-
PDF
- Split View
-
Views
-
Cite
Cite
Álvaro Díaz, Immunology of cystic echinococcosis (hydatid disease), British Medical Bulletin, Volume 124, Issue 1, December 2017, Pages 121–133, https://doi.org/10.1093/bmb/ldx033
Close - Share Icon Share
Abstract
The neglected disease cystic echinococcosis is caused by larval Echinococcus granulosus flatworms, which form bladder-like hydatid cysts in liver, lungs, and other organs.
Published literature.
Establishing larvae are susceptible to antibody-dependent killing, as attested by successful animal vaccination, whereas once established they are partially protected by the so-called laminated layer. Host responses are Th2 dominated, with a Th1 component. Diagnostic antigens from cyst fluid are known, but responses appear absent in one-fifth of patients.
Is evasion mainly based on induction of Th2 or regulatory responses by the parasite?
The parasite induces regulatory responses. The laminated layer has immune-regulatory properties.
Develop tools for functional genomics; characterize immunologically interesting proteins suggested by genomic information; analyse infection in broader context of granulomatous responses; identify molecules secreted/excreted by intact larvae/cysts towards their outside, including diffusible immune-regulators.
Background
Cystic echinococcosis (CE, also called hydatid disease) is a neglected zoonotic tropical disease estimated to be responsible for the yearly loss of 1 million disability-adjusted life years.1 It is caused by infection with the larval stages of platyhelminths (flatworms) of the taenid family, specifically belonging to the genus Echinococcus. The disease is characterized by the growth within internal organs of fluid-filled, bladder-like larvae. These are called hydatid cysts, or more correctly hydatids, as the terms ‘cyst’ encompasses the parasite and the local host reaction.
Human CE is caused by at least three Echinococcus species that are distinguished only by molecular biological tools. Of these, E. granulosus (sensu stricto) and E. canadensis cause almost 90% and 10% of cases, respectively. The species cluster (which includes another two species) is referred to as E. granulosus sensu lato. The genus Echinococcus includes further species, of which the most important is E. multilocularis, causing agent of alveolar echinococcosis, in which the larva invades aggressively the liver parenchyma.2
Echinococcus species have two-host life-cycles.3 For E. granulosus sensu lato, the adult stages (worms measuring only mm in length) develop in the small intestine of carnivore hosts, which are in most cases dogs. Eggs passed out with dog’s faeces infect the so-called intermediate hosts. The intermediate host range is broad, and includes sheeps, cows, pigs, camels, goats, buffaloes and cervids, as well as humans. Eggs hatch in the intestine releasing oncospheres, which penetrate the gut wall, are carried by blood or lymph, and establish in internal organs, developing into hydatids. Within months or years, hydatids may develop within them parasite forms called protoscoleces. The parasites’ life-cycles are completed when dogs feed on infected tissues from intermediate hosts: ingested protoscoleces develop into adult worms in the gut. Each species (and subspecies) within E. granulosus sensu lato has a preferred intermediate host species range.3 The preferred intermediate hosts for E. granulosus sensu stricto are sheep. Humans are considered accidental or aberrant intermediate hosts, since human infection plays no part in maintaining the life-cycles.
