-
PDF
- Split View
-
Views
-
Cite
Cite
Arturo Vera-Ponce de León, Ernesto Ormeño-Orrillo, Shamayim T Ramírez-Puebla, Mónica Rosenblueth, Mauro Degli Esposti, Julio Martínez-Romero, Esperanza Martínez-Romero, Candidatus Dactylopiibacterium carminicum, a Nitrogen-Fixing Symbiont of Dactylopius Cochineal Insects (Hemiptera: Coccoidea: Dactylopiidae), Genome Biology and Evolution, Volume 9, Issue 9, September 2017, Pages 2237–2250, https://doi.org/10.1093/gbe/evx156
Close - Share Icon Share
Abstract
The domesticated carmine cochineal Dactylopius coccus (scale insect) has commercial value and has been used for more than 500 years for natural red pigment production. Besides the domesticated cochineal, other wild Dactylopius species such as Dactylopius opuntiae are found in the Americas, all feeding on nutrient poor sap from native cacti. To compensate nutritional deficiencies, many insects harbor symbiotic bacteria which provide essential amino acids or vitamins to their hosts. Here, we characterized a symbiont from the carmine cochineal insects, Candidatus Dactylopiibacterium carminicum (betaproteobacterium, Rhodocyclaceae family) and found it in D. coccus and in D. opuntiae ovaries by fluorescent in situ hybridization, suggesting maternal inheritance. Bacterial genomes recovered from metagenomic data derived from whole insects or tissues both from D. coccus and from D. opuntiae were around 3.6 Mb in size. Phylogenomics showed that dactylopiibacteria constituted a closely related clade neighbor to nitrogen fixing bacteria from soil or from various plants including rice and other grass endophytes. Metabolic capabilities were inferred from genomic analyses, showing a complete operon for nitrogen fixation, biosynthesis of amino acids and vitamins and putative traits of anaerobic or microoxic metabolism as well as genes for plant interaction. Dactylopiibacterium nif gene expression and acetylene reduction activity detecting nitrogen fixation were evidenced in D. coccus hemolymph and ovaries, in congruence with the endosymbiont fluorescent in situ hybridization location. Dactylopiibacterium symbionts may compensate for the nitrogen deficiency in the cochineal diet. In addition, this symbiont may provide essential amino acids, recycle uric acid, and increase the cochineal life span.
Introduction
The ecological success of insects may be related to their association with different microorganisms. It is estimated that insect microbiota constitute around 10% of host biomass (Douglas 2015). Bacteria and fungi may provide amino acids, pheromones, lipids, or vitamins to their hosts (Adams etal. 2009; Behmer etal. 2011; Douglas 2012, 2016). In other cases, bacteria may compete with pathogens or confer protection to insects against parasites (Xie etal. 2014). Some insects have specialized cells called bacteriocytes in which they harbor maternally inherited bacterial endosymbionts. Only rarely can endosymbionts be cultured in the laboratory (Dale and Maudlin 1999; Matthew etal. 2005; Dale etal. 2006). Consequently, the advent of genomics paved the way to detailed studies on these organisms. The first genome from an endosymbiont (that of Buchnera, the endosymbiont of aphids) was published by Shigenobu etal. (2000). Since then, many genomes have become available from endosymbionts belonging to different bacterial phyla (reviewed in McCutcheon and Moran 2011; Rosas-Pérez etal. 2014; Van Leuven etal. 2014; Martínez-Cano etal. 2015; Bennett etal. 2016). Typically, endosymbionts show a reduction in gene content, genome erosion, and high AT content, as well as over-representation of genes encoding essential functions for their insect host (McCutcheon and Moran 2011).
In most cases, insect gut bacteria are less diverse than mammalian gut bacteria (Degli Esposti and Martínez-Romero 2017). Among gut bacteria, some diazotrophs (nitrogen-fixing bacteria) may alleviate insect nitrogen deficiencies and compensate carbon to nitrogen unbalance. Spirochetes fix nitrogen in termite guts (Ohkuma etal. 1999; Desai and Brune 2012). In ant (van Borm etal. 2002), fruit fly (Behar etal. 2005), cockroach (Tai etal. 2016), and some beetle (Morales-Jiménez etal. 2009, 2013) guts, there are Klebsiella, Kluyvera, and Roultella (gammaproteobacteria) as nitrogen-fixing symbionts (van Borm etal. 2002; Behar etal. 2005; Morales-Jiménez etal. 2009, 2013; Pinto-Tomás etal. 2009). However, diazotrophs have not been reported in hemipterans such as Dactylopius cochineals. Besides nitrogen fixation, nitrogen recycling occurs in insects (Hirayama etal. 1999; Ulyshen 2015); for example, uric acid (UA) may be used by bacteria or fungi in Malpighian tubules (Sabree etal. 2009). Nitrogen recycling seems to occur in the carmine cochineals Dactylopius coccus and Dactylopius opuntiae as well (Vera-Ponce de León etal. 2016).
Dactylopius coccus is the producer of carmine pigment. This scale insect was domesticated by pre-Hispanic Americans and has considerable economic value for the production of natural pigments that are used for dyeing cosmetics, textiles and food (Perez-Guerra and Kosztarab 1992; Campana etal. 2015; Williams and Ben-Dov 2015). Female carmine cochineals spend their entire life on cactus plants and, like other sap-feeding insects, have nutritional deficiencies that may be compensated by their microbial symbionts. It is not unusual to find a diversity of symbiont species in scale insects (Rosenblueth etal. 2017), and this was the case in Dactylopius. At least five species of Dactylopius harbor a betaproteobacterium that we named Candidatus Dactylopiibacterium carminicum (Ramírez-Puebla etal. 2010), hereafter referred to as Dactylopiibacterium. This symbiont was found by a PCR-based approach, also in D. coccus eggs (Ramírez-Puebla etal. 2010). Cochineal insects additionally contain Wolbachia (Ramírez-Puebla etal. 2015, 2016), Spiroplasma (our unpublished results), fungi (Vera-Ponce de León etal. 2016) and proteobacteria commonly associated with plants (James etal. 1997; Kim etal. 1998; López-López etal. 2013), such as Herbaspirillum, Mesorhizobium, Massilia, and Sphingomonas (Ramírez-Puebla etal. 2010). However, the latter were absent in D. coccus and unevenly found in individual cochineals of a few wild species, thereby suggesting that they were transient gut symbionts acquired from the cactus sap.
Cactuses are very efficient in photosynthesis, but their sap is nitrogen-poor (Stintzing and Carle 2005). Thus, we wondered whether the carmine cochineal symbiont Dactylopiibacterium, which is phylogenetically related to diazotrophs such as Azoarcus (Ramírez-Puebla etal. 2010), could fix nitrogen to compensate for the nutritional deficiencies in the cochineals feeding solely on cactus sap. The aim of this study was to analyze the genome sequence and nitrogen-fixing capabilities of Dactylopiibacterium and test its location in cochineal reproductive tissues to assess its maternal transmission.
