Abstract
While there has been progress in making use of breath tests to guide clinical decision making, the full potential of exhaled breath analysis still remains to be exploited. Here we summarize some of the reasons why this is the case, what we have done so far to overcome some of the existing obstacles, and our vision of how we think breath analysis will play a more prominent role in the coming years. In particular, we envision that real-time high-resolution mass spectrometry will provide valuable information in biomarker discovery studies. However, this can only be achieved by a coordinated effort, using standardized equipment and methods in multi-center studies to eventually deliver tangible advances in the field of breath analysis in a clinical setting. Concrete aspects such as sample integrity, compound identification, quantification and standardization are discussed. Novel secondary electrospray ionization developments with the aim of facilitating inter-groups comparisons and biomarker validation studies are also presented.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
No patient will be surprised when the physician, faced with some diagnostic hypothesis, requests a blood or urine analysis to confirm or reject the suspected diagnosis. However, analysis of breath specimens is far less common, and surely would raise the eyebrows of many patients who are confronted with such a request for the first time. In this perspective paper, we summarize why we think breath analysis may soon be routinely used for clinical decision making. Despite the clear advantages that breath poses as an alternative matrix, its full potential remains to be exploited. Nonetheless, a handful of breath tests approved by regulatory authorities already provides a flavor of what the future holds [1]. For example, the 13C-urea breath test for detection of Helicobacter pylori [2, 3], the nitric oxide (NO) breath test for monitoring asthma [4], and the alkanes breath test for heart transplant rejection [5].
2. Biomarker discovery: shortcomings and suggested strategies to overcome them
Clinicians generally base their diagnostic decisions on a 'biological marker' (biomarker), which can be broadly defined as any characteristic which can be measured repeatedly and accurately to indicate the medical state of a patient [6]. The goal of many clinical research projects is to identify biomarkers associated with an underlying pathophysiological mechanism, with the aim to help clinicians improve the diagnostic process and ultimately also the treatment and monitoring of diseases. Unfortunately, despite thousands of biomarker-discovery-related studies, less than a hundred biomarkers are used in the clinical environment [7]. Over time, various reasons have been given to explain this fact [7–10]. Here, we analyze some of them, with a focus on the particularities of metabolic biomarker discovery in breath.
2.1. Preserving the integrity of the sample: real-time analysis
Since the early 1970s, when gas chromatography was used for the separation and detection of ∼250 different components of human breath [11], several other techniques were developed to analyze exhaled breath. These techniques can be categorized into three major groups based on their mode of operations: (i) mass spectrometry (MS)- and ion mobility-based techniques, (ii) chemical sensors-based techniques, and (iii) spectroscopic techniques. Breath samples for these techniques can either be used directly for immediate analysis by a device (on-line sampling) or need to be collected in a bag or tube (off-line sampling) [12–14]. One major problem with sample collection, pre-concentration (a necessity for gas chromatography-MS), and other manipulation is that this may compromise the integrity of the sample. This is not only a problem afflicting gaseous samples, but also liquid samples such as blood. For example, McLerran et al [15, 16] reported on the bias introduced in serum proteomic profiles as a result of subjecting the samples to freeze-thaw cycles. In line with this, concerns about thermal degradation of metabolites during analysis have recently been raised [17]. One way around this problem of compromising the sample is to perform on-line sampling and real-time analysis, which overcomes the abovementioned problems and, for example, also render it possible to measure unstable compounds. Prominent examples of real-time breath analysis techniques are proton transfer reaction-MS (PTR-MS) [18] and selected ion flow tube-MS (SIFT-MS) [19]. Both of these techniques use chemical ionization (CI) of analyte molecules via collision with reactant ions in controlled and well-defined ion-molecule reactions at reduced pressure. After reaction, both reactant and product ions are quantified by downstream mass spectrometry [20].
During recent years, we have concentrated on a third alternative for real-time MS-based techniques called secondary electrospray ionization-MS (SESI-MS) [21]. In SESI-MS, trace analytes from gaseous samples are ionized by interacting with the charges produced by an electrospray plume at atmospheric pressure by a CI-like mechanism [22–25]. The major advantages of SESI are its high ionization efficiency and its flexibility, as it can be interfaced with high-end commercial atmospheric pressure ionization-mass spectrometers. As a result of its advantages and convenience, several groups have incorporated SESI-MS into their research activities [26–33].
