-
PDF
- Split View
-
Views
-
Cite
Cite
Marine Lunven, Michel Thiebaut De Schotten, Clémence Bourlon, Christophe Duret, Raffaella Migliaccio, Gilles Rode, Paolo Bartolomeo, White matter lesional predictors of chronic visual neglect: a longitudinal study, Brain, Volume 138, Issue 3, March 2015, Pages 746–760, https://doi.org/10.1093/brain/awu389
Close - Share Icon Share
Abstract
Chronic visual neglect prevents brain-damaged patients from returning to an independent and active life. Detecting predictors of persistent neglect as early as possible after the stroke is therefore crucial to plan the relevant interventions. Neglect signs do not only depend on focal brain lesions, but also on dysfunction of large-scale brain networks connected by white matter bundles. We explored the relationship between markers of axonal degeneration occurring after the stroke and visual neglect chronicity. A group of 45 patients with unilateral strokes in the right hemisphere underwent cognitive testing for neglect twice, first at the subacute phase (<3 months after onset) and then at the chronic phase (>1 year). For each patient, magnetic resonance imaging including diffusion sequences was performed at least 4 months after the stroke. After masking each patient’s lesion, we used tract-based spatial statistics to obtain a voxel-wise statistical analysis of the fractional anisotropy data. Twenty-seven patients had signs of visual neglect at initial testing. Only 10 of these patients had recovered from neglect at follow-up. When compared with patients without neglect, the group including all subacute neglect patients had decreased fractional anisotropy in the second (II) and third (III) branches of the right superior longitudinal fasciculus, as well as in the splenium of the corpus callosum. The subgroup of chronic patients showed reduced fractional anisotropy in a portion the splenium, the forceps major, which provides interhemispheric communication between regions of the occipital lobe and of the superior parietal lobules. The severity of neglect correlated with fractional anisotropy values in superior longitudinal fasciculus II/III for subacute patients and in its caudal portion for chronic patients. Our results confirm a key role of fronto-parietal disconnection in the emergence and chronic persistence of neglect, and demonstrate an implication of caudal interhemispheric disconnection in chronic neglect. Splenial disconnection may prevent fronto-parietal networks in the left hemisphere from resolving the activity imbalance with their right hemisphere counterparts, thus leading to persistent neglect.
Introduction
Up to 85% of patients in the acute phase of a right hemisphere stroke display signs of left visual neglect (Azouvi et al., 2002). Neglect patients do not orient or respond to stimuli on the left side of space (Heilman and Valenstein, 1979; Parton et al., 2004; Chica et al., 2012; Bartolomeo, 2014a). Neglect represents a major public health problem because of its high frequency and its negative impact on functional recovery (Denes et al., 1982). Despite results indicating a frequent spontaneous recovery in the first weeks after the stroke (Stone et al., 1992; Cassidy et al., 1998; Ringman et al., 2004), at least one-third of neglect patients continue to show important difficulties several months after the lesion (Campbell and Oxbury, 1976; Levine et al., 1986; Cherney and Halper, 2001; Karnath et al., 2011), in spite of intensive rehabilitation programmes (Luauté et al., 2006). Signs of neglect are more likely to persist in elderly, demented or atrophied brains (Levine et al., 1986; Linden et al., 2005), or in patients with anosognosia or hemianopia (Gialanella et al., 2005; Tomaiuolo et al., 2010), or with severe neglect signs during the acute phase (Stone et al., 1992; Karnath et al., 2011).
In the acute phase, visual neglect has often been associated with cortical damage to the inferior parietal lobule, the middle and inferior frontal lobes or the superior temporal gyrus (Vallar and Perani, 1986; Karnath et al., 2001, 2009; Halligan et al., 2003; Mort et al., 2003) as well as to other cortical localizations on the lateral surface of the right hemisphere (Chechlacz et al., 2012a; Molenberghs et al., 2012). Functional neuroimaging studies in acute neglect described bilateral cortical hypoactivation (Pizzamiglio et al., 1998) and decrease of perfusion or glucose metabolism (Perani et al., 1987; Vallar et al., 1988), sometimes with relative hyperactivity of left hemisphere structures (Corbetta et al., 2005). Recovery from neglect seems to be related to restoration of this functional impairment (Perani et al., 1987; Vallar et al., 1988; Pantano et al., 1992; Hillis et al., 2003; Corbetta et al., 2005; Cappa and Perani, 2010) and, in particular, to balanced activity between left and right parietal regions (He et al., 2007). On the other hand, lesional predictors of persistent neglect have been identified in the superior and middle temporal gyri, the inferior parietal lobe, the temporo-parietal junction, and the middle frontal gyrus (Farnè et al., 2004; Karnath et al., 2011; Saj et al., 2012; Thiebaut de Schotten et al., 2014), sometimes with different cortical localizations for different types of neglect (Chechlacz et al., 2012b; Khurshid et al., 2012). These results suggest that persistent neglect results from dysfunction of large-scale networks rather than from focal brain damage.
Dysfunction in fronto-parietal attentional networks seems indeed to be strongly associated with neglect signs (Bartolomeo et al., 2007; Bartolomeo, 2014a), beyond strict cortical localization. Consistent with this hypothesis, fronto-parietal disconnection has been described both in acute neglect (Leibovitch et al., 1998, 1999; Thiebaut de Schotten et al., 2005; Shinoura et al., 2009; Ciaraffa et al., 2013) and in chronic neglect (Doricchi and Tomaiuolo, 2003; Urbanski et al., 2011; Thiebaut de Schotten et al., 2014). Posterior callosal damage has also been associated with acute or subacute (<6 months) neglect (Bozzali et al., 2012; Umarova et al., 2014), but its role in chronic neglect has not been assessed with diffusion MRI.
Severe white matter damage is often related to axonal degeneration of fibre tracts, which occurs days to weeks after a cerebrovascular insult (Thomalla et al., 2005; Jason et al., 2011), similar to other forms of remote neurodegeneration (Viscomi and Molinari, 2014). Axonal degeneration can then be used as a biomarker to identify the disconnected cortical areas. However, its potential relationship with chronic persistence of neglect has never been specifically assessed. Yet, the identification of reliable biomarkers for persistent neglect is a clinically relevant issue, because rehabilitation procedures are costly in terms of time and human resources (Luauté et al., 2006).
In this study, we adopted a prospective, longitudinal approach in order to identify signs of axonal degeneration associated with chronic neglect after a right hemisphere stroke, by using tract-based spatial statistics (TBSS). In particular, we aimed at identifying the lesional predictors of chronic neglect within the major intra- and inter hemispheric association white matter bundles.
Materials and methods
Patients
We recruited 45 patients with a first right hemisphere stroke. Patients were consecutively admitted to the neurologic unit of the Clinique ‘Les Trois Soleils’, Boissise-Le-Roi, France, and underwent high resolution MRI brain scans at the Pitié-Salpêtrière Hospital, Paris according to the protocol established at the local Centre d’Anatomie Cognitive. The protocol was approved by the local ethics committee Ile de France I. The recruited patients did not suffer from impaired vigilance, confusion, general mental deterioration or psychiatric disorders and had no prior history of neurological disease. All patients received standard rehabilitation procedures for up to 6 months post-stroke.