CE is present in all continents except from Antarctica. As expected from the life-cycle pattern described, the infection is heavily associated with livestock rearing in underdeveloped contexts. The regions in which the disease burden is heaviest are located in North Africa, the Near and Middle East, Central Asia, eastern Russia and western China; South America, although still significantly affected, has seen the prevalence of CE fall over the last decades.4,5
The cornerstone of CE control is the regular and supervised dosing of dogs with the anthelmintic drug praziquantel. Additional measures are the state control of livestock slaughter practices, educational measures and reduction of stray dog populations.4 CE control is achievable, as attested by the experience of countries with agricultural economic bases and high development levels (e.g. New Zealand). The control critically requires well-functioning governmental institutions, and in particular, the collaboration of the offices in charge of veterinary health towards alleviating what is primarily a human health problem.4 Indeed, although substantial economic losses due to livestock infection are estimated,2 CE is not an obvious problem for farmers/herders, due to the mostly asymptomatic nature of the infection. Neither is the parasite noticeable in terms of pet well-being, as dog infection causes no symptoms.4
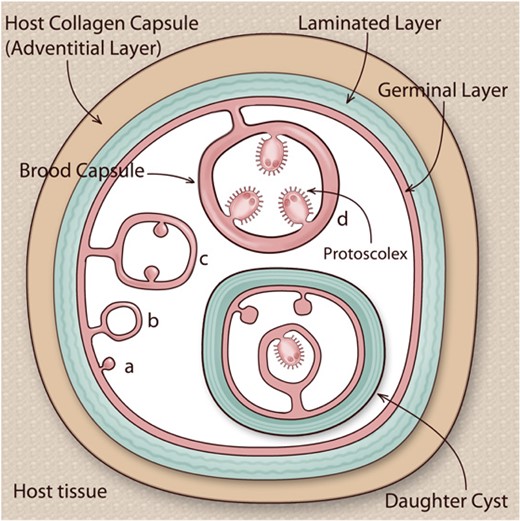
Hydatids are unilocular, subspherical fluid-filled structures (Fig. 1). Each hydatid is bounded by a thin (tens of micrometres) continuous layer of parasite cells (germinal layer). Budding of the germinal layer towards the cyst cavity gives rise to so-called brood capsules, which in turn generate within them protoscoleces. The germinal layer is outwardly protected by a thick (up to 3 mm) acellular coat called the laminated layer (LL), sometimes also called cuticular layer;6,7 germinal layer plus LL form the hydatid wall. In some cases, hydatids develop daughter hydatids within them, which have the same structure. Healthy hydatids are at higher pressure than surrounding tissues. Between the hydatid wall and the organ parenchyma there is the so-called adventitious layer or adventitia, resulting from the local host reaction to the parasite and usually fibrous in nature, further discussed in the immunological section of the article.
Structure of the hydatid cyst. E. granulosus sensu lato larvae (hydatids) are a bladder-like structures lodged in intermediate hosts’ organ parenchymas. Each hydatid is bounded by a hydatid wall, comprising an inner cellular germinal layer and an outer acellular laminated layer. The hydatid is usually surrounded by a host-derived collagen capsule, but can also be encircled by host inflammatory cells, plus varying amounts of fibrous tissue; the area of host reaction, especially when it is fibrous, is called adventitial layer. The hydatid and the local host reaction together form the hydatid cyst. The hydatid is filled with hydatid fluid (hydatid cyst fluid; depicted in white in the figure). After a lapse of months or years, the germinal layer may bud towards the inside, giving rise to brood capsules, which in turn bud towards their inside to generate protoscoleces (phases a–d in the figure). Upon ingestion by a dog or another canid, protoscoleces establish in the intestine, growing into adults that release eggs, in turn capable of giving rise to hydatids if ingested by appropriate hosts. However, protoscoleces are also capable of reserve development into hydatids if released into tissue by cyst rupture. Daughter hydatids (‘daughter cysts’), with their own complete hydatid wall, occasionally form within larger hydatids. Adapted from references7 and 45, with permission.