Materials and Methods
Fluorescent In Situ Hybridization
To identify Dactylopiibacterium location inside the carmine cochineal, fluorescent in situ hybridization (FISH) was performed as described by Koga etal. (2009) with modifications. Adult D. coccus and D. opuntiae females from different life stages (30 and 80 days old) were detached from the host plant and their ovaries were dissected as described (Vera-Ponce de León etal. 2016). Insect tissues were fixed with Carnoy‘s solution and autofluorescence was quenched with 6% H2O2 for 7 days. The designed oligonucleotide fluorescent probes were Cy5-END1-390 (5′-Cy5-AAACCATTTCTTTCCGACTG-3′) that is specific for Dactylopiibacterium and Cy5-END1-1081 (5′-Cy5-CTTGCGTAGCAACTAATGATAAGG-3′) that matches many betaproteobacterial 16S rRNA genes but not those of Herbaspirillum and Massilia (betaproteobacteria) that were found inside the carmine cochineals D. ceylonicus and D. confusus (Ramírez-Puebla etal. 2010). FISH was performed adding the probe (100 nM) diluted with hybridization buffer [Tris–HCl (20 mM); NaCl (180 mM); SDS 10%; formamide 30%] directly to dissected guts and ovaries; slides were incubated overnight at 28°C. After washing with PBS-Tween (0.02%, 40°C for 15 min), the samples were stained with DAPI (24 µg l−1, 15 min) and mounted with citifluor antifade solution (Ted Pella, Inc.). Control experiments were performed without a probe or with RNAse for 45 min. The samples were analyzed under an Olympus FV100 Multi-photonic confocal microscope. Images were processed using FIJI 2.0.0 software (Schindelin etal. 2012).
Genome Sequencing and Metagenomic Binning
Sequencing of the metagenomes from D. coccus was undertaken with different platforms, namely PacBio, pyrosequencing 454 GS-FLX and Illumina HiSeq2000. Illumina and 454 library preparations and sequencing were performed at Macrogen Inc. (Korea) and PacBio at Duke University Genome Sequencing Core Facility (USA). Cells from hemolymph were used for DNA extraction for the PacBio and 454 metagenomic sequencing from 8 and 20 females, respectively. For the third metagenome, obtained with Illumina, DNA was recovered from macerated organs including ovaries, guts and Malpighian tubules from 40 females. Details of the dissection and extraction procedure were as described in Ramírez-Puebla etal. (2016) and Vera-Ponce de León etal. (2016). The SMRT cell from PacBio resulted in a total of 86,880 reads (of 9,627 bp each on average), 454 GS-FLX sequence yielded 811,305 single reads (of 330 bp each on average) and Illumina 58,146,564 reads (pair-end reads of 100 bp). The Dactylopiibacterium whose genome was obtained from the female D. coccus metagenomes was designated NFE1. In addition, two more metagenomes of 50 D. coccus males and of ten females of the wild species D. opuntiae were obtained from whole adult macerates; the dactylopiibacteria whose genomes were obtained from these metagenomes were named NFDCM and NFDO, respectively. Metagenomes were sequenced in Macrogen Inc. (Korea) using Illumina HiSeq 2500 plataform with the TruSeq DNA-PCR free Library kit. D. coccus male and D. opuntiae female metagenomes yielded 251,932,764 and 276,841,126 (pair-end reads of 100 bp), respectively. A metagenomic hybrid assembly from D. coccus female metagenomes was performed with Spades v. 3.7 (Bankevich etal. 2012) using Illumina HiSeq 100 bp pair-end reads, FLX-454 single reads and PacBio long reads, previously reported and deposited in GenBank under BioProject PRJNA349489 (Vera-Ponce de León etal. 2016). The resulting assembly, consisting of 332,915,694 contigs, was binned using MaxBin 2.0 (Wu etal. 2016). A set of four bins was recovered. For taxonomic positioning, Metaxa 2.0 (Bengtsson-Palme etal. 2015) was used for locating the small subunit of ribosomal genes in each bin. To refine the bin containing the Dactylopiibacterium genome, all contigs were blasted against nonredundant (nr) GenBank database using BLAST-X and those contigs annotated as betaproteobacteria with a higher identity value than 90% and e-value <1e−5 in BLAST results were retained in the final assembly. CheckM v1.0.7 (Parks etal. 2015) was used for further assessing bin quality and to discard possible chimeric contamination.
Scaffolding was performed using the resequencing and hybrid-assembly scaffolding protocol on SMRT Portal with PacBio Reads. A subsequent alignment was performed using pair-end Illumina data with Bowtie2 (Langmed and Salzberg 2012) with--very sensitive parameter. The previous scaffold and mapped reads were assembled with Spades using--trusted contigs option. SSPACE (Boetzer etal. 2011) was then used for final scaffold polish with bwa as aligner and Ciclator 1.4.1 (Hunt etal. 2015) was used to find possible circular scaffolds. All reads from D. coccus male and D. opuntiae female metagenomes were mapped against the Dactylopiibacterium female genome that we previously assembled by Bowtie2 (Langmead and Salzberg 2012) with--very-sensitive local option. Properly mapped pair reads were recovered using Samtools utilities (Li etal. 2009). Reads from each metagenome were assembled into contigs with IDBA-UD assembler (Peng etal. 2012) with a k-mer increment of each iteration of 15 (–step arg 15). Scaffolds of the contigs were recovered by SSPACE as above.
Average genome coverage was estimated with coveragebed from bedtools (Quinlan and Hall 2010) using all reads of each metagenome mapped to the final Dactylopiibacterium assembly with Bowtie2 using the--very-sensitive local option.
Dactylopiibacterium Genome Analysis
The RAST server (Meyer etal. 2008) and PROKKA (Seemann 2014) were used for gene prediction and annotation. Manual curation of relevant genes was performed after comparison with sequences deposited in the following databases: nr and Refseq via BLASTP (Camacho etal. 2009), the Conserved Domain Database at GenBank (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), the Protein families (PFAM) database (Finn etal. 2016) and the Transport Classification Database (Saier etal. 2016). In addition, a pathway profiling of metabolic annotation was obtained from all putative coding genes predicted using GhostKoala tool from KEGG (Kanehisa etal. 2016). Manual annotation was further refined using SmartBLAST of the deduced proteins and their hydropathy profile analyzed with the online service of Swissprot TMpred (http://www.ch.embnet.org/cgi-bin/TMPRED_form_parser) as previously described (Degli Esposti etal. 2014). Genome completeness was assessed by the presence of 40 single-copy bacterial widespread orthologue proteins with BUSCO (Simão etal. 2015) and CheckM (Parks etal. 2015). In addition, completeness was assessed by determining that all genes from the Rhodocyclaceae core genome were present in the Dactylopiibacterium genome (see below). Prophage sequences were identified, annotated and visualized by PAHAST and PHASTER tools (Zhou etal. 2011; Arndt etal. 2016). Genome sequences were deposited at DDBJ/ENA/GenBank under accession numbers MQNN00000000, NMRM00000000, and NMRN00000000.