Neglect assessment
Neglect was assessed by using the ‘Batterie d’Evaluation de la Négligence’ (BEN) (Azouvi et al., 2002). The initial behavioural assessment of neglect was carried out in the subacute phase of the stroke [mean 39.56 days post-stroke, standard deviation (SD) 25.83]. All patients were subsequently retested with the same battery in the chronic phase (mean, 515.32 days post-stroke, SD 360.01). The BEN includes a target cancellation task, the bells test (Gauthier et al., 1989), line bisection (two 200-mm lines and two 5-mm lines), the copy of a landscape (Ogden scene; Ogden, 1985), tasks of clock drawing, text reading and writing, and identification of overlapping figures (Gainotti, D'Erme and Bartolomeo, 1991). Pathological scores for each test were based on the cut-off reported by Azouvi et al. (2006). To control for possible test/retest practice effects, in the chronic phase patients performed additional tests, consisting of two further visual search tasks, line cancellation (Albert, 1973) and letter cancellation (Mesulam, 1985), of the bisection of eight lines horizontally disposed in a vertical A4 sheet (Bartolomeo and Chokron, 1999), and of a copy of a linear drawing of a landscape containing five items (Gainotti et al., 1972). New tests were added at the chronic phase to control for possible test-retest effects, which are often observed with repeated use of the same material.
For the first testing (subacute phase), we computed a score of neglect severity by averaging the percentage of left omissions in Bells cancellation, overlapping figures, drawing copy, omitted left-sided words on the reading task, omissions of left-sided digits on clock drawing and percentage of rightwards deviation on line bisection and on the writing test (‘subacute neglect score’). For the chronic phase testing, a similar score was computed by including also the percentage of rightwards deviation on the 8-line bisection test, the left omissions on the letter and line cancellation tasks and the left omissions on the copy of the five-item drawing' (‘chronic neglect score’).
The outcome of longitudinal neglect assessment classified the patients in the following three groups: patients who never showed signs of neglect (hereafter non-neglect patients), patients with persistent neglect (chronic neglect patients), and patients with neglect signs at the subacute phase who had recovered at retest (ex-neglect patients). On the first test at the subacute phase, 27 patients had a pathological performance on at least two neglect tests, while 18 were free of neglect on all of the tests (non-neglect patients). In the chronic phase, 17 patients still showed neglect signs on at least one test (chronic neglect patients), whereas 10 patients had recovered on every test (ex-neglect patients). We carefully checked the clinical reports of all non-neglect patients and found no mention of signs of clinical neglect in the acute phase. Table 1 reports the demographic data and test performance for the 45 patients. The three patient groups (non-neglect, chronic neglect and ex-neglect) did not differ in age [F(2,44) = 1.24; P = 0.30]. The average number of days between stroke and neuropsychological evaluation in the subacute phase (Table 1) did not differ in the three groups (F < 1). Visual field defects were present in seven chronic neglect patients, in one ex-neglect patient and in three non-neglect patients.
Demographic data and performance on neglect’s tests of all 45 right brain damaged patients
| . | Spatial neglect . | . | ||||
|---|---|---|---|---|---|---|
| . | Chronic neglect patients . | Ex-neglect patients . | Non-neglect patients . | |||
| . | Subacute phase . | Chronic phase . | Subacute phase . | Chronic phase . | Subacute phase . | Chronic phase . |
| Demographic data | ||||||
| Patients | 17 (9 M/8 F) | 10 (3 M/7 F) | 18 (12 M/6 F) | |||
| Age | 57.78 (10.84) | 49.39 (17.84) | 52.78 (10.19) | |||
| Volume lesion | 94.19 (106.08) | 54.96 (52.73) | 19.11 (24.32) | |||
| Visual field defects (% present) | 41 | 10 | 17 | |||
| Neuropsychological evaluation | ||||||
| Days post stroke | 42.02 (25.89) | 456.26 (208.87) | 38.80 (22.82) | 458.63 (226.46) | 38.27 (24.20) | 585.25 (190.15) |
| Bell cancellation (Total omission) | 12.12 (9.82) | 5.76 (6.60) | 7.2 (5.33) | 1.65 (1.16) | 1.5 (1.95) | 1.39 (2.19) |
| Letter cancellation (Total omission) | – | 6.81 (6.84) | – | 2.9 (3.18) | – | 1.44 (2.06) |
| Line cancellation (Total omission) | – | 1.47 (2.32) | – | 1 (3.16) | – | 0 (0) |
| Copy of Ogden Scene (/4) | 1.94 (1.71) | 0.47 (0.94) | 1 (1.24) | 0 (0) | 0 (0) | 0 (0) |
| Copy of Gainotti scene (/6) | – | 4.76 (1.15) | – | 6 (0) | – | 6 (0) |
| Line bisection /Line 5 cm (mean deviation, mm) | −0.12 (2.67) | −0.53 (2.25) | 0.65 (2.13) | −0.8 (0.74) | 0.07 (1.09) | −0.45 (1.86) |
| Line bisection /Line 20 cm (mean deviation, mm) | 8.09 (13.55) | 6.09 (6.5) | 7.85 (13.96) | −2.7 (4.99) | 0.56 (4.94) | −1.68 (4.14) |
| Line bisection 8-lines (% deviation) | – | 3.83 (2.81) | – | 3.12 (1.95) | – | 3.32 (2.65) |
| Clock drawing (/2) | 0.44 (0.51) | 0 (0) | 0.2 (0.422) | 0 (0) | 0 (0) | 0 (0) |
| Overlapping figures (Total omissions) | 2.60 (3.70) | 0 (0) | 0.3 (0.48) | 0 (0) | 0 (0) | 0 (0) |
| Text reading (Total omissions) | 17 (28.42) | 1.64 (2.90) | 0.56 (1.67) | 0 (0) | 0 (0) | 0 (0) |
| Writting (left margin, cm) | 5.59 (3.33) | 3.83 (2.81) | 3.75 (2.79) | 2.76 (1.68) | 2.1 (1.40) | 3.32 (2.65) |
| Neglect Score (%) | 29.38 (20.59) | 10.82 (7.90) | 14.40 (7.86) | 2.86 (1.44) | 3.65 (2.15) | 3.41 (1.90) |
| . | Spatial neglect . | . | ||||
|---|---|---|---|---|---|---|
| . | Chronic neglect patients . | Ex-neglect patients . | Non-neglect patients . | |||
| . | Subacute phase . | Chronic phase . | Subacute phase . | Chronic phase . | Subacute phase . | Chronic phase . |
| Demographic data | ||||||
| Patients | 17 (9 M/8 F) | 10 (3 M/7 F) | 18 (12 M/6 F) | |||
| Age | 57.78 (10.84) | 49.39 (17.84) | 52.78 (10.19) | |||
| Volume lesion | 94.19 (106.08) | 54.96 (52.73) | 19.11 (24.32) | |||
| Visual field defects (% present) | 41 | 10 | 17 | |||
| Neuropsychological evaluation | ||||||
| Days post stroke | 42.02 (25.89) | 456.26 (208.87) | 38.80 (22.82) | 458.63 (226.46) | 38.27 (24.20) | 585.25 (190.15) |
| Bell cancellation (Total omission) | 12.12 (9.82) | 5.76 (6.60) | 7.2 (5.33) | 1.65 (1.16) | 1.5 (1.95) | 1.39 (2.19) |
| Letter cancellation (Total omission) | – | 6.81 (6.84) | – | 2.9 (3.18) | – | 1.44 (2.06) |
| Line cancellation (Total omission) | – | 1.47 (2.32) | – | 1 (3.16) | – | 0 (0) |
| Copy of Ogden Scene (/4) | 1.94 (1.71) | 0.47 (0.94) | 1 (1.24) | 0 (0) | 0 (0) | 0 (0) |
| Copy of Gainotti scene (/6) | – | 4.76 (1.15) | – | 6 (0) | – | 6 (0) |
| Line bisection /Line 5 cm (mean deviation, mm) | −0.12 (2.67) | −0.53 (2.25) | 0.65 (2.13) | −0.8 (0.74) | 0.07 (1.09) | −0.45 (1.86) |
| Line bisection /Line 20 cm (mean deviation, mm) | 8.09 (13.55) | 6.09 (6.5) | 7.85 (13.96) | −2.7 (4.99) | 0.56 (4.94) | −1.68 (4.14) |
| Line bisection 8-lines (% deviation) | – | 3.83 (2.81) | – | 3.12 (1.95) | – | 3.32 (2.65) |
| Clock drawing (/2) | 0.44 (0.51) | 0 (0) | 0.2 (0.422) | 0 (0) | 0 (0) | 0 (0) |
| Overlapping figures (Total omissions) | 2.60 (3.70) | 0 (0) | 0.3 (0.48) | 0 (0) | 0 (0) | 0 (0) |
| Text reading (Total omissions) | 17 (28.42) | 1.64 (2.90) | 0.56 (1.67) | 0 (0) | 0 (0) | 0 (0) |
| Writting (left margin, cm) | 5.59 (3.33) | 3.83 (2.81) | 3.75 (2.79) | 2.76 (1.68) | 2.1 (1.40) | 3.32 (2.65) |
| Neglect Score (%) | 29.38 (20.59) | 10.82 (7.90) | 14.40 (7.86) | 2.86 (1.44) | 3.65 (2.15) | 3.41 (1.90) |
Standard deviations are presented in parenthesis.
