-
PDF
- Split View
-
Views
-
Cite
Cite
E. Codner, P.M. Merino, M. Tena-Sempere, Female reproduction and type 1 diabetes: from mechanisms to clinical findings, Human Reproduction Update, Volume 18, Issue 5, September/October 2012, Pages 568–585, https://doi.org/10.1093/humupd/dms024
Close - Share Icon Share
Abstract
The functional reproductive alterations seen in women with type 1 diabetes (T1D) have changed as therapy has improved. Historically, patients with T1D and insufficient metabolic control exhibited a high prevalence of amenorrhea, hypogonadism and infertility. This paper reviews the impact of diabetes on the reproductive axis of female T1D patients treated with modern insulin therapy, with special attention to the mechanisms by which diabetes disrupts hypothalamic–pituitary–ovarian function, as documented mainly by animal model studies.
A comprehensive MEDLINE search of articles published from 1966 to 2012 was performed. Animal model studies on experimental diabetes and human studies on T1D were examined and cross-referenced with terms that referred to different aspects of the gonadotropic axis, gonadotrophins and gonadal steroids.
Recent studies have shown that women with T1D still display delayed puberty and menarche, menstrual irregularities (especially oligomenorrhoea), mild hyperandrogenism, polycystic ovarian syndrome, fewer live born children and possibly earlier menopause. Animal models have helped us to decipher the underlying basis of these conditions and have highlighted the variable contributions of defective leptin, insulin and kisspeptin signalling to the mechanisms of perturbed reproduction in T1D.
Despite improvements in insulin therapy, T1D patients still suffer many reproductive problems that warrant specific diagnoses and therapeutic management. Similar to other states of metabolic stress, T1D represents a challenge to the correct functioning of the reproductive axis.
Introduction
Type 1 diabetes (T1D) has a sustained increasing incidence worldwide, of 2–3% every year, reaching the highest rates in Finland and Newfoundland, Canada, with 40 new reported cases every 100 000 children younger than 14 years old per year (Vehik and Dabelea, 2010). As discussed in this review, up to 40% of these female patients will display menstrual disturbances, hyperandrogenism or early menopause at certain moment of their life, thus representing a significant health problem.
Admittedly, the reproductive problems of patients with T1D have experienced dramatic changes recently along with improvements in therapy. Prior to the use of insulin therapy, severe hypogonadism and low fertility rates were observed in T1D patients. After the introduction of insulin in 1923, menstrual cycles and fertility improved in T1D women, but primary and secondary amenorrhea and severe pubertal delay remained (Gilbert and Dunlop, 1949; Bergqvist, 1954). Before 1993, the standard therapy for T1D patients consisted of twice-daily insulin injections, a treatment that frequently did not attain optimal metabolic control and was associated with a high prevalence of the aforementioned reproductive problems (Griffin et al., 1994; Mestman, 2002; Codner and Cassorla, 2009).
The publication of the landmark Diabetes Control and Complications Trial showed that intensive insulin treatment with the aim of achieving near-normal glucose prevents the onset and progression of chronic complications (The Diabetes Control and Complications Trial Research Group, 1993). However, different abnormalities in gonadal function associated with sub-optimal blood glucose levels and non-physiological insulin replacement are still observed in T1D patients (Eyzaguirre and Codner, 2006; de Beaufort et al., 2007).
Intensive insulin therapy, with multiple daily insulin injections or continuous subcutaneous insulin infusion and intensive education, has become the standard therapy, allowing improvement in metabolic control and reproductive function in women with T1D.
This paper reviews the current knowledge of the reproductive problems observed in T1D patients and examines recent developments, coming mostly from animal studies, on the mechanistic basis of these reproductive abnormalities that affect different levels of the hypothalamic–pituitary–ovarian axis. In addition, reproductive function changes during different life stages of women with T1D will be summarized, as most research in the area has focused in the female.
Methods
A systematic review of the literature was conducted in September 2011 and updated on March 2012. A MEDLINE search of articles published from 1966 to 2012 was performed. The MESH terms included for the animal studies were: hypothalamus, hypothalamic hormones/GnRH, gonadotrophins/pituitary, FSH, LH, insulin, Kiss1/kisspeptin, leptin, adipokines, ovary, gonadal steroid hormones, estrogens, ovulation, puberty, sexual maturation, menopause and hypogonadism. This literature search was cross-referenced with an additional search on experimental diabetes mellitus, streptozotocin diabetes and alloxan diabetes.
The following MESH terms were included for the clinical studies: gonadotrophins/pituitary, FSH, LH, KISS1/kisspeptin, leptin, insulin, ovary, gonadal steroid hormones, testosterone, androgens, estrogens, ovulation, puberty, menarche, fertility, menopause and hypogonadism. This literature search was cross-referenced with an additional search on T1D (including several synonyms).