Phylogenetics, Phylogenomics, and Comparative Analyses
16S rRNA sequences were identified from genome contigs using the Metaxa2 pipeline (Bengtsson-Palme etal. 2015). Sequences were aligned using ClustalX 2.0 (Larkin etal. 2007) with other 16S rRNA sequences from GenBank. A phylogenetic tree was generated from a maximum likelihood analysis using PhyML 3.1 (Guindon etal. 2010). JModelTest 2.1.10 (Posada 2008) was used to select appropriate models of sequence evolution by the AIC. Model K80 + I (P-inv = 0.772) was the chosen model for the 16S rRNA genes.
For comparative genomics, a set of 31 Rhodocyclaceae genomes were retrieved from the NCBI genome database (https://www.ncbi.nlm.nih.gov/genome/). Open reading frames and coding sequences (CDS) of all genomes were predicted using GeneMark (Besemer and Borodovsky 2005). The pangenome and core genome of all strains were obtained by GETHOMOLOGUES version 2.0 software (Contreras-Moreira and Vinuesa 2013), with -A -c -t 0 -M -n 35 parameters. Single copy orthologous genes from the core genome-matrix were aligned with Clustal Omega version 1.2.1 (Sievers etal. 2011) and Prottest3 version 3.4.2 (Darriba etal. 2011) was used to select the best amino acid substitution model. A maximum likelihood phylogeny tree was constructed using PhyML 3.1 (Guindon etal. 2010) with the WAG model and the Shimodaira–Hasegawa like procedure for measuring internal branch support (Shimodaira and Hasegawa 1999).
Average amino acid identity (AAI) between Dactylopiibacterium genome and other genomes was calculated using all core genes with AAI calculator enveomics collection tools (Rodriguez-R and Konstantinidis 2016). In addition, eight selected genomes were aligned with Nucmer and plotted with Mummer plot (Delcher etal. 2002) to determine syntenic blocks of genes between Dactylopiibacterium and other Rhodocyclaceae.
To generate a nifHDK phylogeny, Dactylopiibacterium CDS predicted and annotated as nitrogenase-iron protein, nitrogenase molybdenum–iron protein alpha chain and nitrogenase molybdenum-iron protein subunit beta were aligned to sequences of the corresponding genes from other bacteria deposited in GenBank using Clustal Omega (Sievers etal. 2011). The concatenated alignments of the three nif genes were generated in BioEdit version 7.2.5 (Hall 1999) and a maximum likelihood phylogenetic tree was obtained as described above using the LG + I+G + F model (α = 0.836 for gamma distribution; P-inv = 0.225).
In addition, to recover the nitrogenase genes from two available D. coccus metagenomes obtained elsewhere from the whole body of cochineals from Oaxaca and Peru (hereafter called DCoax and DCperu metagenomes, respectively, deposited in GenBank under BioProject PRJNA244295, Campana etal. 2015), all reads greater than 100 bp were retrieved from GenBank fastq records and amino acid translations of these sequences were obtained using FragGeneScan v 1.20 (Rho etal. 2010). Nitrogenases genes (nifH and nifD) from the Campana etal. (2015) filtrated metagenome were searched by BLASTP against Dactylopiibacterium nif genes.
Dactylopiibacterium nifH Gene Expression in D. coccus
To detect expression of nif genes we used reverse transcription of RNA extracted from ten adult cochineal females (∼90 days old), previously externally disinfected with 70% ethanol. Hemolymph, guts and ovaries from D. coccus were dissected as described (Vera-Ponce de León etal. 2016) and placed separately in 200 µl of RNAlater® Solution (Life Technologies, Carlsbad, CA, USA). Samples were centrifuged 1 min at 9,300 × g. RNAlater® was removed and RNA was isolated with TRIzol reagent (Invitrogen) following the manufacturer’s instructions, digested with DNAse and cleaned with the RNeasy column kit (Qiagen, Germantown, MD, USA). RNA purity and integrity was checked by gel electrophoresis and absorbance ratios (260/280 and 260/230). cDNA was synthesized for RT-PCR from purified RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. cDNA was used for PCR amplification with 0.8 pmol of primers NiFHBeta-9 (5′-AACGTCAATGCGCAATTTACG-3′) and NifHBeta-805 (5′-ATGATGCCGAACTCCATCAG-3′) (this study). The final concentration for 50 µl PCR was 0.2 mM dNTPs, 2.5 mM MgCl2, 0.5 U Taq polymerase and 1× Taq polymerase buffer (Invitrogen Life Technologies, Sao Paulo, Brazil). The cycling conditions were 94°C for 5 min; 35 cycles of 60 s at 94°C, 60 s at 58°C, and 90 s at 72°C and final extension at 72°C for 10 min. PCR products were purified using the High Pure PCR Product Purification Kit (Roche) and sequenced by Macrogen Inc. (Seoul, Korea). Concentrated RNAs were used as negative controls and genomic DNA of D. coccus was used as positive control.
Assay for Nitrogen Fixation
To biochemically evaluate nitrogen fixation, we used the conventional acetylene reduction assays with different tissues of D. coccus. Five adult females (∼60 days old) were detached from the host plant and rinsed in 100% ethanol for wax removal. Insects were then washed twice with sterile deionized water and placed in sterile PBS. 1 µl of hemolymph from each individual was obtained by making a fine puncture in the third segment of the insect, the oozing fluid was collected with a micropipette and placed in 10 ml vessels with 5 ml of semisolid nitrogen-free medium (KH2P04 1.1 g l−1; K2HPO4 2.2 g l−1; MgSO4 0.2 g l−1; NaCl 0.1 g l−1; CaCl2 0.05 g l−1.; FeSO4 0.05 g l−1; Na2MoO4 0.002 g l−1; agar 3 g l−1; fructose 0.25 g l−1; sucrose 0.15 g l−1, and trehalose 0.1 g l−1 and 1.0 ml of a vitamin solution (10 mg of biotin and 20 mg of pyridoxin hydrochloride in 100 ml of distilled water). Ovaries from the same individual were dissected as described earlier (Vera-Ponce de León etal. 2016) and were submerged in the media. Samples were incubated at 28°C for 48 h and acetylene-enriched atmosphere was injected to achieve a final concentration of 10% acetylene. Media with autoclaved organs (121°C for 15 min at 15 psi) were used as negative controls, while positive controls were cultures of Klebsiella variicola 6A3 (Rosenblueth etal. 2004). Ethylene formed by nitrogenase was measured as described by Morales-Jiménez etal. (2013) in five replicates for each treatment. The normal distribution of the results was tested using the Shapiro–Wilk test. Differences in ethylene production with respect to sterile controls were analyzed by planned pair comparisons using the Mann–Whitney test. Statistical analysis was performed with the GraphPad prism v. 7.2 software.