2.2. Compound identification
Different combinations of endogenous volatile organic compounds (VOCs) are responsible for the 'smell' of breath, which has been used for centuries to diagnose diseases: the smell of acetone for diabetes, a fishy smell for liver disorders, a urine-like smell for kidney failure, or a sewer-like smell for lung abscesses. There are also many examples where animals with powerful olfactory system, such as dogs and rats, were trained and then used to diagnose various cancers, infectious and metabolic diseases as reviewed by Bijland et al [34]. However, for breath analysis to be useful in clinical practice, identification of various components responsible for a characteristic smell is absolutely necessary.
This is also reflected by the statement of Xia et al [35], arguing that 'Biomarkers must consist of positively identified compounds. Unknowns or tentatively identified features cannot (and never will) be approved for clinical laboratory testing'. We support this statement and think that high-resolution mass spectrometry (HRMS) with tandem MS (MS/MS) capabilities is among the most powerful methods for unambiguous compound identification. Complementary techniques to real-time analysis, like UPLC-MS/MS of exhaled breath condensate, are also very useful to characterize breath metabolites [36–39]. The latter is especially important to characterize isomeric structures that are indistinguishable by real-time mass spectrometric analysis.
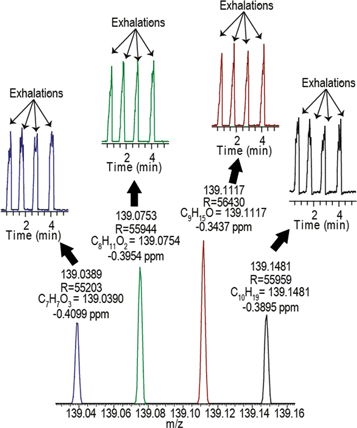
To address the requirements of sensitivity and separation power, we have opted to use commercially available atmospheric pressure ionization-mass spectrometers like the Q Exactive Plus (Thermo Fisher). Modern Orbitrap mass analyzers have limits of detection in the range of 500 fg (S/N 100:1), a mass accuracy of ≤1 ppm, and a mass resolution up to 140 000 (at m/z = 200 and a scan rate of 1.5 Hz). They also possess fragmentation (i.e. MS/MS) capabilities to assist compound identification. In addition, it offers the possibility of operating in negative ion mode, covering complementary molecules as compared to positive ion mode (i.e. metabolites prone to deprotonate such as fatty acids [40, 41]). We take advantage of this powerful technology to couple it with SESI to provide real-time breath analysis with outstanding mass spectrometric performance [42, 43]. To the best of our knowledge, this is the only available option to perform real-time breath analysis using commercial equipment with a mass resolution of >30 000 and on-line MS/MS for compound identification. Figure 1 illustrates the benefits of real-time breath analysis with high resolution (i.e. ∼55 000) and high mass accuracy (i.e. <1 ppm).
Figure 1. High-resolution and high-accuracy mass spectrometry assists for high metabolome coverage and for compound identification. Breath mass spectrum obtained by combining SESI and an Orbitrap mass analyzer. It illustrates how high resolution is crucial to obtain clearly defined features (in this case four clear peaks at nominal m/z 139), as well as how high mass accuracy provides nearly unambiguous molecular formulae. The corresponding time-traces for four exhalation cycles are also shown.
Download figure:
Standard image High-resolution image2.3. Quantification
In clinical practice, decision making using the positive or negative outcome of a test is often based on a cut-off value of a parameter, which maximizes both sensitivity and specificity of the test. Hence, it is imperative that absolute concentration data is reported for positively identified biomarkers [14, 35]. SIFT-MS delivers accurate real-time quantitation of selected volatiles with concentrations down to parts-per-billion. PTR-MS provides semi-quantitative results, but at lower detection limits approaching low parts-per-trillion. At the moment, determining absolute concentration using SESI-MS is challenging (for different reasons, such as dependence on the MS instrument used, complex ionization mechanism, etc). Nevertheless, quantification is feasible by constructing calibration curves for a given mass spectrometric set-up [44, 45].