M = male; F = female.
Demographic data and performance on neglect’s tests of all 45 right brain damaged patients
| . | Spatial neglect . | . | ||||
|---|---|---|---|---|---|---|
| . | Chronic neglect patients . | Ex-neglect patients . | Non-neglect patients . | |||
| . | Subacute phase . | Chronic phase . | Subacute phase . | Chronic phase . | Subacute phase . | Chronic phase . |
| Demographic data | ||||||
| Patients | 17 (9 M/8 F) | 10 (3 M/7 F) | 18 (12 M/6 F) | |||
| Age | 57.78 (10.84) | 49.39 (17.84) | 52.78 (10.19) | |||
| Volume lesion | 94.19 (106.08) | 54.96 (52.73) | 19.11 (24.32) | |||
| Visual field defects (% present) | 41 | 10 | 17 | |||
| Neuropsychological evaluation | ||||||
| Days post stroke | 42.02 (25.89) | 456.26 (208.87) | 38.80 (22.82) | 458.63 (226.46) | 38.27 (24.20) | 585.25 (190.15) |
| Bell cancellation (Total omission) | 12.12 (9.82) | 5.76 (6.60) | 7.2 (5.33) | 1.65 (1.16) | 1.5 (1.95) | 1.39 (2.19) |
| Letter cancellation (Total omission) | – | 6.81 (6.84) | – | 2.9 (3.18) | – | 1.44 (2.06) |
| Line cancellation (Total omission) | – | 1.47 (2.32) | – | 1 (3.16) | – | 0 (0) |
| Copy of Ogden Scene (/4) | 1.94 (1.71) | 0.47 (0.94) | 1 (1.24) | 0 (0) | 0 (0) | 0 (0) |
| Copy of Gainotti scene (/6) | – | 4.76 (1.15) | – | 6 (0) | – | 6 (0) |
| Line bisection /Line 5 cm (mean deviation, mm) | −0.12 (2.67) | −0.53 (2.25) | 0.65 (2.13) | −0.8 (0.74) | 0.07 (1.09) | −0.45 (1.86) |
| Line bisection /Line 20 cm (mean deviation, mm) | 8.09 (13.55) | 6.09 (6.5) | 7.85 (13.96) | −2.7 (4.99) | 0.56 (4.94) | −1.68 (4.14) |
| Line bisection 8-lines (% deviation) | – | 3.83 (2.81) | – | 3.12 (1.95) | – | 3.32 (2.65) |
| Clock drawing (/2) | 0.44 (0.51) | 0 (0) | 0.2 (0.422) | 0 (0) | 0 (0) | 0 (0) |
| Overlapping figures (Total omissions) | 2.60 (3.70) | 0 (0) | 0.3 (0.48) | 0 (0) | 0 (0) | 0 (0) |
| Text reading (Total omissions) | 17 (28.42) | 1.64 (2.90) | 0.56 (1.67) | 0 (0) | 0 (0) | 0 (0) |
| Writting (left margin, cm) | 5.59 (3.33) | 3.83 (2.81) | 3.75 (2.79) | 2.76 (1.68) | 2.1 (1.40) | 3.32 (2.65) |
| Neglect Score (%) | 29.38 (20.59) | 10.82 (7.90) | 14.40 (7.86) | 2.86 (1.44) | 3.65 (2.15) | 3.41 (1.90) |
| . | Spatial neglect . | . | ||||
|---|---|---|---|---|---|---|
| . | Chronic neglect patients . | Ex-neglect patients . | Non-neglect patients . | |||
| . | Subacute phase . | Chronic phase . | Subacute phase . | Chronic phase . | Subacute phase . | Chronic phase . |
| Demographic data | ||||||
| Patients | 17 (9 M/8 F) | 10 (3 M/7 F) | 18 (12 M/6 F) | |||
| Age | 57.78 (10.84) | 49.39 (17.84) | 52.78 (10.19) | |||
| Volume lesion | 94.19 (106.08) | 54.96 (52.73) | 19.11 (24.32) | |||
| Visual field defects (% present) | 41 | 10 | 17 | |||
| Neuropsychological evaluation | ||||||
| Days post stroke | 42.02 (25.89) | 456.26 (208.87) | 38.80 (22.82) | 458.63 (226.46) | 38.27 (24.20) | 585.25 (190.15) |
| Bell cancellation (Total omission) | 12.12 (9.82) | 5.76 (6.60) | 7.2 (5.33) | 1.65 (1.16) | 1.5 (1.95) | 1.39 (2.19) |
| Letter cancellation (Total omission) | – | 6.81 (6.84) | – | 2.9 (3.18) | – | 1.44 (2.06) |
| Line cancellation (Total omission) | – | 1.47 (2.32) | – | 1 (3.16) | – | 0 (0) |
| Copy of Ogden Scene (/4) | 1.94 (1.71) | 0.47 (0.94) | 1 (1.24) | 0 (0) | 0 (0) | 0 (0) |
| Copy of Gainotti scene (/6) | – | 4.76 (1.15) | – | 6 (0) | – | 6 (0) |
| Line bisection /Line 5 cm (mean deviation, mm) | −0.12 (2.67) | −0.53 (2.25) | 0.65 (2.13) | −0.8 (0.74) | 0.07 (1.09) | −0.45 (1.86) |
| Line bisection /Line 20 cm (mean deviation, mm) | 8.09 (13.55) | 6.09 (6.5) | 7.85 (13.96) | −2.7 (4.99) | 0.56 (4.94) | −1.68 (4.14) |
| Line bisection 8-lines (% deviation) | – | 3.83 (2.81) | – | 3.12 (1.95) | – | 3.32 (2.65) |
| Clock drawing (/2) | 0.44 (0.51) | 0 (0) | 0.2 (0.422) | 0 (0) | 0 (0) | 0 (0) |
| Overlapping figures (Total omissions) | 2.60 (3.70) | 0 (0) | 0.3 (0.48) | 0 (0) | 0 (0) | 0 (0) |
| Text reading (Total omissions) | 17 (28.42) | 1.64 (2.90) | 0.56 (1.67) | 0 (0) | 0 (0) | 0 (0) |
| Writting (left margin, cm) | 5.59 (3.33) | 3.83 (2.81) | 3.75 (2.79) | 2.76 (1.68) | 2.1 (1.40) | 3.32 (2.65) |
| Neglect Score (%) | 29.38 (20.59) | 10.82 (7.90) | 14.40 (7.86) | 2.86 (1.44) | 3.65 (2.15) | 3.41 (1.90) |
Standard deviations are presented in parenthesis.