When discussing the pathology of CE in humans, three major facts have to be taken into account. The first of these is that the larvae can develop in almost any tissue compartment of the human body (this of course meaning to exclude the lumen of gastrointestinal, respiratory and genitourinary tracts). The second fact is that larvae grow slowly (mm to tens of mm per year2,3) but, unless intervened upon, may reach tens of centimetres in diameter. The third fact is that the parasite is superbly adapted to minimizing the inflammation it elicits, although this adaptation does not cover the possibility of cyst rupture. These three facts determine that: (i) infections are usually asymptomatic for years; (ii) symptoms, when arising, are largely caused by organ compression by the viable larva, or complications resulting from spillage of cyst contents; (iii) symptoms are highly variable depending on infection site and particular structures being compressed, or location of cyst content spillage if fissure/rupture takes place. The liver is the most common infection site (2/3–4/5 of cases), followed by lungs (around one-fifth of cases).1,3,5 Patients harbouring uncomplicated liver or lungs cysts may experience abdominal or chest pain respectively and/or other non-specific symptoms, or no symptoms at all. Spillage of cyst contents generally causes allergic symptoms, including anaphylactic shock.2,8 For all cyst locations, a serious potential complication of cyst content spillage is secondary infection. This is the development of hydatids from protoscoleces, which are capable of reverse development when freed into a tissue site. Secondary CE is often serious because of the presence of multiple cysts, the result of the successful development of a small fraction of the thousands of protoscoleces usually contained in a single hydatid.3
Diagnosis of CE is mainly based on imaging techniques, with ultrasound as the main tool. When cyst location precludes ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and conventional chest radiography are used. Ultrasound images (and by extension other types of images, especially from MRI) are classified according to guidelines drawn by a World Health Organization (WHO) workgroup, to help guide patient management.1,2,9 This classification distinguishes two sub-stages of ‘active’ (CE1 and CE2), two sub-stages of ‘transitional’ (CE3a and CE3b) and two sub-stages of ‘inactive’ or dead (CE4 and CE5) hydatids. In addition it incorporates the category ‘cystic lesion’ (CL), for those images that may or may not correspond to CE. Serology, which plays an ancillary but nonetheless important role in diagnosis,10 will be discussed in the immunological section of this Review.
CE is managed through: (i) chemotherapy, (ii) percutaneous therapy, (iii) surgery and/or (iv) observation without intervention.2,9 Chemotherapy employs benzimidazole anthelminthics, most usually albendazole or mebendazole. As sole measure, chemotherapy is recommended in cases of inoperable and multiple cysts, and can be used on certain sub-stages of active and transitional hydatids. Chemotherapy is additionally used as an adjunctive treatment to surgery or percutaneous therapy, helping to reduce the risk of secondary CE. Percutaneous therapy techniques are based on puncture of the cyst and killing of the parasite, by aspiration of cyst fluid, injection of parasiticidal solutions (hypertonic saline or ethanol) and/or mechanical disruption of the cyst wall. Technical improvements in these techniques have allowed them to grain ground with respect to surgery, which was formerly the gold standard. Surgical techniques aim to completely remove the parasite proper, and they differ in whether the cyst is opened or not, and in whether the adventitia is partially or totally removed; radical surgery is used in some cases. Observation without intervention is recommended for inactive cysts. Independently of the management option(s) taken, patients require long-term follow-up, which is essentially based on imaging.
Sources of data
This review is essentially based on published primary and secondary literature. As a consequence of journal limitation to the number of references, primary papers are cited only when they have not been covered by the reviews being cited or when they are especially important to clarify a particular point. In particular for the background section above, the author found much support in the reviews published in two recent issues of Advances in Parasitology2–4,8,10 and in the excellent mini-review by Agudelo-Higuita and colleagues.1
Areas of agreement
The establishing larval parasite is susceptible to immune mechanisms in primed hosts
As mentioned, the parasite stage that establishes in human (or livestock animal) organs is the oncosphere. The oncosphere, measuring 28μm in diameter, is motile and bears hooks, features that allow it to actively penetrate the gut wall. In ~1 week after reaching the target organ, the oncosphere loses its hooks and muscles, generates a central cavity, and starts deploying a LL, thus becoming a very small hydatid. Animals vaccinated with oncosphere antigens are protected against infection, and this protection is thought to be mostly due to destruction of the establishing oncospheres by antibodies and complement.11 Antigenic preparations from other parasite stages do not confer similar protection.11 It is thought that in non-primed hosts, by the time an adaptive immune response is mounted, the parasite no longer expresses oncospheral antigens and it is protected by the LL, as expanded below. Nothing is known of the mechanisms that allow the establishing oncosphere to evade innate immunity in non-primed hosts during the time window that precedes LL deployment.