Results
Dactylopiibacterium in Carmine Cochineal Ovaries
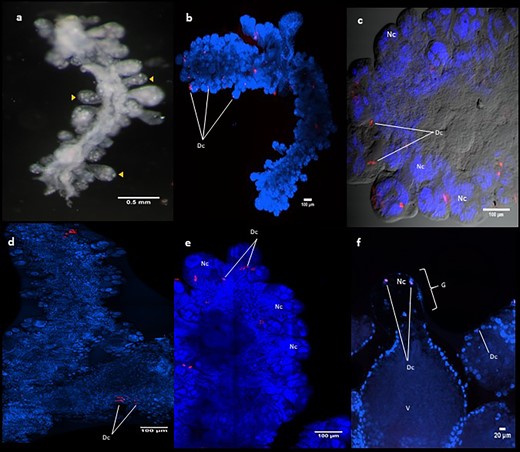
Dactylopius coccus and D. opuntiae cochineals are shown in figure 1. To verify the presence of Dactylopiibacterium in the insects we performed FISH experiments and fluorescent signals were detected in ovaries from the two tested species (fig. 2). Two probes were used, one specific for Dactylopiibacterium and the other specific to betaproteobacteria, which gave similar results. RNAse treated samples were devoid of signals. Probe signal was frequently observed in ovarioles, in some cases in their distal part (fig. 2b and d) and magnification showed signals inside nurse cells of the germarium either in 30- or 80-day-old insects (fig. 2c, e, and f).
—Dorsal view of adult Dactylopius with wax removed with 96% ethanol. (a) D. coccus, (b) D. opuntiae.
—(a) Ovary dissected from 30-day-old D. coccus nymph showing ovarioles in yellow arrowheads. (b–f) FISH using the designed probe Cy5-END1-1081, red and blue signals indicate Dactylopiibacterium and insect nuclei, respectively. (b, c) 30-day-old D. coccus ovaries, (d, e) 30-day-old D. opuntiae ovaries, (f) 80-day-old D. coccus ovariole and germarium. Nc, nurse cell; V, vitellarium, G, germarium, Dc, Dactylopiibacterium.
Genome Analysis of Dactylopiibacterium
The genome of the new endosymbiont Dactylopiibacterium was obtained from metagenomic data. One bin obtained from D. coccus metagenomes, composed of 193 contigs and 3,694,534 assembled bases, showed the presence of a 16S rRNA gene sequence that was 99–100% identical to our previously reported sequences of the Dactylopius betaproteobacterium (supplementary fig. S1, Supplementary Material online) (Ramírez-Puebla etal. 2010). After BLAST annotation of all sequences obtained, we found that 122 contigs of this bin, summing ca. 3.6 Mb (table 1), have betaproteobacterial sequences as their closest matches. Quality check of the 122 contigs by CheckM showed no contaminant sequences. The combined contigs represented a genome with a size of 3,649,266 bases, which was covered 18×, 1×, and 2× times with Illumina, 454-FLX and PacBio metagenomic reads, respectively. Additional Dactylopiibacterim genomes (with 21× and 23× coverage) were recovered from D. coccus male and D. opuntiae female metagenomes in 136 and 150 contigs, respectively. Genome statistics are shown in table 1.
Genome Features of Dactylopiibacterium from D. coccus and D. opuntiae
| Feature . | D. coccus Females (NFE1) . | D. coccus Males (NFDCM) . | D. opuntiae Females (NFDO) . |
|---|---|---|---|
| Genome accession | MQNN00000000 | NMRN00000000 | NMRM00000000 |
| Number of contigs | 122 | 136 | 150 |
| Number of scaffolds | 107 | 120 | 124 |
| N50 (bp) | 50,206 | 44,208 | 42,791 |
| Estimated genome size (bp) | 3, 589, 384 | 3,578,638 | 3,550,510 |
| G+C content (%) | 62.74 | 62.74 | 62.73 |
| CDS | 3,488 | 3,421 | 3,396 |
| rRNA genes | 3 | 3 | 3 |
| tRNAs | 46 | 46 | 47 |
| Pseudogenes | 248 | 202 | 216 |
| Genome completeness | 100% | 97% | 97% |
| Feature . | D. coccus Females (NFE1) . | D. coccus Males (NFDCM) . | D. opuntiae Females (NFDO) . |
|---|---|---|---|
| Genome accession | MQNN00000000 | NMRN00000000 | NMRM00000000 |
| Number of contigs | 122 | 136 | 150 |
| Number of scaffolds | 107 | 120 | 124 |
| N50 (bp) | 50,206 | 44,208 | 42,791 |
| Estimated genome size (bp) | 3, 589, 384 | 3,578,638 | 3,550,510 |
| G+C content (%) | 62.74 | 62.74 | 62.73 |
| CDS | 3,488 | 3,421 | 3,396 |
| rRNA genes | 3 | 3 | 3 |
| tRNAs | 46 | 46 | 47 |
| Pseudogenes | 248 | 202 | 216 |
| Genome completeness | 100% | 97% | 97% |
Genome Features of Dactylopiibacterium from D. coccus and D. opuntiae
| Feature . | D. coccus Females (NFE1) . | D. coccus Males (NFDCM) . | D. opuntiae Females (NFDO) . |
|---|---|---|---|
| Genome accession | MQNN00000000 | NMRN00000000 | NMRM00000000 |
| Number of contigs | 122 | 136 | 150 |
| Number of scaffolds | 107 | 120 | 124 |
| N50 (bp) | 50,206 | 44,208 | 42,791 |
| Estimated genome size (bp) | 3, 589, 384 | 3,578,638 | 3,550,510 |
| G+C content (%) | 62.74 | 62.74 | 62.73 |
| CDS | 3,488 | 3,421 | 3,396 |
| rRNA genes | 3 | 3 | 3 |
| tRNAs | 46 | 46 | 47 |
| Pseudogenes | 248 | 202 | 216 |
| Genome completeness | 100% | 97% | 97% |
| Feature . | D. coccus Females (NFE1) . | D. coccus Males (NFDCM) . | D. opuntiae Females (NFDO) . |
|---|---|---|---|
| Genome accession | MQNN00000000 | NMRN00000000 | NMRM00000000 |
| Number of contigs | 122 | 136 | 150 |
| Number of scaffolds | 107 | 120 | 124 |
| N50 (bp) | 50,206 | 44,208 | 42,791 |
| Estimated genome size (bp) | 3, 589, 384 | 3,578,638 | 3,550,510 |
| G+C content (%) | 62.74 | 62.74 | 62.73 |
| CDS | 3,488 | 3,421 | 3,396 |
| rRNA genes | 3 | 3 | 3 |
| tRNAs | 46 | 46 | 47 |
| Pseudogenes | 248 | 202 | 216 |
| Genome completeness | 100% | 97% | 97% |
The program BUSCO (Simão etal. 2015) calculated a genome completeness of 97–100%, indicating that the recovered contigs could represent most of the Dactylopiibacterium genome. Further analyses indicated that Dactylopiibacterium genomes contained the core genome of the Rhodocyclaceae family (29 strains considered) comprising 293 single copy genes (supplementary table S1, Supplementary Material online), further supporting genome completeness. In total, the Dactylopiibacterium genome encoded around 3500 CDS genes, 79% of which could be assigned to a known putative function (tables 1 and 2, supplementary table S2, Supplementary Material online). After scaffolding, a set of 107, 120, and 124 scaffolds were retrieved from Dactylopiibacterium genomes of D. coccus females, D. coccus males, and D. opuntiae females, respectively.