M = male; F = female.
Imaging data acquisition and lesion analysis
In the chronic phase of the stroke (mean days post-stroke, 444.80, range 114–2300) each patient underwent a high resolution brain MRI scan including T1 3D anatomical SPGR (spoiled gradient recalled) images (repetition time: 7164 ms; echo time: 3124 ms; inversion time: 380 ms; flip angle: 15°; coronal orientation perpendicular to the double echo sequence; acquisition matrix: 288 × 256; voxel resolution: 0.5 × 0.5 ×1.2 mm3). In addition, a diffusion tensor sequence was also performed using echo-planar imaging (repetition time: 14 s, echo time: 75.8 ms; flip angle: 90°; acquisition matrix: 128 × 128; per cent phase field of view = 100; slice thickness = 3 mm; no gap; voxel resolution = 1, 1, 3 mm3). Fifty diffusion images weighted with a b-value of 1000 s/mm2 and three volumes with no diffusion gradient were acquired.
Grey matter lesion analysis
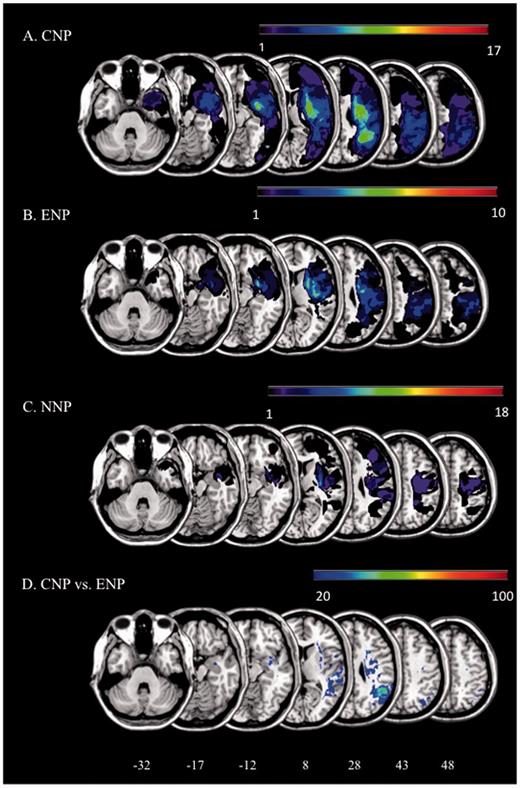
Lesion masks of patients were first drawn on the native 3D T1 images by using the MRIcron software (Rorden et al., 2007) and a graphic tablet (WACOM Intuos A6, Vancouver, Washington, USA). T1 images were normalized to a standard brain template (Montreal Neurological Institute) using rigid and elastic deformation tools provided in the software package Statistical Parametric Mapping 8 (SPM8, http://www.fil.ion.ucl.ac.uk/spm) running under Matlab 2009b (http://www.mathworks.com). Deformations were applied to the whole brain except for the voxels contained in the lesion mask to avoid deformation of the lesioned tissue (Brett et al., 2001; Volle et al., 2008). Finally, patients’ lesions were manually segmented a second time on the normalized images by a neuropsychologist (M.L.) trained to lesion analysis and reviewed by an expert neurologist (R.M.). Figure 1 illustrates the lesion distributions for the three groups of patients. As expected, the three groups differed in lesional volume [F(2,44) = 4.81; P = 0.013]. Post hoc comparisons revealed that patients with chronic neglect had larger lesions as compared with non-neglect patients [t(42) = 3.102; P = 0.003]; they had, however, similar lesion volumes as patients who eventually recovered from neglect [t(42) = 1.375; P = 0.176]. The probability map of the ex-neglect patients was subtracted from the probability map of the chronic neglect patients. Only areas that after subtraction were damaged at least 20% more often in chronic neglect patients than ex-neglect patients were considered for descriptive purposes.
Lesion overlap. (A–C) Overlap of the lesions for all the included right brain-damaged patients. (D) Results of the subtraction of the probability map of the ex-neglect group from the probability map of the chronic neglect group. Only areas that were damaged 20% more frequently in chronic neglect patients than in ex-neglect patients are reported in the figure. NNP = non-neglect patients.
Several studies have raised concerns on the use of lesion mapping to assess anatomical correlates of vascular lesions, because of biases induced by the architecture of the vascular tree (Godefroy et al., 1998; Bartolomeo, 2011; Mah et al., 2014). Despite this, voxel-wise analyses remain largely used in the literature; thus, we conducted voxel-wise (topological) lesion-deficit analyses on our data.
We first conducted four voxel-based lesion behaviour analyses by using the non-parametric Liebermeister test implemented in MRIcron toolbox (Rorden et al., 2007), to identify the voxels whose damage might predict subacute or chronic neglect. For each voxel, we compared presence/absence of lesion in (i) all subacute neglect versus non-neglect patients; (ii) chronic neglect versus non-neglect patients; (iii) chronic neglect versus ex-neglect patients; and (iv) ex-neglect versus non-neglect patients. Only voxels damaged in at least 10% of patients were included in the analysis. For all analyses, we controlled for multiple comparisons using permutation-based thresholding (Kimberg et al., 2007) with 1000 iterations. The significant results survived a 5% permutation-based false positive probability threshold.
As a second step, we assessed the relationship between the lesioned voxels and the severity of neglect in the subacute and in the chronic phase. Two logistic regressions were performed using MRIcron software (Rorden et al., 2007) with three independent variables: lesion volume (continuous measure), visual field defects (binary measure), and whether or not each single voxel was damaged in each patient (binary measure). Next, we calculated whether these three variables were able to predict the severity of neglect (continuous measure) in the subacute phase (‘subacute neglect score’) or in the chronic phase (‘chronic neglect score’).
White matter lesion analysis
DTI preprocessing
As a first step, we corrected diffusion data sets simultaneously for motion and geometrical distortions by using ExploreDTI (Leemans and Jones, 2009). The tensor model was fitted to the data by using the Levenberg-Marquardt non-linear regression (Marquardt, 1963). Fractional anisotropy maps were extracted by using the function ‘extract stuff’, which is part of the ExploreDTI software package.
TBSS analysis
We used a whole-brain voxel-wise analysis of the fractional anisotropy data carried out with TBSS (Smith et al., 2006), as included in the FSL software package (Smith et al., 2004; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL). All fractional anisotropy maps were aligned to an averaged fractional anisotropy template into 1 × 1 × 1 mm MNI-152 standard space using a non-linear registration. To avoid deformation of the lesioned tissue during the registration, for each patient we used her/his segmentation of lesion as mask during this step. Correct registration was visual checked for each patient. Next, an average fractional anisotropy map was created and a skeleton map representing the centre of the white matter (fractional anisotropy > 0.2) common to all patients, computed. Finally, patient’s registered fractional anisotropy maps were projected into the skeleton.
First, we identified the white matter areas whose damage predicted subacute neglect and its recovery by statistically comparing fractional anisotropy values between: (i) all subacute neglect versus non-neglect patients; (ii) chronic neglect versus non-neglect patients; (iii) chronic neglect versus ex-neglect patients; and (iv) ex-neglect versus non-neglect patients.
