptor BMPR2 in Pulmonary Arterial Hypertension. Hum Mutat. 27(2), 121–132 (2006).
Girerd, B. et al. Characteristics of pulmonary arterial hypertension in affected carriers of a mutation located in the cytoplasmic tail of bone morphogenetic protein receptor type 2. Chest. 147(5), 1385–94 (2015).
Chan, M. C. et al. A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Mol Cell Biol. 27(16), 5776–89 (2007).
Leyton, P. A. et al. Deletion of the sequence encoding the tail domain of the bone morphogenetic protein type 2 receptor reveals a bone morphogenetic protein 7-specific gain of function. PLoS One. 8(10), e76947 (2013).
Liu, D. et al. BMPR2 mutations influence phenotype more obviously in male patients with pulmonary arterial hypertension. Circ Cardiovasc Genet. 5(5), 511–8 (2012).
Galiè, N. et al. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 46(4), 903–75 (2015).
Deng, Z. et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 67, 737–44 (2000).
Berg, J. N. et al. The Activin Receptor-Like Kinase 1 Gene: Genomic Structure and Mutations in Hereditary Hemorrhagic Telangiectasia Type 2. Am J Hum Genet. 61, 60–67 (2007).
Gallione, C. J. et al. Mutation and expression analysis of the Endoglin Gene in Hereditary Hemorrhagic Telangiectasia reveals null alleles. Hum Mutat. 11, 286–294 (1998).
Yang, T., Yang, P., Roden, D. M. & Darbar, D. A novel KCNA5 Mutation Implicates Tyrosine Kinase Signaling in Human Atrial Fibrillation. Heart Rhythm. 7(9), 1246–1252 (2010).
Adzhubei, I., Jordan, D. M. & Sunyaev, S. R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 7(7), 20 (2013).
Ferrer-Costa, C., Orozco, M. & de la Cruz, X. Sequence-based prediction of pathological mutations. Proteins. 57(4), 811–9 (2004).
Kumar, P., Henikoff, S. & Ng, P. C. Predicting the effects of coding nonsynonymous variants on protein function using the SIFT algorithm. Nat Protoc. 4(7), 1073–81 (2009).
Schwarz, J. M., Cooper, D. N., Schuelke, M. & Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 11(4), 361–362 (2014).
Desmet, F. O., Hamroun, D., Lalande, M., Collod-Béroud, G., Claustres, M. & Béroud, C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 37(9), e67 (2009).
Thanaraj, T. A. Positional characterisation of false positives from computational prediction of human splice sites. Nucleic Acids Res. 28(3), 744–54 (2000).
Acknowledgements
We are grateful to the patients who participated in our research, we thank the physicians who participated in the collection of patients and data (Carlos Vilariño Pombo, José Manuel Cifrián, Olalla Añón) and, finally, we acknowledge the collaboration of Asociación Española de Hipertensión Pulmonar. Programa de Apoyo a las Capacidades Biomédicas (BIOCAPS) FP7-REGPOT316265. This study was supported by grants IN-202-05 from SOGAPAR, CO-0085-10 from Actelion Pharmaceuticals and INBIOMED 2009-063 Xunta de Galicia.
Author information
Authors and Affiliations
Contributions
G.P. conceived of the study and its design, performed genetic research, data statistical analysis and interpretation, correlation genotype/phenotype and draft the manuscript. A.B. realized the collection of patients and data, statistical analysis and drafts the manuscript. D.V. conceived the study and participated in its design and coordination and draft the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pousada, G., Baloira, A. & Valverde, D. Complex inheritance in Pulmonary Arterial Hypertension patients with several mutations. Sci Rep 6, 33570 (2016). https://doi.org/10.1038/srep33570
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33570
This article is cited by
-
Computed tomographic findings in TBX4 mutation: a common cause of severe pulmonary artery hypertension in children
Pediatric Radiology (2024)
-
Molecular and functional characterization of the BMPR2 gene in Pulmonary Arterial Hypertension
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.