Comparison of Dactylopiibacterium Genome Features with Related Betaproteobacteria
| Bacterial Genome . | Dactylopiibacterium NFE1 . | Dactylopiibacterium NFDO . | U. gangwonense . | Azoarcus sp. BH72 . | Candidatus Tremblaya princeps . | Candidatus Zinderia insecticola . | P. rhizoxinica . |
|---|---|---|---|---|---|---|---|
| Accession number | MQNN00000000 | NMRM00000000 | GCA_000373965 | GCF_000061505 | GCF_000219195 | GCA_000147015 | GCA_000198775 |
| Size (Mb) | 3.6 | 3.5 | 5 | 3.9 | 0.139 | 0.209 | 3.8 |
| G+C (%) | 62.74 | 62.73 | 56.57 | 67.92 | 58.83 | 13.54 | 60.7 |
| CDSs | 3,488 | 3,396 | 4,717 | 3,992 | 140 | 202 | 3,878 |
| Host | Domesticated carmine cochineal (D. coccus) | Nondomesticated carmine cochineal (D. opuntiae) | None | Kallar grass (Leptochloa fusca) | Mealybug (Planococcus citri) | Spittlebug (Clastoptera arizonana) | Zygomycete (Rhizopus microsporus) |
| Life style | Insect endosymbiont | Insect endosymbiont | Free-living | Grass endophyte | Insect endosymbiont | Insect endosymbiont | Fungi endosymbiont |
| Diazotroph | Yes | Yes | Yes | Yes | No | No | No |
| Bacterial Genome . | Dactylopiibacterium NFE1 . | Dactylopiibacterium NFDO . | U. gangwonense . | Azoarcus sp. BH72 . | Candidatus Tremblaya princeps . | Candidatus Zinderia insecticola . | P. rhizoxinica . |
|---|---|---|---|---|---|---|---|
| Accession number | MQNN00000000 | NMRM00000000 | GCA_000373965 | GCF_000061505 | GCF_000219195 | GCA_000147015 | GCA_000198775 |
| Size (Mb) | 3.6 | 3.5 | 5 | 3.9 | 0.139 | 0.209 | 3.8 |
| G+C (%) | 62.74 | 62.73 | 56.57 | 67.92 | 58.83 | 13.54 | 60.7 |
| CDSs | 3,488 | 3,396 | 4,717 | 3,992 | 140 | 202 | 3,878 |
| Host | Domesticated carmine cochineal (D. coccus) | Nondomesticated carmine cochineal (D. opuntiae) | None | Kallar grass (Leptochloa fusca) | Mealybug (Planococcus citri) | Spittlebug (Clastoptera arizonana) | Zygomycete (Rhizopus microsporus) |
| Life style | Insect endosymbiont | Insect endosymbiont | Free-living | Grass endophyte | Insect endosymbiont | Insect endosymbiont | Fungi endosymbiont |
| Diazotroph | Yes | Yes | Yes | Yes | No | No | No |
Comparison of Dactylopiibacterium Genome Features with Related Betaproteobacteria
| Bacterial Genome . | Dactylopiibacterium NFE1 . | Dactylopiibacterium NFDO . | U. gangwonense . | Azoarcus sp. BH72 . | Candidatus Tremblaya princeps . | Candidatus Zinderia insecticola . | P. rhizoxinica . |
|---|---|---|---|---|---|---|---|
| Accession number | MQNN00000000 | NMRM00000000 | GCA_000373965 | GCF_000061505 | GCF_000219195 | GCA_000147015 | GCA_000198775 |
| Size (Mb) | 3.6 | 3.5 | 5 | 3.9 | 0.139 | 0.209 | 3.8 |
| G+C (%) | 62.74 | 62.73 | 56.57 | 67.92 | 58.83 | 13.54 | 60.7 |
| CDSs | 3,488 | 3,396 | 4,717 | 3,992 | 140 | 202 | 3,878 |
| Host | Domesticated carmine cochineal (D. coccus) | Nondomesticated carmine cochineal (D. opuntiae) | None | Kallar grass (Leptochloa fusca) | Mealybug (Planococcus citri) | Spittlebug (Clastoptera arizonana) | Zygomycete (Rhizopus microsporus) |
| Life style | Insect endosymbiont | Insect endosymbiont | Free-living | Grass endophyte | Insect endosymbiont | Insect endosymbiont | Fungi endosymbiont |
| Diazotroph | Yes | Yes | Yes | Yes | No | No | No |
| Bacterial Genome . | Dactylopiibacterium NFE1 . | Dactylopiibacterium NFDO . | U. gangwonense . | Azoarcus sp. BH72 . | Candidatus Tremblaya princeps . | Candidatus Zinderia insecticola . | P. rhizoxinica . |
|---|---|---|---|---|---|---|---|
| Accession number | MQNN00000000 | NMRM00000000 | GCA_000373965 | GCF_000061505 | GCF_000219195 | GCA_000147015 | GCA_000198775 |
| Size (Mb) | 3.6 | 3.5 | 5 | 3.9 | 0.139 | 0.209 | 3.8 |
| G+C (%) | 62.74 | 62.73 | 56.57 | 67.92 | 58.83 | 13.54 | 60.7 |
| CDSs | 3,488 | 3,396 | 4,717 | 3,992 | 140 | 202 | 3,878 |
| Host | Domesticated carmine cochineal (D. coccus) | Nondomesticated carmine cochineal (D. opuntiae) | None | Kallar grass (Leptochloa fusca) | Mealybug (Planococcus citri) | Spittlebug (Clastoptera arizonana) | Zygomycete (Rhizopus microsporus) |
| Life style | Insect endosymbiont | Insect endosymbiont | Free-living | Grass endophyte | Insect endosymbiont | Insect endosymbiont | Fungi endosymbiont |
| Diazotroph | Yes | Yes | Yes | Yes | No | No | No |
Phylogenomics and Comparative Genomics
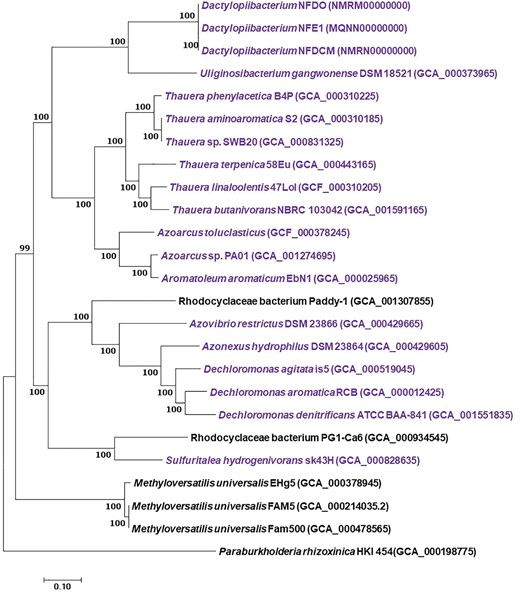
A phylogenetic analysis of 602 concatenated orthologous genes showed that Dactylopiibacterium is a new lineage most closely related to Uliginosibacterium gangwonense isolated from a wetland in Korea (Weon etal. 2008). Other Uliginosibacterium isolates with no available genomes are plant endophytes (Li etal. 2011, 2013). Both Uliginosibacterium and Dactylopiibacterium belong to the order Rhodocyclales and have Azoarcus (a rice endophyte) and Thauera as close genera (fig. 3). The AAI analysis showed that core genome sequences of Dactylopiibacterium and Uliginosibacterium have 75% identity, but Dactylopiibacterium has a smaller genome than that of U. gangwonense (Weon etal. 2008), which is ∼5 Mb in size (table 2). However, the latter has an unusual large genome compared to those from other Rhodocyclales. Most of U. gangwonense unique genetic material comprises transposable elements, prophages and a large plasmid (Weon etal. 2008) which was not found in the Dactylopiibacterium genome.