Second, we conducted regression analyses including all the 45 patients to identify the white matter areas contributing to the severity of neglect in the subacute and in the chronic phase. Voxel-wise associations were investigated between patients’ fractional anisotropy and the ‘subacute neglect score’ and ‘chronic neglect score’, respectively. These scores were demeaned before being entered into the general linear model. We also performed regression analyses between patients’ fractional anisotropy and their performance on line bisection and target cancellation task in the subacute and chronic phases, respectively.
In all comparisons or regressions, we used the ‘Lesion Masking’ tool implemented in FSL software to exclude directly damaged areas from the TBSS analysis for each patient (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise/UserGuide). To exclude confounding factors that might determine spurious differences between the three patient groups, we also included the lesion volume (as a continuous measure) and the presence/absence of chronic visual field defects (as a binary measure) as co-variables of non-interest. These values were demeaned and entered into the general model.
All statistics were assessed by using the function ‘Randomise’ (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise/UserGuidetool) implemented in FSL, with 5000 random permutation tests and a Threshold-Free Cluster Enhancement option (Smith and Nichols, 2009). Results were adjusted for family wise error (FWE) corrections for multiple comparisons and thresholded at P < 0.025. Results were interpreted by using a diffusion atlas of white matter fibre tracts (Thiebaut de Schotten et al., 2011b) and the labels defined by the (JHU ICBM)-DTI white matter atlas (Mori et al., 2008).
Tractography in healthy subjects based on the TBSS results in chronic neglect versus non-neglect patients
TBSS analysis revealed a significant fractional anisotropy decrease in voxels localized in the splenium of corpus callosum for the contrast chronic neglect < non-neglect patients. To identify the cortical projections of the disconnected splenial fibres in chronic neglect compared to non-neglect patients, we conducted a tractography study on high resolution diffusion imaging data sets from 40 adult healthy control subjects from the Human Connectome Project (HCP) database (http://www.humanconnectome.org, Release Q2) (see Supplementary material for detailed description of the methods). We created percentage overlap maps by adding the normalized visitation maps from each subject at each point in the MNI space. A similar approach has been followed by Thiebaut de Schotten et al. (2011b). We present results from 50–100% of overlap.
Results
Lesion mapping analysis
Examination of lesion overlap plots (Fig. 1) revealed a maximum overlap in the subcortical white matter in all three patient groups. However, white matter damage appeared to be much more extensive in the chronic neglect group. It is also important to note that lesions can disconnect the same tracts at different levels, without demonstrating any overlap (Catani and Mesulam, 2008; Thiebaut de Schotten et al., 2014). We then subtracted the lesion probability map of ex-neglect patients from the lesion probability map of chronic neglect patients (Fig. 1D). This analysis revealed regions damaged more frequently in chronic neglect patients, such as the angular gyrus and the superior temporal gyrus near the temporo-parietal junction, together with a peak in the fronto-parietal white matter.
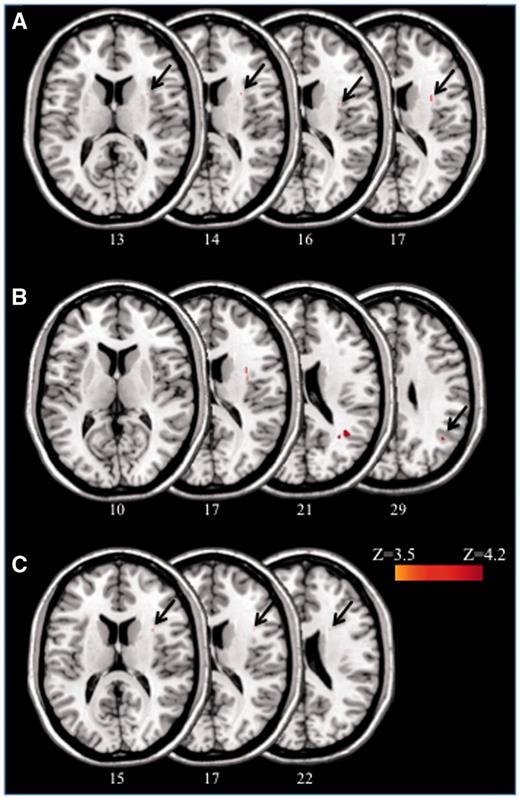
Figure 2A–C displays the location of those voxels whose damage was significantly associated with the presence of neglect in the subacute and in the chronic phase. The very conservative Liebermeister Test only demonstrated few significantly damaged voxels, similar to previous results (Kopp et al., 2013). Most of the damaged voxels were localized in the white matter, both for subacute and chronic neglect. In the subacute phase (Fig. 2A), the analysis revealed a small area around MNI coordinates x = 31, y = 9, z = 14, which corresponds to a white matter region around the putamen and the insula. Damage to white matter underlying the angular gyrus and the middle temporal gyrus was associated with chronic neglect (Fig. 2B), as compared to patients who never showed neglect (area around MNI coordinates x = 34, y = −56, z = 20). Comparison between ex-neglect and non-neglect patients (Fig. 2C) revealed a small area around MNI coordinates x = 32, y = 10, z = 15, similar to subacute neglect (Fig. 2A). Finally, no significant results emerged for the comparison between ex-neglect and chronic neglect patients.
Anatomical results obtained from the voxel-based lesion-behaviour mapping. (A) MNI voxels predicting the presence versus absence of subacute neglect. (B) MNI voxels predicting the presence versus absence of chronic neglect (ex-neglect patients excluded). (C) MNI voxels predicting the presence versus absence of neglect when chronic patients were excluded. Only results corrected for multiple comparisons are presented.
Logistic regressions were performed between the damaged voxels and the severity of neglect, respectively in the subacute and in the chronic phase. There were no significant results after correction for lesion volume, consistent with previously reported evidence (Karnath et al., 2004; Thiebaut de Schotten et al., 2014).
Tract-based spatial statistics
Voxel-wise analysis using TBSS showed significant differences in mean fractional anisotropy outside the area damaged by the stroke for each patient in the comparisons between all subacute neglect and non-neglect patients, between non-neglect and ex-neglect patients, and between chronic and non-neglect patients. The difference did not reach significance in the ex-neglect versus chronic neglect comparison. In the following section these results will be described in detail.
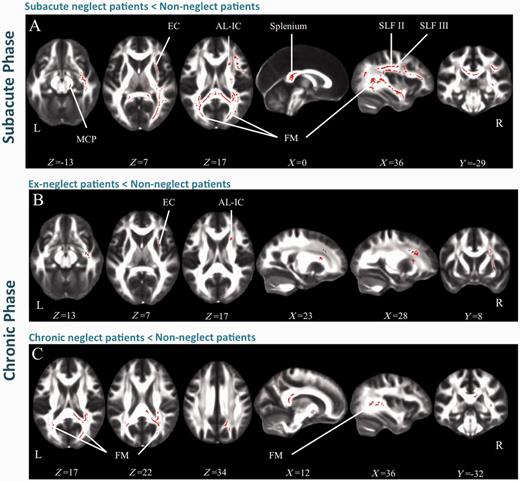
For all patients with neglect in the subacute phase, fractional anisotropy was globally decreased in the right hemisphere, in comparison with non-neglect patients. In particular, fractional anisotropy was lower in the splenium of the corpus callosum and in its bilateral projections towards the occipital, parietal and temporal white matter (forceps major) (Fig. 3A). There was also evidence of microstructural alteration in the second and third branches of the superior longitudinal fasciculus (SLF II and III) in the right hemisphere, in the right superior cerebellar peduncle (containing corticospinal and cortico-ponto-cerebellar tracts) and in the right frontal white matter, including the external capsule.