Mechanistic studies on the metabolic control of reproduction: implications for T1D
Reproductive impairment in poorly controlled T1D results from perturbations at different levels of the gonadotropic axis, including the hypothalamus/pituitary and ovary (Codner and Cassorla, 2009). Such perturbations stem from the combined effects of insulin deficiency and hyperglycaemia that disrupt the physiological functioning of various metabolic signals participating in the regulation of the reproductive system (Fernandez-Fernandez et al., 2006; Hill et al., 2008; Roa et al., 2010).
Overview of the hypothalamic–pituitary–gonadal axis, focusing on the control of gonadotrophins
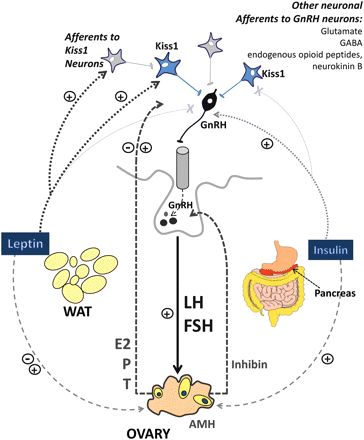
Reproduction is governed by the joint action of several neural and hormonal signals (Roa et al., 2008). In this neurohormonal system, a scarce neuronal population in the hypothalamus, which releases the decapeptide gonadotrophin-releasing hormone (GnRH), forms the major hierarchical node for the central control of reproduction (Constantin, 2011). Pulsatile secretion of GnRH drives the function of downstream elements of the hypothalamus–pituitary–gonadal (HPG) axis by dictating the secretion of pituitary gonadotrophins, LH and FSH. These hormones, acting in concert, are the major driving force for gonadal development and function in both males and females. In turn, gonadal hormones, mainly sex steroids but also peptides provide feedback to the upper levels of the HPG axis to dynamically regulate the function of this neurohormonal axis (Fig. 1; Roa et al., 2008; Uenoyama et al., 2009; Garcia-Galiano et al., 2012).
Neuroendocrine regulation of the HPG axis, with special attention to the roles of gonadal and metabolic factors and the involvement of Kiss1 neurons. The pituitary gonadotrophins LH and FSH are the major driving force of ovarian development and cyclic function from puberty onwards. In turn, pulsatile secretion of gonadotrophins is driven by the hypothalamic decapeptide GnRH, whose release is stimulated by kisspeptins produced by discrete populations of Kiss1 neurons (among other factors). Ovarian steroids, mainly estradiol (E2) and progesterone (P) but also testosterone (T) and peptides such as inhibins, provide feedback to the upper levels of the HPG axis and dynamically regulate GnRH and/or gonadotrophin secretion; sex steroids have negative- or positive-feedback effects depending on the stage of the cycle. Other secretory products of the ovary include AMH, which provides a reliable estimate of small, growing follicles. In turn, metabolic hormones, such as leptin from white adipose tissue (WAT) and insulin from the pancreas, participate in the control of the HPG axis. Many of the effects of these metabolic factors are mediated at the central (hypothalamic) level, where leptin, either directly or indirectly, modulates Kiss1/kisspeptin expression (the indirect leptin action is denoted by as yet uncharacterized neurons up-stream of the Kiss1 neurons). Moreover, Kiss1-independent actions of leptin on the GnRH neurons have been suggested (not depicted). In turn, insulin may directly regulate the function of GnRH neurons. In addition, the direct ovarian effects of leptin and insulin may contribute to the metabolic regulation of female gonadal function. Note that the different populations of Kiss1 neurons (i.e. ARC versus AVPV) are not distinguished in this scheme. Note also that other important neuronal populations and neurotransmitters, including glutamate, GABA, NPY and POMC-derived peptides, are involved in the neuroendocrine control of the HPG axis but for the sake of simplicity are not depicted here. For further details, see Sections ‘Overview of the hypothalamic–pituitary–gonadal axis, focusing on the control of gonadotropins’ and ‘Reproductive impairment in animal models of T1D’.
GnRH neurons integrate and transmit the biological messages conveyed by many key modulators of reproduction, including neurotransmitters, peripheral hormones and environmental cues (Fig. 1; Roa et al., 2008; Constantin, 2011; Roa et al., 2011). Kisspeptins, products of the Kiss1 gene, are central regulators of puberty and reproduction because of their ability to potently activate GnRH neurons. Kisspeptin neurons have been described in the hypothalamus. In rodents, two major groups of hypothalamic Kiss1 neurons have been mapped: one in the arcuate nucleus (ARC); another in a more rostral location, mainly in the anteroventral periventricular nucleus (AVPV; Roa et al., 2008; Oakley et al., 2009).