—Maximum likelihood tree (−ln L = −1403085.372840) of 602 orthologous of Dactylopiibacterium and other Rhodocyclaceae genomes. The genome of Paraburkholderia rhizoxinica was used as an outgroup. Scale bar indicates 10% estimated sequence divergence. SH-like support values ≥50% are indicated. In purple are genomes with nitrogen-fixing genes.
Comparative genomics applied to Dactylopiibacterium and close Rhodocyclaceae bacteria indicated that the core genome contains genes for nitrogen fixation and regulation, among others (supplementary table S1, Supplementary Material online). Specifically, 973 Dactylopiibacterium genes from NFE1 were not found in the closest relative U. gangwonense. Some of these genes encoded for flagella synthesis, pectin and polygalacturonic catabolism and urea metabolism (supplementary table S3, Supplementary Material online).
Dactylopiibacterium was not phylogenetically related to endosymbionts from other families of betaproteobacteria such as those found in mealybugs (López-Madrigal etal. 2011), spittlebugs (McCutcheon and Moran 2011), leafhoppers (Bennett etal. 2016), and in the crypts of stinkbugs (Kikuchi etal. 2012).
Metabolic Capabilities and Other Dactylopiibacterium Characteristics Inferred from Genomic Data
The genome of Dactylopiibacterium encoded complete metabolic pathways for the biosynthesis of biotin, thiamine, ubiquinone, cobalamine, heme, coenzyme B12, riboflavin, pyridoxine, NAD(P), folate, lipoic, acid and coenzyme A (supplementary table S2, Supplementary Material online). Moreover, it encoded complete pathways for the biosynthesis of all amino acids except asparagine. However, the genome encoded for an Aspartyl-tRNA(Asn) synthetase, thereby suggesting the ability to produce charged asparaginyl-tRNA from aspartyl-tRNA (supplementary table S2, Supplementary Material online). While the pathways for the biosynthesis of purines and pyrimidines were present, key enzymes for glycolysis and gluconeogenesis appeared to be absent. The pentose phosphate and Entner–Doudoroff pathways were instead fully represented, thus providing alternative routes for carbon metabolism. All genes of the tricarboxylic acid cycle were also present (supplementary table S2, Supplementary Material online). Intriguingly, all the three Dactylopiibacterium genomes that we report here, had genes for polygalacturonase enzymes, which are absent in the genomes of other members of the Rhodocyclaceae family (supplementary tables S1–S3, Supplementary Material online).
Anaerobic Metabolism in Dactylopiibacterium
The new endosymbiont Dactylopiibacterium appears to have distinctive features of anaerobic metabolism. For instance, its genome contains the genes for pyruvate formate-lyase and lactate dehydrogenase, suggesting that the endosymbiont could perform fermentation reactions (supplementary table S1, Supplementary Material online). Other genes associated with anaerobic metabolism include those for nitrite respiration to nitrous oxide (supplementary table S1, Supplementary Material online), as well as two terminal oxidases which are active under very low concentrations of oxygen: the bd-type ubiquinol oxidase and the cbb3-type cytochrome c oxidase (supplementary table S2, Supplementary Material online) (Degli Esposti etal. 2014). Both of these oxidases are over-expressed in proteobacteria that fix N2 in symbiosis with plants (Degli Esposti and Martínez Romero 2016). Conversely, only a few, noncatalytic genes for the subunits of COX operons encoding cytochrome c oxidase were found (supplementary table S2, Supplementary Material online). Nitrite reductase and lactate dehydrogenase are present among the 973 Dactylopiibacterium unique genes but absent in the core genome of other Rhodocyclaceae (supplementary tables S2 and S3, Supplementary Material online).
The genome also includes the entire operon of fumarate reductase (supplementary table S2, Supplementary Material online), an enzyme that recycles the reduced membrane quinones that are produced by complex I and other dehydrogenases and is typically present in facultative anaerobic bacteria such as Escherichia coli. This anaerobic trait is present in other members of the Rhodocyclaceae family such as Azoarcus (Degli Esposti and Martínez Romero 2017).
Cell Surface and Secretion Systems
Inspection of genes related to membrane and cell envelope biosynthesis indicated that Dactylopiibacterium could produce membranes with phosphatidylglycerol, phosphatidylserine, phosphatidylethanolamine, and cardiolipin but not phosphatidylcholine (supplementary table S2, Supplementary Material online). Its genome encodes genes for lipopolysaccharide biosynthesis, including the O-antigen, a normal peptidoglycan layer and some form of exo- or capsular polysaccharide. Dactylopiibacterium was deduced to be potentially motile because its genome has two sets of genes for the flagellar machinery (supplementary tables S2 and S3, Supplementary Material online) and one gene set for chemotaxis. A set of eight genes that encode FlhA, FlhB, FliH, FliI, FliO, FliP, FliQ, and FliR components of the flagellum-specific protein export apparatus and for type IV pilus biogenesis were also found (supplementary tables S2 and S3, Supplementary Material online).