Immune protection by crude oncosphere preparations is imitated by vaccination with a single protein antigen, called Eg95, in native or recombinant form.11 The biological function of Eg95, expressed only by the oncosphere stage, is unknown.12 In spite of its remarkable efficiency in experiments in sheep and cattle, development of this vaccine (for veterinary use) has been hampered by economical hurdles. The vaccine is presently undergoing a field trial in sheep in Argentina.4 The possibility of a human vaccine is remote, also because of economic reasons, since the burden of human CE in the developed world is small.4
The established parasite is shielded against immune attack by the laminated layer, but this shielding is not perfect
Once established in an organ, the larval parasite does not move or reproduce (except for the generation of protoscoleces within it, and the possibility that these give rise to secondary infections). In contrast to the E. multilocularis larval mass, which usually includes necrotic portions coexisting with thriving ones that continue colonizing the host organ, the E. granulosus (sensu lato) hydatid is a sitting target for the immune system. Also, there is no evidence whatsoever in Echinococcus of antigenic variation akin to that employed by some protozoan parasites. Therefore the major survival mechanism employed by E. granulosus is to shield itself against immune effector mechanisms, which it does by means of the LL.
The LL is a meshwork of highly glycosylated proteins of the mucin type, with interspersed deposits of a calcium salt of a highly phosphorylated compound, inositol hexakisphosphate.7,13 It is elastic and mechanically resistant, allowing healthy hydatids to be under hydrostatic pressure as mentioned. It acts as a filter that protects the germinal layer from contact by host cells, yet allows the passage of molecules. The LL, and at least in some situations, the underlying germinal layer, become opsonised with host antibodies.6,14 These antibodies could activate complement, which could directly damage the germinal layer and/or promote inflammation in the parasite’s vicinity. The LL has complement avoidance mechanisms, although these are not fully understood.6
Established hydatids are not fully insensitive to immune effector mechanisms. The immune system could well be involved in pushing the infection to the transitional and inactive stages (CE3–CE5) mentioned above. Even stage CE2 hydatids, considered as ‘active’ in the WHO classification, are proposed to derive from stage CE1 parasites as a consequence of these sustaining damage.15,16 Whether immune mechanisms participate in such damage is not known, yet is likely. Observations on infections across the spectrum of animal intermediate hosts point in the same direction: the maintenance of local inflammation is associated with low parasite vitality, and inflammatory resolution is associated with thriving larvae.6 The effectors responsible for such a proposed immune control of parasite vitality are not known. Nitric oxide, a toxic mediator capable of considerable diffusion,17 is a candidate, and eosinophil-derived effectors should also be considered.6,18
The local response against the established parasite is subdued
As mentioned above, the LL does not afford the larva full protection against host-derived effectors. Thus the parasite’s survival strategy does not rely solely on deploying a physical shield: it additionally relies on down-regulating host responses. This is most obvious from the histopathology of CE. In humans in particular, inflammation is mild in comparison to what may be expected of the reaction against such a large pathogen lodged in tissues. Further, the most common situation is that the hydatid is immediately surrounded by a non-infiltrated layer of collagen, with some inflammatory foci found more distally to the parasite.6,19,20
The systemic adaptive immune response is dominantly Th2 but has a Th1 component
The T-cell immunological profile of CE patients has been analysed in terms of serum cytokines, cell-surface and intracellular markers detected in peripheral blood mononuclear cells (PBMCs) by flow cytometry and cytokines elicited from PBMCs by hydatid fluid antigens. The most general findings are elevations in parameters indicative of a Th2 response. However, in many cases, Th1-associated parameters are also found elevated in relation to healthy controls.8,21,22
Diagnostic antigens are well described but not all patients have responses against them