Results of TBSS. FA differences (P = 0.025) are represented in red. (A) contrast subacute neglect < non-neglect patients; (B) contrast recovered neglect < non-neglect patients; (C) contrast chronic neglect < non-neglect patients. SCP = superior cerebral peduncle; FM = forceps major; SLF = superior longitudinal fasciculus; EC = external capsule; AL-IC = anterior limb of the internal capsule.
The comparison between ex-neglect and non-neglect patients showed decreased fractional anisotropy in the anterior limb of the internal capsule and the extreme/external capsule (Fig. 3B). The chronic neglect versus non-neglect comparison revealed microstructural alterations in the callosal splenium and in the forceps major (Fig. 3C).
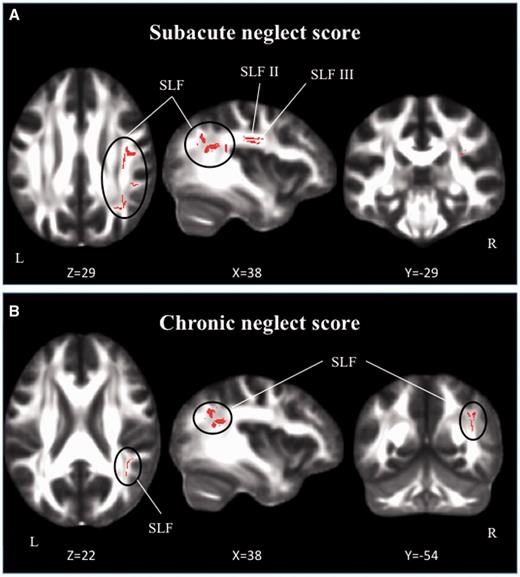
The TBSS regression analysis demonstrated a strong association between the severity of neglect in all patients and decreased fractional anisotropy values in the right SLF II and III (Fig. 4A). The severity of chronic neglect was instead only associated with lower fractional anisotropy values in the posterior part of the SLF (Fig. 4B), where SLF II and III run together (Thiebaut de Schotten et al., 2011a). An additional TBSS analysis, performed after having excluded the new tests administered at the chronic phase, obtained similar results, highlighting the caudal portion of the SLF, where SLF II and III run together (Supplementary Fig. 1). There was no significant association between fractional anisotropy values and patients’ performance on the Bells cancellation task or on line bisection, either in the subacute or in the chronic phase.
Relationship between white matter damage and severity of neglect. (A) Subacute phase; (B) chronic phase. Results of TBSS regression analyses are represented in red (P = 0.025), corrected for multiple comparisons.
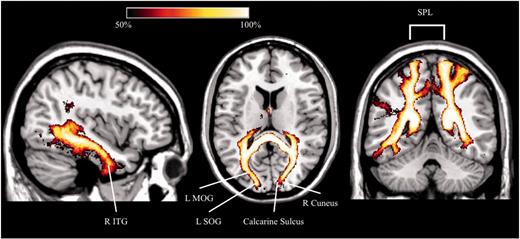
The analysis performed in normal subjects revealed that the fibres running through the voxels with lower fractional anisotropy in patients with chronic neglect mostly connect regions of the occipital lobes (middle and superior occipital gyri, cuneus), the superior parietal lobule, the precuneus, and the inferior temporal gyri (Fig. 5). Seven of 17 patients with chronic neglect suffered from visual field defects. Although these deficits were entered as a regressor in the TBSS analysis, to further assess the potential role of visual field defects in neglect persistence we performed a further TBSS analysis comparing non-neglect patients to chronic patients without visual field defects. Results revealed again microstructural alterations in the callosal splenium for chronic patients (Supplementary Fig. 2). We can thus safely conclude that the splenial disconnection we observed in chronic neglect did not depend on the presence of visual field defects. In addition, decreased fractional anisotropy also occurred in more anterior regions of the corpus callosum (rostrum and genu), in the second branch of the SLF, in the posterior limb of the internal capsule, in the external capsule, in the superior corona radiata and in the cerebral peduncle. We also performed a tracking analysis in healthy subjects (Supplementary material), to identify the cortical projections of the disconnected fibres in chronic neglect without visual field defects, as compared to non-neglect patients. Concerning the callosal splenium, voxels with decreased fractional anisotropy contain fibres connecting the cuneus, the fusiform and lingual gyri, the middle and superior occipital lobes, and the precuneus. In the rostrum and genu, the affected fibres connect the superior and middle frontal gyri and the gyrus rectus.
Results of TBSS. Percentage map in healthy subjects (n = 40) showing the cortical projections of the splenial voxels (forceps major) identified by TBSS analysis in chronic neglect patients compared to non-neglect patients. L = left; MOG = middle occipital gyrus; R = right; SOG = superior occipital gyrus; SPL = superior parietal lobule; ITG = inferior temporal gyrus.
Extraction of fractional anisotropy values
In the present patient sample, TBSS did not reveal any significant difference between chronic and ex-neglect patients. This comparison may have failed to reach significance because of the relatively small size of the groups, and because of the inclusion of the lesion as a voxel-dependent covariable of non-interest. Having excluded the lesioned regions from the analysis may have decreased the sensitivity of the comparison.
We specifically tested whether the fractional anisotropy values in the forceps major, SLF II and III (whether lesioned or not) can predict neglect recovery. We extracted the mean fractional anisotropy values of the tracts identified by TBSS analysis, which were in the forceps major, SLF II and SLF III. Following common procedures (Scanlon et al., 2013; Thiebaut de Schotten et al., 2014), the percentages of tract presence derived from the atlases were thresholded at a probability superior to 20% for the (JHU ICBM)-DTI atlas (Mori et al., 2008), or to 50% for the atlas by Thiebaut de Schotten et al. (2011b). The percentages were consequently binarized to create appropriate regions of interest to extract tract-specific mean fractional anisotropy values for each patient. Mean differences were assessed by using a univariate analysis of variance (ANOVA), corrected for lesion volume and for the presence of visual field defects using SPSS software (http://www-01.ibm.com/software/fr/analytics/spss/). Finally, the same tracts were also examined in the left, intact hemisphere as an intra-subject control and in order to explore potential re-organization of white matter microstructure in the contralesional hemisphere. We also performed partial correlations, corrected for lesion volume and for the presence of visual field defects, between fractional anisotropy values for each tract and severity of neglect in the subacute and in the chronic phase.
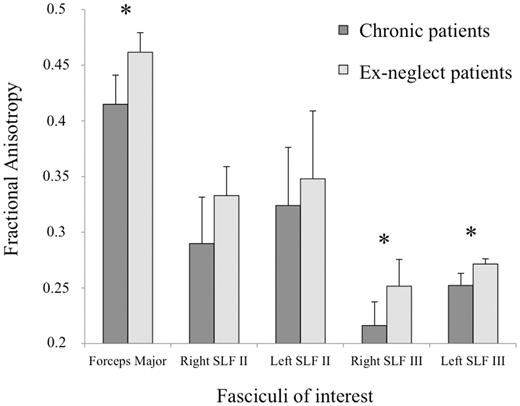
Results (Fig. 6) showed lower fractional anisotropy values in the forceps major of the corpus callosum for chronic neglect patients than for ex-neglect patients [F(1,23) = 6.343, P = 0.019]. Finally, chronic patients showed lower fractional anisotropy values in both the right and left SLF III [F(1,23) = 4.584, P = 0.043; F(1,23) = 10.505, P = 0.004, respectively]. By contrast, there was no significant difference for the right or left SLF II [F(1,23) = 0.455, P = 0.506; F(1,23) = 0.30, P =0.863, respectively].