Secretion systems are present in this genome, including tra/trb genes for the mating-pair formation apparatus of a type IV secretion system (T4SS) (supplementary table S2, Supplementary Material online), which are closely related to those found in the Integrative Conjugative Elements inserted in the chromosomes of beta and gammaproteobacteria (Burrus and Waldor 2004). Dactylopiibacterium genome also contains genes for type I and II secretion systems (supplementary table S2, Supplementary Material online).
Prophages
Analysis by PHASTER identified at least five prophage regions (scaffolds) in the Dactylopiibacterium genome from D. coccus. Two of these regions encoded intact phage sequences. Prophages showed >90% similarity to Burkholderia phage BcepMu and Xylella phage Xfas53. Other incomplete prophages were similar to Bacillus phage G, Burkholderia phage KL1 and Pseudomonas phage LPB1. Moreover, at least three scaffolds in Dactylopiibacterium genome from D. opuntiae were similar to phages sequences.
Nitrogen Metabolism in Carmine Cochineals
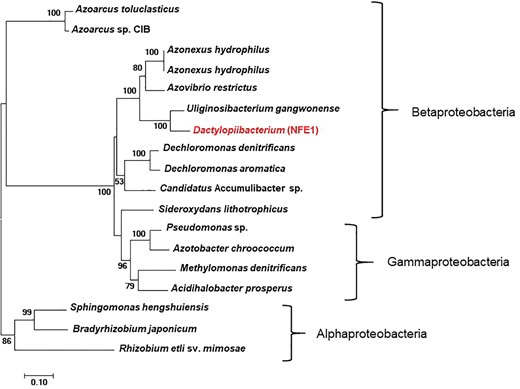
We found that the Dactylopiibacterium genomes contain nitrogenase genes that were phylogenetically related to those of betaproteobacteria (fig. 4), in particular, they were most closely related to Uliginosibacterium nif genes either in concatenated nifHDK (fig. 4) or in individual nif gene phylogenies (supplementary fig. S2, Supplementary Material online). nif gene phylogenies showing Uliginosibacterium as the closest relative to Dactylopiibacterium were congruent with whole core gene phylogenies (figs. 3 and 4). The genes encoding for Mo-Fe nitrogenase (nifD–nifK), nitrogenase reductase (nifH), nitrogen regulatory proteins and transporters specific for nitrogenase cofactors (nifQ–nifA and 4Fe–4S ferredoxin) were found assembled into four contigs of Dactylopiibacterium. In addition, we recovered a nifH gene sequence from the DCoax metagenome and a nifD gene sequence from the DCperu metagenome (Campana etal. 2015) and their phylogenetic analysis indicated that they belonged to Dactylopiibacterium (supplementary fig. S3, Supplementary Material online). No other nif gene sequences were found in either our samples or in DCoax or DCperu metagenomes.
—Maximum likelihood tree (−ln L = −16717.43208) of concatenated nifHDK genes of Dactylopiibacterium and other related sequences from GenBank. Scale bar indicates 10% estimated sequence divergence. SH-like support values ≥50% are indicated.
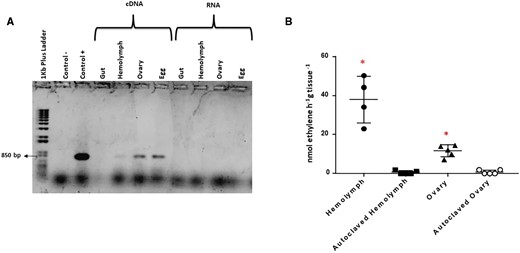
RT-PCR showed expression of Dactylopiibacterium nifH gene in D. coccus hemolymph, ovaries, and eggs, but not in guts (fig. 5a). Accordingly, acetylene reduction activity, the standard assay for nitrogen fixation, was detected in hemolymph and ovaries of D. coccus (38 ± 5.98 and 11.58 ± 1.38 nmol ethylene h−1 tissue g−1, respectively). These values were statistically significant when compared with those obtained in autoclaved controls of the same tissues (Mann–Whitney test, fig. 5b).
—(a) Amplicons of RT-PCR from Dactylopiibacterium nifH transcript. D. coccus total DNA as positive control and no-DNA template as negative control. (b) Acetylene reduction activity detected in D. coccus tissues. Bar indicates media ± SEM. Red asterisks show significant differences between tissues and autoclaved controls and nonsterile tissues (Mann–Whitney test P < 0.05).
The endosymbiont genome showed UA recycling genes. UA is the main waste compound of nitrogen catabolism in D. coccus (Vera-Ponce de León etal. 2016). The genome of Dactylopiibacterium had genes for nitrogen recycling of purine waste products and UA degradation to urea. All genes for urea metabolism and transport of its metabolites were predicted and annotated (supplementary table S2, Supplementary Material online).
Discussion
The carmine cochineal was the red gold of the Americas and there is still a large economic interest in carmine even today. Approaches are being considered to produce carmine using novel biotechnological procedures. For a long time, it has been hypothesized that microbes could be involved in carmine production (reviewed in Margulis 2010). To study carmine cochineal microbiota, we undertook a 16S rRNA gene diversity analysis using different Mexican Dactylopius species including the domesticated D. coccus and the wild species D. ceylonicus, D. confusus, D. opuntiae, and D. tomentosus (Ramírez-Puebla etal. 2010). In all the cochineals studied, we uncovered a novel betaproteobacterial 16S rRNA gene sequence and we designated the putative novel symbiont as Candidatus Dactylopiibacterium carminicum. Here, we expanded the knowledge on dactylopiibacteria showing their location inside the host, genome sequences, putative metabolic traits, and nitrogen-fixing capabilities.
Until now we have been unable to grow Dactylopiibacterium in culture media in the laboratory. Notably, the betaproteobacterium Azoarcus which is phylogenetically not distantly related to Dactylopiibacterium, attains a nonculturable state in plants (Hurek etal. 2002) and a similar situation may occur in the carmine cochineals with Dactylopiibacterium. Consequently, we recovered its genome from different metagenomes obtained from tissues of female D. coccus, from whole male cochineals and from female D. opuntiae. The Dactylopiibacterium genomes (3.6 and 3.5 Mb from D. coccus and D. opuntiae, respectively) turned out to be larger than those of other endosymbionts (ranging from 0.1 to 2 Mb, table 2). However, the genome was comparable in size to those of gammaproteobacterial symbionts such as Sodalis or Arsenophonus which are maternally transmitted and considered as secondary (Gil etal. 2008; Oakeson etal. 2014) or primary endosymbionts in some insects (Oakeson etal. 2014). Sodalis was considered as an in statu nascendi symbiont in the insect Cicadella viridis (Michalik etal. 2014) and different Sodalis species or strains have variable genome sizes (reviewed in Rosas-Pérez etal. 2017) that may be up to 4.5 Mb in Sodalis pierantonius (Oakeson etal. 2014).