Detection of specific antibodies has an important role in the diagnosis of CE.2 Current guidelines require a positive serological results in two tests for CE patients to be defined as ‘probable’ or ‘confirmed’, together with different clinical, epidemiological, imaging and/or parasitological evidence.2,9 Serology may also be used to monitor for CE recurrence after treatment.2,10 Specific IgG is the parameter most usually measured; commonly the more sensitive ELISA tests are used first, followed by less sensitive but more specific immunoblotting techniques.10
Serological diagnosis of CE is problematic. Sensitivity is acceptable for liver infections but poor for lung ones, and very low for infections in immune-privileged sites such as brain or eye.1,9,10 Sensitivity also tends to be lower for early (CE1) and inactive hydatids (CE4 and CE5) than for the intermediate stages. Therefore, although contradictory with the consensus guidelines, negative serology does not in practice preclude the presence of CE.1,9 Cross-reactivity with other flatworm infections, i.e. sub-optimal specificity, is an additional problem.1,10 Serology for patient follow-up is also problematic, as antibody titres can persist for a long time, independently of treatment outcome.10
The antigens used for CE serodiagnosis are derived from hydatid fluid.10 The cyst wall has generally not been taken into account as a source of diagnostic antigens; synthetic carbohydrates based on the described structures of LL mucin carbohydrates remain a possibility, which has only been explored for alveolar echinococcosis.13 Decades of study have shown that the main antigenic proteins in hydatid fluid are the so-called antigen 5 and antigen B, both of which are well characterized in molecular terms.10,12 Antigen 5, the function of which is unknown, is antigenic mainly because of a particular carbohydrate chain it carries.12 Antigen B comprises several related proteins encoded by a gene family that additionally shows considerable polymorphism among parasite isolates.12,23 Evolutionary studies on the gene family suggest that its variability was shaped by pressure by the host immune system.24 Antigen B proteins occur within lipoprotein particles possibly akin to human plasma lipoproteins. It is proposed to participate in lipid acquisition by the parasite, with support from evidence on a homologous protein of larval Taenia, and in immune modulation.8,23,25
It was hoped that the use of defined antigens would improve CE serology. Recombinant antigen B proteins perform similarly to crude hydatid fluid in patient diagnosis, which is good news, given the standardization problems posed by the former.10 However, neither the use of single recombinant antigens nor the combination of testing against two or more antigens surmount the problem that approximately one-fifth of total patients test negative.10
The improvement of CE serology will require large inter-centre studies, of which few have been conducted.10 The recently started HERACLES project (http://www.heracles-fp7.eu/) has such studies within its aims.10
Areas of controversy
Is the Th2 response responsible for immune evasion in CE?
CE patients that do not respond well to chemotherapy have responses against CE antigens that are Th2-skewed in comparison to those of patients cured by chemotherapy, whose responses are more Th1-biased.8 A parasite-damaging role for Th1 responses is also suggested by the hints that nitric oxide, a mediator normally associated with the Th1 profile, participates in decreasing parasite vitality.6,18 Also, in mouse models, interventions that boost Th1 responses decrease parasite burden, whereas those that enhance Th2 responses have the opposite effect.26 These observations, placed in a conceptual framework centred in the Th1–Th2 dichotomy as was dominant in the 1990s, lead to the notion that Th2 responses allow larval E. granulosus to escape from host-protective Th1 responses.8,27
On the other hand, helminth immunologists (most of which work on roundworms and Schistosoma) have largely reached a consensus view in which worms evade the immune system by exacerbating their hosts’ regulatory responses.28–30 Regulatory responses employ regulatory T cells and suppressive cytokines such as IL-10 (previously considered specifically a Th2 cytokine) and TGF-β to restrain all effector responses. In the view being exposed, Th2 responses did evolve to deal with helminths, and thus include helminth-damaging mechanisms together with mechanisms for repairing the tissue damage caused by helminths.28 Also in this view, Th2 responses can damage helminths if released from the restrain imposed by regulatory responses. As discussed below, it is increasingly clear that regulatory responses are strong in CE. Also, the histopathology of CE lesions, previously discussed, is more compatible with a strong regulatory response than with a full-fledged Th2 response, which may be expected for example to result in strong eosinophil infiltration.