2.4. Standardization
Standardization at all levels (e.g. instrumentation, sampling strategies, data analysis, etc) is a critical aspect that needs to be addressed collectively [46–49]. We echo one of the reviewers' comments on this issue: 'In order to achieve standardization we need to agree the key performance indicators that we will adopt collectively to apply to our instruments and methodologies, and it is the lack of objective criteria in this work that detracts from its impact'. For this reason, we put together an interdisciplinary team of engineers and scientists over the past few years in order to address this important aspect. In a first stage, we developed a universal SESI source, designed to maximize ionization efficiency and to be compatible with commercial mass spectrometers [50]. The main goal being to offer the possibility of upgrading high-resolution mass spectrometers into sensitive real-time sniffers. This development allowed us to showcase the power of SESI-HRMS [51] and, more importantly, we learned what improvements were required for this technique to be broadly used: namely, reducing the background levels and memory effects so as to properly identify low volatility species, and easing operation and maintenance procedures. For this reason, a fully redesigned ionizer has been developed by Fossil Ion Technology (FIT, Malaga, Spain). Two key requirements were considered for this engineering endeavor: it had to be easy to clean, and it would not use electrodes to control the flow of ions, thereby reducing geocmain as high as previous prototypes. This required a very careful fluid mechanics design. For this reason, we developed a numerical method to simulate the flows of gases and ions within the ionization region. Compared with previous numerical methods, the new method incorporates the exchange of kinetic momentum between the ions and the neutral gases. Figure 2 shows a numerical simulation of the ion source, whereby the geometry and operational conditions (i.e. electric and flow fields) have been fully optimized. An important element that the simulation unveiled was the existence of a toroidal vortex at the core of the ionization region produced by the friction between the ions and neutral gases. The numerical simulation allowed us to find the optimal geometry to stabilize the toroidal vortex, maximizing the ionization efficiency and the flow of ions transferred to the MS. In addition, a fraction of the gas is deflected backwards so that vapors released from the inner walls of the ionizer do not reach the ionization region. This helps to reduce background signals, which can otherwise be very high because the temperature in the ionization region is limited by the boiling point of the electrospray. This configuration, which does not incorporate any internal electrode, can be easily opened and reassembled for cleaning. Figure 3 shows the resulting source (Super SESI, FIT) as designed for Thermo instruments. Supplementary figure S1 is available online at stacks.iop.org/JBR/12/027113/mmedia and shows two Super SESI sources that work with the same principles, having very similar internal geometries. The housing and the interface with the MS differs to make it compatible with either Thermo or Sciex mass spectrometers.
Figure 2. Numerical modeling assists optimization of SESI. Detail of the numerical simulation of an ion source (FIT, Spain), as optimized for Thermo instruments. Left: Sectional view of the ionization core; Center: streamlines, and concentration profile of molecules and ions introduced through the sample inlet. Right: Concentration profile of molecules released from the inner walls of the ionizer, and the corresponding ions.
Download figure:
Standard image High-resolution imageFigure 3. Optimized SESI source compatible with commercial mass spectrometers. Left: Overall view of the source including the breath sampling interface. Right: Details of the nano-electrospray system, an open view of the ionization chamber ready for cleaning, and the interface to the MS.
Download figure:
Standard image High-resolution imageThe sampling interface and associated exhalation maneuvers are also critical aspects [52, 53]. For this reason, the ion source comes with a sampling interface in order to standardize all the technical and sampling procedures. The subject exhales through a disposable mouthpiece featuring a one-way valve that retains saliva. Both the flow and exhalation pressure are controlled. The subject keeps the pressure within a comfortable range by monitoring an analogic manometer. This helps to stabilize the pressure of operation, but humans are not particularly good at regulating the pressure of their breath. Especially, people with lung diseases, elderly people and infants can have serious problems to provide a steady pressure. For this reason, our ionization system incorporates a regulator that stabilizes the flow passed into the ionizer—matching that found in the numerical simulations to be optimal—and keeps it constant. When the person exhales, the pressure within the regulator increases, and an internal weight is lifted by this pressure. As it slides up, it opens an aperture for some exhaled breath to exit to the room, thus reducing the pressure in the regulator. When the exhaled flow changes, the aperture changes accordingly. As a result, the pressure in the regulator and the flow of breath passed to the ionizer are automatically stabilized. Figure 4 shows the complete on-line breath analysis set-up, including the sample inlet regulation system and the ion source interfaced to a high-resolution mass spectrometer (Thermo).