In view of the large Dactylopiibacterium genomes and similarity, we suggest that dactylopiibacteria may have been recently acquired without much time to evolve reduced or divergent genomes. However, considering that the symbiont was found in all Dactylopius species tested, Dactylopiibacterium symbiosis was probably established before Dactylopius cochineal speciation, unless it is periodically obtained from plants. Recurrent plant acquisition would be not required if the symbiont is maternally transmitted by cochineals to their progeny.
Maternal transmission of bacteria is commonly found in insects, especially with primary endosymbionts that have largely reduced genomes and complete host dependence (McCutcheon and Moran 2011). Vertical inheritance suggests that there is a benefit from the symbiont to the host as the latter is committed to retain the bacterium for future generations. For maternal inheritance, it seems that only few symbionts would be needed to be transferred to the progeny. Bacteria in germarium and in nurse cells have been found in other scale insects like Pseudococcus and Icerya (Shinji 1919), as well as in D. coccus (Ramírez-Cruz 2012). The presence of Dactylopiibacterium in ovaries and eggs in the carmine cochineal (though in low numbers) does suggest maternal transmission, while the expression of nif genes and nitrogenase activity in ovary constitute distinctive characteristics of this species. Since Dactylopiibacterium looks like a newly acquired symbiont and it is probably maternally inherited, we suggest that maternal inheritance is an early and key step to further evolve specific and highly adapted symbionts. In other endosymbionts, for maternal transmission, flagella and type IV pili are essential for the colonization of host embryos (Dulla etal. 2012; Wang etal. 2013). Indeed, we found these systems encoded in the genome of Dactylopiibacterium, suggesting they may participate in the bacterial colonization of ovaries and eggs, as well as in exporting molecules into the cochineal host mediating its symbiotic interactions, as reported for some parasitic or free-living bacteria (Macnab, 2003; Zhou etal. 2015).
A major finding of this study is that bacterial endosymbionts of cochineal insects have the capacity to fix nitrogen, thereby identifying Dactylopiibacterium as a novel nitrogen fixing symbiont. The only nif gene sequences found in all carmine cochineal metagenomes were those from Dactylopiibacterium and included all genes required for nitrogen fixation. Nitrogen-fixing bacteria previously reported to be associated with insects such as ants, bark beetles and fruit flies, are gut symbionts (van Borm etal. 2002; Behar etal. 2005; Pinto-Tomás etal. 2009; Morales-Jiménez etal. 2009, 2013; Desai and Brune 2012). Only in the case of the wood-feeding insect Anoplophora glabripennis, nif genes, and transcripts from gut bacteria were also found in eggs (Ayayee etal. 2014). In D. coccus guts no Dactylopiibacterium nifH transcripts were detected by RT-PCR. This result and the very few Dactylopiibacterium transcripts found in guts (our unpublished transcriptome results) suggest that dactylopiibacteria are not principally gut symbionts. We further suggest that these bacteria are not directly and continuously acquired from the sap diet, since adult male Dactylopius cochineals contain Dactylopiibacterium even if they do not exhibit any feeding behavior since pupae.
Genomic analysis indicated that the symbiont is well adapted to a low oxygen life style that is compatible with nitrogen fixation, which is oxygen sensitive. Moreover, the presence of glutamine synthetase genes in the genome of the endosymbiont suggests that nitrogen fixation follows the transformation from NH3 to glutamine. Ammonium assimilation constitutes the first step towards the biosynthesis of amino acids. In scale insects, essential amino acids are commonly produced by flavobacteria and gammaproteobacteria (Rosenblueth etal. 2012; Rosas-Pérez etal. 2014; Skidmore and Hansen 2017). Since flavobacteria and enterobacteria are not found in Dactylopius, Dactylopiibacterium, which has all genes for essential amino acid biosynthesis, might in turn provide essential amino acids to the host. Genes for nonessential amino acids are not found in the aphid endosymbiont Buchnera, as nonessential amino acids are provided by the insect host. Dactylopiibacterium only lacks one of the biosynthetic genes for the nonessential amino acid, asparagine, which may well be provided by the insect host, or obtained from the plant sap. Alternatively, asparagine may be obtained within the endosymbiont by the conversion of asparagine-charged tRNAs as described in other bacteria (Bailly etal. 2007).
Another notable feature of the Dactylopiibacterium genome is that it encodes genes typical of bacterial association with plants, such as those for the catabolism of salicylate (key molecule in plant defense, Klessig etal. 2000), pectinases, polygalacturonases (to degrade plant cell walls), and auxin (plant hormone) transport (supplementary table S3, Supplementary Material online). Genes involved in salicylate and pectin degradation, as well as auxin transport, may be reminiscent of a previous Dactylopiibacterium life in plants. Alternatively, their presence may suggest a potential dual life style in symbiosis with insects and plants, as unexpectedly found in other insect endosymbionts (Frago etal. 2012). Bacteria closely related to Dactylopiibacterium as Uliginosibacterium and Azoarcus are grass plant endophytes (Li etal. 2011, 2013; Krause etal. 2006). It is worth noting that nitrogen-fixing bacteria are commonly minor components of bacterial communities (Church etal. 2005; Morris etal. 2012), mainly due to the energy cost of fixing nitrogen. A similar argument may be used to explain the limited numbers of Dactylopiibacterium cells that were found inside carmine cochineals.
Finally, we speculate that Dactylopiibacterium provides benefits to the host that may extend even to an increase in cochineal longevity for it may produce nitric oxide (NO) via partial denitrification, a process encoded in its genome. NO can act as a signal molecule in plants and animals (Wendehenne etal. 2001) and has been found to extend the life span of worms (Heintz and Mair 2014). In sum, this study has revealed a novel nitrogen fixing endosymbiont of arthropods that may be evolved from an earlier symbiosis with plants and may fulfill various roles in its association with cochineal insects.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Acknowledgments
The authors thank CONACyT Basic Science (grant 253116) and PAPIIT-UNAM (IN207615). They also thank Pilar González-Román for help with dissections; María J. Palma-Martínez for help with RT-PCR; Arturo Ramírez Cruz for his discussions on microscopy from cochineal ovaries; José Luis Marquina for providing D. coccus; and Alejandro Sánchez-Flores, Víctor del Moral, Alfredo Hernández and Romualdo Zayas for bioinformatics and computing support. All bioinformatic analyses were performed on CCG-UNAM servers.
Literature Cited
Author notes
Associate editor: Bill F. Martin
Data deposition: This project has been deposited at DDBJ/ENA/GenBank as Bioproject PRJNA355137 under accesion numbers MQNN00000000 Candidatus Dactylopiibacterium carminicum NFE1, NMRM00000000 Candidatus Dactylopiibacterium carminicum NFDO, NMRN00000000 Candidatus Dactylopiibacterium carminicum NFDCM.