Growing points
The LL contributes to immune-regulation
It has been suspected for some time that in addition to providing physical protection the LL must convey anti-inflammatory signals to the host immune system.6 Immune cells can come into contact with the LL itself and also in all likelihood with particles shed from it as a necessity for larval growth.12 Our group has recently shown that LL particles inhibit, in a mouse model, the proliferation of macrophages,31 which is a newly recognized, important component of inflammation in Th2 settings.32 Also, an LL extract can ameliorate inflammation in a mouse model of colitis.33
Regulatory T cell responses are strong in CE
In spite of the disagreement on the role of Th2 responses, it is widely accepted that CE spurs strong regulatory responses.8,12 CE patients significantly elevated percentages of regulatory T cells in blood and significantly elevated serum levels of TGF-β1 and IL-10 8,22. Also, the IgG responses of patients with CE1-3 hydatids (but not those of patients with ‘inactive’ cysts) are mainly of the IgG4 subisotype, normally associated with regulatory responses.10 Results from experimental mouse infections also support strong regulatory responses.12,34,35
Areas timely for developing research
Development of much needed tools for basic research
The genome of E. granulosus senso stricto was sequenced by two groups, one of which also obtained a higher-quality genome for E. multilocularis.36 The use of genomic information to understand immune evasion by these parasites is severely hampered by the lack of so-called functional genomic tools, such as the possibility to delete specific genes to study the function of the proteins they encode.37 Certain advances in this direction have been obtained with E. multilocularis.37 The description of an E. granulosus sensu stricto cell line derived from the germinal layer37 is a step towards functional genomics in this species. A crucial needed development is the description of culture conditions in which cells would develop into vesicles similar to hydatids.
Unless a method is devised to grow micro-hydatids from cell lines, a very important development would be a method to grow micro-hydatids in vitro from protoscoleces with higher efficiency than currently achieved.38 Either development would pave the way to the wider use of an infection model based on injection in mice of small hydatids.38 This model has important advantages over the model based on injection of protoscoleces, used for the overwhelming majority of experimental in vivo E. granulosus research. Valuable advances have been made with protoscolex injection.27 However, a very large (and variable) proportion of protoscoleces are killed before they develop into hydatids, which then only occurs after 5–7 months. In addition, the early host response is probably much shaped by stimulation by the protoscolex glycocalyx, evolutionarily perfected for protecting the parasite in the dog’s gut, and by dead protoscolex debris. Neither of the models mentioned allows analysis of oncosphere establishment, but efficient models based on injection of micro-hydatids would allow analysis of the chronic phase of infection with much more ease and certainty than currently possible.
Immune-regulating activities of interesting proteins suggested by genomic information
Even in the absence of functional genomics tools, a wealth of information is already available from genomic and transcriptomic data. Transcriptomic data give reasonable clues on which proteins are preferentially expressed by the parasite stages/tissues most important for CE (oncosphere and germinal layer). Protein-coding sequences provide clues on proteins likely to be secreted. Proteins that exist as families the size of which is larger in a parasite than in its free-living relatives may be particularly important for the parasitic lifestyle. Some Echinococcus protein families of potential interest based on these criteria have been pointed at,12,36,37 these probably representing only the tip of the iceberg. Part of the immune-evasion proteins are probably flatworm-specific (or even Echinococcus-specific) and thus their biochemical function cannot be guessed from knowledge on other organisms. Even when no biochemical information is available, novel proteins can be recombinantly expressed and tested for activity in relevant immunological assays, in vitro (with molecules or cells) and in vivo (typically in models of inflammatory diseases in mice). This kind of strategy has the potential of providing clues on immune-evasion by the crucial oncosphere and early post-oncosphere stages, much understudied because of the difficulty to obtain and work with oncospheres. This kind of research is very much in line with the proposal that helminths can be mined for molecules with therapeutic potential for conditions in which the immune system over-reacts, such as allergies, autoimmune diseases and transplantation.29
Investigations on granulomatous inflammation
Persistent biological or non-biological foreign entities lodged in tissues normally elicit granulomas.39 These incompletely understood immune reactions appear to develop under the influence of opposing signals favouring on the one side persistence of inflammation and on the other side inflammatory resolution accompanied by fibrosis (from the deposition of collagen). Granulomas usually feature a central area (neighbouring the foreign body) where macrophages are dominant, surrounded by a cellular cuff rich in lymphocytes. Variable amounts of collagen appear in the periphery of the lesions, reflecting the trend towards resolution/fibrosis. The local response in CE is normally tilted towards fibrosis but it clearly has the potential for granulomatous inflammation, which in fact is the norm for some E. granulosus sensu lato species–host species combinations.6 Macrophages in granulomas adopt some stereotyped, poorly understood phenotypes, called epithelioid cells and giant multinucleated cells, both of which can be seen in responses to larval Echinococcus.6,8E. granulosus proteins, chosen as discussed in the previous section, could be tested in in vivo models of (non-infectious) granuloma.