Mean fractional anisotropy extraction values. Mean fractional anisotropy extraction values (with 95% confidence intervals) of fasciculi of interest for chronic and recovered patients. *P < 0.05.
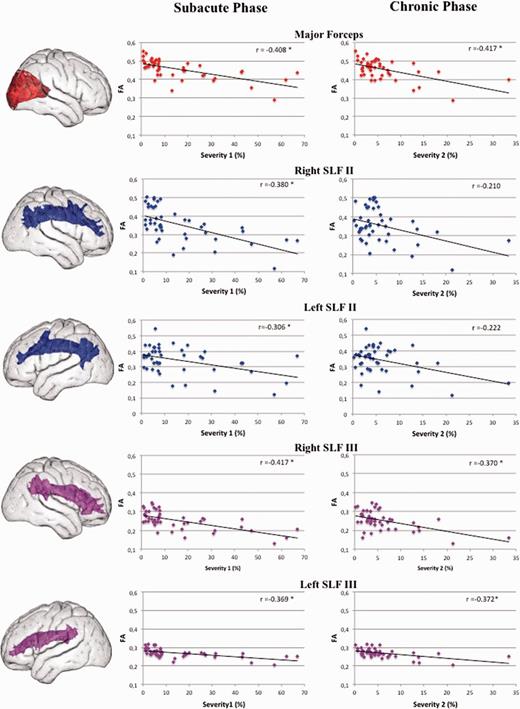
Partial correlations between mean extracted fractional anisotropy of all the 45 patients and their neglect scores (Fig. 7) suggested a role for damage to the right SLF II (r = −0.38, P = 0.012), left SLF II (r = −0.31, P = 0.046), right SLF III (r = −0.42, P = 0.005), left SLF III (r = −0.306, P = 0.015), and forceps major (r = −0.41, P = 0.007) in the severity of neglect during the subacute phase (Fig. 5). Concerning the chronic phase, fractional anisotropy in the left and right SLF III (r = −0.37, P = 0.014; r = −0.37, P = 0.015), and in the forceps major of the corpus callosum (r = −0.42, P = 0.005) correlated with the neglect score (Fig. 7). There was no significant correlation between mean fractional anisotropy in the right or left SLF II and the severity of neglect in the chronic phase (r = −0.21, P = 0.177; r = −0.22, P = 0.153, respectively).
Correlations between the fractional anisotropy values extracted from the fasciculi of interest and the severity of neglect in the subacute and chronic phases. *P < 0.05 after covarying out for the presence/absence of visual field defects and the lesion size. FA = fractional anisotropy.
Discussion
This prospective, longitudinal study assessed the anatomical predictors of neglect recovery after vascular strokes in the middle cerebral artery territory. We discovered subcortical connections whose damage is likely to simultaneously disrupt the functioning of large-scale brain networks, thus decreasing the possibility for compensation of deficits (Bartolomeo et al., 2007, 2012). Two main findings emerged. First, we showed for the first time that micro-structural alteration of white matter in the splenium of the corpus callosum is related to the persistence of neglect in the chronic phase (interhemispheric disconnection). Second, we confirmed that damage to white matter fronto-parietal pathways is a crucial determinant of emergence of spatial neglect (intrahemispheric disconnection).
Our chronic neglect patients had predominantly post-Rolandic lesions. Damage to the white matter underlying the parietal lobe, where SLF II and III run together, may produce severe and persisting signs of neglect because it can jointly disrupt the functioning of both the ventral attention network (through SLF III disconnection) and its communication with the dorsal attention network (through SLF II damage). More rostral lesions are, instead, less likely to damage both of these SLF branches, because their trajectories diverge in more anterior regions (Bartolomeo et al., 2012). As described by Thiebaut de Schotten et al. (2011a), SLF II and III share common cortical projections at the level of the supramarginal gyrus. They also project together to the inferior parietal cortex and to the postcentral gyrus. SLF II, SLF III and the callosal splenium seem to share common cortical projections in the supramarginal gyrus and in the postcentral gyrus.
Thus, splenial disconnection has a negative prognostic value for neglect when associated with damage to fronto-parietal networks in the right hemisphere, presumably because it further isolates the left hemisphere from left-sided visual input. The fibres damaged in our chronic patients mostly connect the occipital and superior parietal lobes. Thus, two possible mechanisms leading to chronic neglect are (i) the impossibility for the left hemisphere to take into account visual information processed by the right hemisphere; and (ii) a persistent imbalance between left and right attentional networks (He et al., 2007). This imbalance may depend on mere interhemispheric diaschisis in acute patients (Carrera and Tononi, 2014), but can persist in the chronic phase in cases of severe degeneration of the forceps major.
Signs of axonal degeneration affecting the medial callosal fibres in the chronic phase after stroke are associated with impaired interhemispheric connectivity (Chen and Schlaug, 2013; Dick et al., 2013). Also consistent with our results, in eight subacute stroke patients (1–6 months from stroke) neglect severity correlated with decreased fractional anisotropy values in the splenium of the corpus callosum (Bozzali et al., 2012). Previous evidence in monkeys (Gaffan and Hornak, 1997) and in patients with strokes in the posterior cerebral artery territory (Bird et al., 2006; Lunven et al., 2014; Park et al., 2006; Rode et al., 2010; Tomaiuolo et al., 2010) also indicated a role for splenial disconnection in neglect, when coupled with disconnection of the optic tract or of the optic radiations. Our results indicate that splenial disconnection may contribute to the chronic persistence of neglect even in patients with strokes in the middle cerebral artery territory, without direct damage to the visual pathways, provided that concurrent lesions are present in the fronto-parietal attentional pathways (Rode et al., 2010).
Our results are in apparent contrast with evidence that left neglect can improve as a consequence of a second lesion in the left hemisphere (Vuilleumier et al., 1996), or after interference with left hemisphere functioning induced by transcranial magnetic stimulation (Oliveri et al., 2001; Koch et al., 2008) in acute patients. Also, a study using magneto-encephalograpy on neglect patients (Rastelli et al., 2013) found evidence of left frontal activity selectively preceding omissions of left-sided targets. These results seem consistent with the inter hemispheric rivalry hypothesis of neglect (Kinsbourne, 1987), and with functional MRI results linking acute neglect with relative hyperactivity of the left undamaged hemisphere (Corbetta et al., 2005), sustained by decreased callosal inhibition from the damaged right hemisphere (Koch et al., 2011). However, recovery from acute neglect was found to be associated with improvement of the interhemispheric correlation in blood oxygen level-dependent signal between the left and the right posterior parietal cortices (He et al., 2007), perhaps supported by relatively unimpaired interhemispheric communication.
Other findings also suggest that hypotheses uniquely based on interhemispheric rivalry are insufficient to explain the occurrence and evolution of neglect signs (Heilman and Adams, 2003). Evidence indicates that the undamaged hemisphere may in fact participate in the recovery from neurological signs (Carter et al., 2010; Wilke et al., 2012), thus assuming a compensatory role (Bartolomeo, 2014a, b). Recovery from neglect signs seems to correlate with restoration of normal metabolism not only in the unaffected regions of the right hemisphere, but also in the left hemisphere (Pantano et al., 1992; Perani et al., 1993). More recently, functional MRI before and after prism adaptation in neglect patients demonstrated increased blood oxygen level-dependent signal with adaptation-induced recovery not only in the damaged right fronto-parietal networks, but also in the homologue left-hemisphere structures (Saj et al., 2013). These results suggest that an isolated left hemisphere may fail to compensate neglect because it cannot take into account left-sided objects (Geschwind, 1965; Bartolomeo et al., 2007; Bartolomeo, 2014a, b). On the contrary, the presence of intact posterior callosal fibres may allow the left fronto-parietal attentional networks to compensate for the failure of their right hemisphere counterparts, thus perhaps increasing the ability of the left hemisphere to learn to take into account information coming from the left hemispace. This process may become difficult or impossible if the left hemisphere ventral fronto-parietal network is inadequate, or if interhemispheric connections are disrupted by the lesion. Our results also call for caution in attempting to interfere with the function of the left hemisphere by using non-invasive brain stimulation techniques in patients with chronic neglect, because these attempts might potentially jeopardize adaptive plastic reorganization leading to neglect compensation.