Figure 4. Complete system enabling real-time breath analysis by SESI-HRMS in a standardized fashion. The exhalation pressure is monitored with an analog manometer. In addition, the regulator features a variable opening that further stabilizes the pressure and the flow passed into the analyzer. This flow matches the optimal one found by the numerical simulations (figure 2).
Download figure:
Standard image High-resolution image2.5. Additional important factors in translation of any mass spectrometric system to a clinical environment
2.5.1. Human
Training: Our own experience is that, with highly standardized equipment as the one presented here (figure 4), there is no need for skilled mass spectrometrists to acquire routine breath analyses. This task is now being taken over by research nurses, leaving more time to mass spectrometrists to interpret data and perform more demanding tasks such as quantification and compound identification.
Maintenance: Our experience is that little maintenance is needed, being the cleaning of the core of the ion source the main task. We recommend doing the operation at least every week. The fact that the core is easily removable and can be sonicated, eases the operation and can be accomplished in around 30 min.
Patient acuity: Our own experience, with hundreds of subjects measured over the last years, is that patient compliance with the breath test is very high. This applies to children down to five years old, as well as patients with severe lung diseases. The procedure is certainly less invasive than routine spirometries, which are sometimes difficult to accomplish.
Time per sample: every patient coming to our SESI-HRMS premises is asked to provide around ten replicate exhalations, whereby positive and negative ion mode spectra are acquired. The total time spent is typically around 10 min.
2.5.2. Environmental
One of the limitations of HRMS is that the ultrahigh mechanical precision of the ion path, and the very high vacuum required, come at the expense of bulky structures and pumping systems anchored to specialized laboratories. HRMS has very restraining installation requirements that limit the possibilities of performing large real-time breath analysis campaigns, as the patients need to approach the SESI-HRMS instrument. For this reason, in order to transport the instrument to the patient, we are developing a rack system that houses all the elements needed to perform SESI-HRMS analysis on the road (supplementary figure S2).
2.5.3. Clinical
Infection control: Dealing with patients suspected of suffering from an infection poses additional challenges. In our case, the exhaled air goes directly into the exhaust of the building, rather than to the room. Importantly, the only direct contact with the SESI source is through a disposable mouthpiece. In addition, the fact that the ion source is easily cleanable allows for rapid disinfection of the sampling tube and ionization core.
Sample consumables: Consumables are restricted to disposable mouthpieces and electrospray emitters.
Background volatiles: The new generation of ion sources (figures 2–4) have been specifically designed to minimize this issue: (i) the system operates at high temperature, (ii) complex geometries vulnerable to contamination have been eliminated, (iii) the ionizer and sampling tube are coated with analytical grade silica, (iv) contaminants released from the inner walls of the system are carried away and not ionized, (v) the electrospray region is constantly bathed with high purity nitrogen, which in addition is passed through active charcoal filter before entering the ionizer, minimizing as a result the chemical noise. In addition, the ambient air of the room is constantly renewed with filtered air. In any case, we recommend excluding from the data analysis known contaminants ubiquitously present in mass spectrometry (e.g. polysiloxanes [54, 55]).
Matrix effects: Our experience is that ion suppression occurs when highly abundant exogenous species are present in the oral cavity, depleting as a result the available primary reactant ions. For this reason, our protocol requires no food, drinks (except for water), tooth brushing and chewing gum at least one hour before the breath test, as well as to refrain from using lipstick or other lip moisturizers.
2.5.4. Operational
Reproducibility and quality control: Background chemical noise can be used for twofold purposes. Firstly, it can be used as internal standard for locking the mass range to achieve accuracies within 2 ppm. In addition, because the electrospray is bathed constantly with ultrapure nitrogen, the intensity profile is rather constant, offering the opportunity to use it as an objective measure of inter-day reproducibility. Deviations of explained variance of the first score, after principal component analysis, beyond +/−2 standard deviations and coefficient of variations above 20% should rise a warning about the quality of the spectra. In classical liquid phase-based metabolomics, pooling samples and injecting them at regular intervals is common practice for quality control [56]. In the case of gaseous samples like breath, this is impractical. For this reason, we are currently developing a gas sample delivery system, covering typical chemical species found in breath. The idea is to introduce trace amounts of vapors at known concentrations intercalated in between breath analyses.