A better understanding of the function of granuloma macrophages may contribute to resolve the apparent contradiction between Th1- and Th2-mediated mechanisms against larval Echinococcus.8 For example, epithelioid cells in tuberculosis granulomas can express nitric oxide synthases (the enzymes producing nitric oxide, associated with Th1), even if they concomitantly express arginase (an antagonistic enzyme, associated with Th2).40 The careful description in cellular and molecular terms of the local host reaction in CE is one of the areas in which close collaboration between clinicians and basic researchers is necessary. Tools such as immunohistochemistry, in-situ hybridization, and qPCR and proteomics analysis of tissue samples can be powerful.41 The most meaningful results require that basic scientists have access to human samples on which there is complete information about clinical history, lesion stage, intervention procedure used, and form of sample preservation.15 The HERACLES project mentioned is already filling this gap in Europe.
Molecules released by intact live hydatids
We are still lacking a characterization by proteomics methods of the proteins released by intact hydatids towards their outside. Firstly, this may indicate which parasite proteins are constitutively exported from the hydatid, which is an important consideration for diagnostic antigens, classical or new. With respect to the classical diagnostic antigens, as already mentioned a sizable proportion of CE patients appear not to have antibodies against them.10 It is still a possibility that antigen 5 and antigen B are secreted only towards the hydatid fluid, and we do not understand the (bi-directional) movement of molecules between this compartment and host extracellular fluids.12 Thus some authors discuss the possibility that these antigens make contact with the immune system only as a result of (presumably mostly minor) events of hydatid fluid leakage.37 For antigen B in particular, this seems at odds with considerations mentioned above.23,24
Crucially, analysing the culture supernatants of live hydatids may provide clues on the immune-modulators secreted by this parasite stage. These must exist, as the properties of the LL alone are highly unlikely to maintain inflammatory control, as hinted by the observation that dead hydatids (in which the LL persists) are associated with local inflammation. Even in the absence of proteomics analysis, the immune-regulating activities present in crude larval secretions should be analysed, as it has started to be done for E. multilocularis.37
Interestingly in this context, in two non-Echinococcus flatworm species, extracellular vesicles (containing known diagnostic antigens among other proteins) are released from live larvae, whereas in E. multilocularis the analogous vesicles do not reach culture supernatants because they cannot cross the LL in the outward direction.42 Of course this does not preclude the likely possibility of proteins being conventionally secreted (by exocytosis) by the germinal layer towards the host side.12
Importantly, it should be borne in mind that molecules released by the larva into surrounding host tissues may well include low molecular mass metabolites, and these can be immune-regulatory. For example, the cyst fluid, by virtue of its volume, is a massive reservoir of lactate and succinate,43 recently discovered to have immune-regulatory effects.44 This is an area that is awaiting analysis.
Discussion
CE is an important neglected disease. It can be controlled through well-known measures that do not require immunological research and that depend on overall improvements in human development. Immunological research could on the other hand bring needed improvements into CE (sero)diagnosis. Beyond CE control and diagnosis, the astonishing control of host responses in tissue sites achieved by larval E. granulosus represents a worthwhile subject for immunological research. Independently of the whether Th2 responses help or harm the parasite, it is almost certain that the evasion mechanisms will be found to involve regulatory responses. Identification of the molecules released by the parasite ultimately responsible for eliciting or amplifying these regulatory responses holds promise for the therapy of autoimmune and allergic disorders, the prevalence of which of course increases with Western-type human development.
Funding
This work was funded by CSIC Grupos project No. 977 (to AD together with Ana M. Ferreira).
Conflict of interest statement
The authors have no potential conflicts of interest.
Glossary
- Alveolar echinococcosis
Infection with the larval stage of Echinococcus multilocularis. It is characterized by massive parasite infiltration of the liver parenchyma accompanied by florid granulomatous inflammation and fibrosis. This disease is only found in areas of Central Europe, North America and Northern and Central Asia.
- Cystic echinococcosis
Infection with the larval stage of parasites belonging to the Echinoccocus sensu lato species group. Also called hydatid disease.