Concerning intrahemispheric disconnection, the present results are in full agreement with previous evidence that emergence of spatial neglect or its severity in the subacute and chronic phase of the stroke are associated with damage to the right SLF (Leibovitch et al., 1998, 1999; Doricchi and Tomaiuolo, 2003; Thiebaut de Schotten et al., 2005; Bartolomeo et al., 2007, 2012; Urbanski et al., 2008, 2011; Shinoura et al., 2009), in accordance with a network-based model of neglect (Bartolomeo et al., 2007). Damage to SLF III has been previously shown as a lesional correlate of visual neglect (Doricchi et al., 2008; Urbanski et al., 2008). Neuroimaging studies indicated that two distinct but interacting networks subserve visuo-spatial attention (Corbetta et al., 2005; He et al., 2007; Carter et al., 2010; Daitch et al., 2013). SLF III connects the ventral attentional network (Thiebaut de Schotten et al., 2011a); lesions of this pathway may create a functional imbalance between the left and right dorsal fronto-parietal networks, with relative overactivity of the left-sided network during the acute phase (Corbetta et al., 2005). SLF II links the caudal nodes of the ventral attentional network to the frontal nodes of the dorsal network, thus establishing a direct communication between dorsal and ventral attentional networks (Thiebaut de Schotten et al., 2011a). Its damage is also linked to neglect signs (Thiebaut de Schotten et al., 2005, 2014), which can thus result not only from disconnection within fronto-parietal networks in the right hemisphere, but also between ventral and dorsal attentional networks.
By extracting the fractional anisotropy for the tracts of interest in each patient, and by identifying the resulting locations with white matter atlases, we also observed decreased fractional anisotropy in the SLF III in the undamaged left hemisphere of chronic neglect patients, consistent with previous results on acute/subacute patients (Umarova et al., 2014). Caution is needed in the interpretation of this result, because tract measurements in the undamaged hemisphere can change simply as a result of damage to the contralateral hemisphere (Ahlhelm et al., 2002; Dell’Acqua et al., 2013). Nevertheless, patients who had decreased microstructural integrity of the left SLF III, for reasons independent of the right hemisphere stroke, might also be more likely to suffer from persistent neglect because of lack of possible compensation from those damaged left-hemisphere networks (Thiebaut de Schotten et al., 2011a). In agreement with our observation, the presence and extent of leukoaraiosis can predict the occurrence and severity of neglect in acute stroke patients, independent of lesion volume (Bahrainwala et al., 2014). Also, recent evidence on recovery from aphasia after vascular strokes (Forkel et al., 2014) stressed the importance of white matter connections within the healthy right hemisphere.
Degeneration of white matter tracts at a distance from a primary lesion is increasingly recognized as a major cause of neurological symptoms (Coleman and Perry, 2002). After cortical damage, several mechanisms, including trans-synaptic degeneration and inflammation, can contribute to axonal degeneration. Thus, axonal degeneration can ultimately be more extensive than predicted from neuronal body loss (Moxon-Emre and Schlichter, 2010). Consistent with these notions, evidence showed that white matter degeneration may affect the healthy hemisphere in stroke patients, and may inversely correlate with recovery (Buffon et al., 2005; Dacosta-Aguayo et al., 2014; Forkel et al., 2014). Moreover, axonal degeneration might also occur because of abnormal activity in preserved cortical areas (Moritani et al., 2005). If so, then persistence of inappropriate left parietal activity (Corbetta et al., 2005; He et al., 2007) might in the long term aggravate callosal disconnection and thus determine a durable impairment of interhemispheric integration with consequent signs of chronic neglect.
At variance with previous studies on the anatomy of chronic neglect, here we used TBSS, which has repeatedly been shown to be apt at evaluating white matter integrity changes in brain-damaged patients (Messé et al., 2011; Bozzali et al., 2012; Chen and Schlaug, 2013; Yin et al., 2013). TBSS does not require smoothing, with its associated partial volume averaging effects (Jones et al., 2005). Thus, TBSS can lead to more spatially precise results in neurological populations as compared, for example, to voxel-based morphometry (Afzali et al., 2011). Nevertheless, despite the fact that we included lesioned areas during registration in TBSS preprocessing, and checked the final results for each patient, we cannot exclude that lesions generally reduced the efficiency of the TBSS preprocessing steps, which are optimized for brains without macroscopic lesions. Another limitation of our method is the use of non-isotropic voxels in MRI data acquisition, which is not optimal for diffusion-derived measure.
In addition to TBSS, we also used atlas-based fractional anisotropy extraction to assess damage of white matter bundles. Atlas-based fractional anisotropy extraction, at variance with TBSS, allowed us to observe differences between chronic and ex-neglect patients. A similar outcome occurred in a recent study on patients with multiple sclerosis (Gobbi et al., 2014), where tract-specific analysis with atlas-based fractional anisotropy extraction disclosed significant differences between two patient groups, whereas TBSS failed to do so and thus proved to be less sensitive. This could in part result from the fact that TBSS is based on a fibre skeleton and not on the whole bundle; as a consequence, sometimes only a few voxels per bundle are available for analysis. On the other hand, atlas-based fractional anisotropy extraction could be strongly affected by the presence of chronic lesions where tissue is completely missing. However, fractional anisotropy values extracted in the left, unaffected hemisphere, which also demonstrated differences between chronic and ex-neglect patients, are of course unlikely to suffer from this potential problem.
For the present study, we adopted a clinical definition of neglect based on patients’ performance on commonly used paper-and-pencil tests, because our primary focus was on anatomical predictors of chronic neglect. Future research should enlarge the scope of longitudinal explorations to specific forms or components of neglect, such as neglect for the bodily or external space, motor-intentional forms of neglect, or the importance for chronicity of vestibular components, extinction and anosognosia. Finally, in the present study, patients underwent only one MRI scan acquisition in the chronic stage. Future studies should investigate possible changes in fractional anisotropy between the acute and chronic phases of neglect, and their potential relation with neglect persistence.
In conclusion, our results stress the importance of network-based integration, both within and across the hemispheres, to compensate for neglect signs. Attempts at improving cooperation within these networks should become a focus of translational research on this disabling condition.
Acknowledgements
We thank Bénédicte Batrancourt for help with MRI acquisition within the framework of the MRI database for the Centre for Cognitive Anatomy (CAC; http://bit.ly/1nJC6Er).
Funding
The research leading to these results has received funding from a PhD CIFRE grant with the Clinique les Trois Soleils to ML, an AP-HP translational research grant to PB, the ANR ‘Phenotypes’ ANR-13-JSV4-0001-01 to MTS and the programme ‘Investissements d’Avenir’ ANR-10-IAIHU-06. Part of the data on normal subjects was provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657), funded by the 16 NIH Institutes and Centres that support the NIH Blueprint for Neuroscience Research and by the McDonnell Centre for Systems Neuroscience at Washington University.
Supplementary material
Supplementary material is available at Brain online.
Abbreviations
- DTI
diffusion tensor imaging
- SLF
superior longitudinal fasciculus
- TBSS
tract-based spatial statistics