Mean-time between failure: Occasional clogging of the electrospray emitters can occur during operation, which can usually be remedied quickly by increasing the pressure of the ESI to 1 bar for 30 s. After around ten days of operation ultrasonic capillary cleaning might be required, however by using a set of multiple emitters, only minimal downtime due to capillary clogging can be expected.
2.5.5. Financial
The capital investment of a brand new mass spectrometer and a SESI source is in the order of 800 000 Eur. Taking a reasonable life span of 5 years, and considering two shifts of 8 h (i.e. 16 h of breath tests per day), we can estimate that the salaries of two full time employed research nurses over five is in the order of 1 million Eur. This gives an investment figure of 1 800 000 Eur, and adding a 25% overheads sums 2 250 000 Eur over five years. Now, in five years one could carry out 115 200 breath tests (i.e. 10 min per breath test), which gives an estimation of 20 Eur per breath test. In the case in which the annual salary of a research nurse is in the order of 50 000 Eur, the final figure would be 14 Eur per test.
2.6. Follow-up studies beyond the pilot phase
One of the main limitations in biomarker discovery efforts is that putative markers identified in pilot studies are rarely validated in follow-up studies, contributing to the notion that biomarker discovery should be addressed in a more comprehensive manner. Our strategy foresees to validate putative markers by independent groups. Thus, we have established a network of users of standardized SESI-HRMS in Switzerland (e.g. Zurich Exhalomics network; http://hochschulmedizin.uzh.ch/en/projekte/zurich-exhalomics/projekt-details.html). More recently, groups in Peru (Pontifical Catholic University of Peru) and Italy (National Cancer Institute) have joined this endeavor, and two centers in China will shortly be added, which opens exciting opportunities to conduct multi-center studies for breath biomarker validation. Obviously, our network is open for other groups willing to participate. During the last years we have concentrated on phenotyping respiratory diseases such as chronic obstructive pulmonary disease (COPD) [57] and obstructive sleep apnea (OSA) [58] by exhaled breath analysis. These pilot studies, using a limited number of patients, resulted in panels of exhaled metabolites that serve to predict the presence or absence of disease, but also allow to gain insight into pathophysiological aspects of the disease. We are currently validating the prediction power of these metabolites in completely independent larger cohorts of patients (typically including around 150 subjects). The results for COPD are very encouraging (i.e. prediction accuracy in the range of 75%), whereby complete series of dicarboxylic acids seem to play a major role [59, 60]. OSA validation studies are currently ongoing. Similarly, following preliminary animal studies suggesting that semi-volatile drugs and their metabolites can be tracked in breath, mirroring plasma levels [61, 62], we are currently validating these findings to determine the feasibility of using breath analysis for therapeutic drug monitoring in children. Moreover, the close collaboration between clinicians and the experts in analytical chemistry will allow to characterize children's respiratory diseases by measuring other lung function parameters (e.g. lung volume and ventilation homogeneity) [63] with exquisite precision, as we have also broad epidemiological and lung physiological experts on-site. This information, combined with information at the metabolic level obtained from SESI-HRMS, will enable a more comprehensive understanding on the pathophysiology of respiratory diseases.