- Definitive host
The host that harbours the adult form of a parasite. For E. granulosus sensu lato, definitive hosts are essentially canids.
- Echinococcus granulosus sensu lato
The group of species that cause cystic echinococcosis, and was formerly considered a single species. It includes E. granulosus sensu stricto, E. ortleppi, E. canadensis, E. equinus and E. felidis.
- Echinococcus granulosus sensu scricto
The Echinococcus species that used known as the ‘sheep strain’, before E. granulosus was recognized to comprise several species.
- Echinococcus multilocularis
Parasite the larval stage of which causes alveolar echinococcosis. Its life-cycle involves foxes and other canids as definitive hosts and rodents as intermediate hosts. Humans are accidental intermediate hosts.
- Germinal layer
Thin (tens of micrometres) layer of parasite cells that surrounds the central cavity of the hydatid.
- Hydatid (cyst) fluid
Liquid contained within the hydatid.
- Hydatid cyst
Fluid-filled structure, lodged in tissues, comprising the Echinoccocus sensu lato larva and the local host reaction that it elicits.
- Hydatid
Bladder-like larva of parasites belonging to the Echinoccocus sensu lato species group. It is also often referred to as metacestode; this is a more general term that describes the larval stages of cestodes (tapeworms), the platyhelminth class that encompasses the genera Echinococcus and Taenia among others.
- Intermediate host
The host that harbours the larval form of a parasite. E. granulosus sensu lato intermediate hosts include a range of ungulates, and accidentally, humans.
- Laminated layer
Thick (hundreds of micrometres to millimetres) acellular layer that outwardly protects the germinal layer.
- Oncosphere
Life-cycle stage of Echinococcus or other cestodes released upon egg hatching and capable of penetrating the gut wall and developing in internal organs into a metacestode (hydatid in the case of Echinococcus).
- Primary cystic echinococcosis
The biologically and medically most important form of cystic echinococcosis, resulting from the ingestion of eggs, which release oncospheres. Each oncosphere generates a single hydatid.
- Protoscolex (protoscoleces)
Life-cycle stage of Echinococcus or other cestodes similar to adult worm heads, bearing suckers and hooks, capable of establishment in the small intestine of dogs (and other caniid) definitive hosts. Echinococcus protoscoleces are also capable of reverse development to hydatids when released in a tissue site. A single hydatid can contain thousands of protoscoleces.
- Regulatory responses
Name given to the arm of the immune system that stops effector responses (Th1, Th2 and other) from damaging the host. Its central cells are regulatory T cells, and its central cytokine is TGF-β, which is immune-suppressive, and promotes inflammatory resolution and fibrosis.
- Secondary cystic echinococcosis
Medically significant form of cystic echinococcosis that results from the release of protoscoleces in tissue sites, as the result of trauma or medical intervention. It is also often used to generate experimental infections, usually by intraperitoneal inoculation of protoscoleces in mice, since protoscoleces are much easier to obtain and safer to work with than oncospheres.
- Th1 responses
Adaptive immune responses centrally characterized by the production of the cytokine interferon-γ, which classically activates macrophages, maximizing their output of free radical-based and other microbicides.
- Th2 responses
Adaptive immune responses usually observed in helminth infections and allergic reactions, of which the central cytokine is IL-4, which causes alternative activation and local proliferation of macrophages. Th2 responses also cause eosinophil recruitment.
- TLR
Toll-like receptors. The most well understood receptors of innate immunity, the activation of which usually starts Th1 and other strongly inflammatory response types.
- Zoonosis
An infectious disease of animals that can be transmitted to humans.
Acknowledgements
The author is grateful to Gustavo Salinas, Ana M. Ferreira, Cecilia Fernández and Cecilia Casaravilla (from the same Department) for useful discussions.
References
- lung
- biological response modifiers
- immune response
- functional genomics
- antigens
- cestoda
- cyst fluid
- cysts
- genome
- larva
- parasites
- platyhelminthes
- vaccination
- infections
- antibodies
- urinary bladder
- diagnosis
- echinococcosis
- liver
- echinococcus granulosus
- echinococcus granulosus infections
- immunology
- molecule
- killing
- host (organism)