3. Conclusion
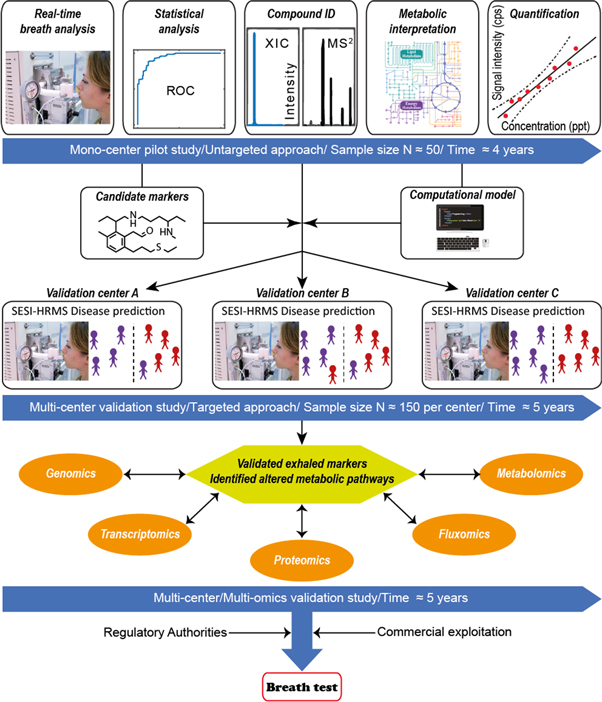
The comprehensive description of the human volatile phenome has been a landmark for the community [64]. However, there are still many challenges to tackle before the field of exhaled breath analysis will be fully developed and validated for clinical practice. For this reason, we feel it is absolutely necessary that clinicians, analytical scientists, bioinformaticians and engineers work closely together in order to successfully develop a truly translational research strategy. We have taken important steps in this direction over the last years, providing very promising results using the SESI-HRMS technique in a clinical context. We are currently bringing this program to the next level by establishing standardized instrumentation and procedures across research groups in the Americas, Europe and Asia. Figure 5 summarizes our comprehensive strategy, whereby untargeted pilot studies are subsequently validated. Unambiguous compound identification will enable to pinpoint altered metabolic pathways, that could explain the mechanistic link between clinical symptoms and the observation of derangement of exhaled metabolites. Furthermore, we are planning to incorporate a multi-omics approach to provide a broader mechanistic perspective. During the final phase of the workflow, approval of the regulatory authorities and commercialization will be sought, as these are essential steps to translate the biomarkers into clinical practice [65–67].
Figure 5. Workflow aiming to bring breath biomarkers into clinical practice. Untargeted approach (∼4 years): the process starts in a mono-center pilot study, whereby typically a case-control study with limited sample sizes (n ∼ 30–50 patients per arm) are conducted. In this early exploratory phase, our approach is untargeted, meaning that we screen for differences of all detectable metabolites, rather than concentrating in a subset of expected altered metabolites. Thus, the subjects exhale into a standardized SESI-HRMS system in full MS mode, both in positive and negative ion mode. In the same visit, the subjects provide a breath condensate (EBC) sample, which is immediately stored at −80 °C; Once the fingerprinting for all subjects has been concluded we conduct a rigorous statistical analysis to highlight the most promising mass spectral features, while controlling for false discovery rate. Typically, a subset of around 10 features or predictors are used to construct a classification model. The prediction accuracy of the model is assessed via cross-validations, estimating as a result of the classification performance parameters such as the area under the ROC curve. If the model suggests that at this point unknown metabolites hold promise for diagnosis then we pull out the EBC samples collected previously and run classical high performance liquid chromatography-HRMS to determine the structure of these unknown metabolites. Retention time and fragmentation spectra are matched against chemical standards to ensure high degree of confidence in compound identification. Additionally, we run real-time tandem MS/MS analysis to confirm that the on-line breath measurements also show the most diagnostic fragments; once the metabolites have been annotated, we interpret them in their biological context and follows a calibration procedure to estimate absolute quantification. Targeted approach (∼5 years): at this point we initiate a further multi-center validation study. This is a targeted approach whereby different centers across Switzerland and elsewhere using the same standardized experimental and data analysis procedures confirm whether or not the panel of ∼10 exhaled metabolites identified previously, truly have classification power in real populations respecting the disease prevalence. Multi-omics approach (∼5 years): if the results in the previous phase are positive, we would then move to a more comprehensive validation strategy whereby genomics, transcriptomics, proteomics and classical metabolomics approaches would be combined to provide a global understanding of why these exhaled metabolites are indeed altered under certain pathophysiological conditions. Finally, this would lead to a new phase where approval from regulatory authorities and commercialization would be sought.
Download figure:
Standard image High-resolution imageAcknowledgments
PMLS gratefully acknowledges the financial support of the Fondation Botnar (Switzerland). This work is part of the Zurich Exhalomics project under the umbrella of University Medicine Zurich/Hochschulmedizin Zürich. The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7-2013-IAPP) within the project Analytical Chemistry Instrumentation Development' (609691).