Peripheral hormones participate also in tuning GnRH neurosecretory activity. These include gonadal hormones, but also numerous metabolic factors from key tissues, such as adipose tissue, and the pancreas and gut. These metabolic signals are essential for the joint regulation of energy homeostasis and reproduction (Fernandez-Fernandez et al., 2006; Roa et al., 2008; Pralong, 2010; Roa and Tena-Sempere, 2010). Among these, insulin is an important regulator of the HPG axis. Animal models of neuronal-specific insulin receptor deletion display severe metabolic disruption, hypogonadotropic hypogonadism and infertility (Bruning et al., 2000); the latter being due to GnRH deficiency. Studies using primary hypothalamic cultures and cell lines have suggested that insulin can directly stimulate GnRH secretory activity (Salvi et al., 2006; Pralong, 2010). Murine studies using hyperglycaemic clamps have shown that increased insulin can stimulate LH secretion, regardless of the associated eu-, hyper- or hypo-glycaemic condition (Burcelin et al., 2003). Thus, insulin may directly target GnRH neurons to modulate their secretory function and, therefore, the gonadotropic axis.
The adipose hormone, leptin, signals the level of body fat and participates in the integral control of energy balance and reproduction. Leptin deficiency, as observed in animals genetically null for leptin or its receptor, is associated with severe hypogonadism. The reproductive effects of leptin at the central hypothalamic levels are predominantly permissive (Cunningham et al., 1999; Tena-Sempere, 2007). This permissive nature is clearly illustrated at puberty, when threshold leptin levels are required for puberty to proceed (Cunningham et al., 1999; Tena-Sempere, 2007) but leptin per se does not operate as the trigger of puberty.
Reproductive impairment in animal models of T1D
Genetic and pharmacological models of T1D in rodents have allowed the definition of the reproductive deficits of uncontrolled diabetes and their potential underlying mechanisms. In this context, rodent models of T1D induced by acute administration of streptozotocin (STZ), which causes the rapid and selective elimination of pancreatic β cells and severe insulinopenia, have revealed that male and female animals with uncontrolled diabetes display a profound hypogonadotropic state, characterized by low basal levels of gonadotrophins and sex steroids, reduced LH pulsatility and defective gonadotrophin responses to gonadectomy, an index of disturbed negative-feedback responses (Katayama et al., 1984; Spindler-Vomachka and Johnson, 1985; Bowton et al., 1986; Steger et al., 1989; Chandrashekar et al., 1991; Dong et al., 1991; Valdes et al., 1991; Kienast et al., 1993; Steger et al., 1993; Sexton and Jarow, 1997; Steger and Rabe, 1997; Chang et al., 2005; Castellano et al., 2006, 2009). In diabetic females, disruption of positive-feedback effects of estradiol, delayed or absent pre-ovulatory LH surges and anovulation are observed (Katayama et al., 1984; Spindler-Vomachka and Johnson, 1985; Bowton et al., 1986; Valdes et al., 1991; Kienast et al., 1993; Steger et al., 1993). These abnormalities are at least partially reversed after insulin administration (Bestetti et al., 1987; Steger et al., 1989).
Compelling evidence suggests that some of the reproductive deficits associated with T1D may stem from alterations in the ovary. Abnormalities in follicular growth and survival, including increased follicular and granulosa cell apoptosis, as well as impairment of oocyte-to-granulosa communication, oocyte maturation and ovarian follicular development occur in animal models of T1D (Chang et al., 2005; Chabrolle et al., 2008). Perturbation of ovarian steroidogenesis and ovulation was also observed in diabetic female mice. In addition, insulin deficiency has been associated with defective ovulation, which can be reversed by insulin treatment in diabetic rodents (Powers et al., 1996; Poretsky et al., 1999). Glycation of ovarian proteins has been described in non-diabetic hamsters and mice (Chaplen et al., 1998; Diamanti-Kandarakis et al., 2007a) and has a potential impact on ovarian ageing in mice (Tatone et al., 2010). The above observations suggest direct deleterious effects of low insulin levels and hyperglycaemia on ovarian functions.
Metabolic sensing and reproduction: roles of kisspeptins and leptin in T1D and other conditions of metabolic stress
As stated above, body energy stores and metabolism influence puberty onset and fertility, but how this occurs had remained contentious (Hill et al., 2008; Castellano et al., 2010a; Roa and Tena-Sempere, 2010). Compelling evidence has now demonstrated that uncontrolled T1D (Castellano et al., 2006, 2009) and other conditions of metabolic stress and negative energy balance, such as short-term fasting in rodents and primates (Castellano et al., 2005, 2010b; Kalamatianos et al., 2008; Wahab et al., 2011), chronic dietary restriction in sheep (Backholer et al., 2010a) and acute inflammation in rats (Castellano et al., 2010b), can cause hypogonadism due to suppression of hypothalamic Kiss1/kisspeptin expression, suggesting that Kiss1 neurons are sensitive to changes in metabolic status.
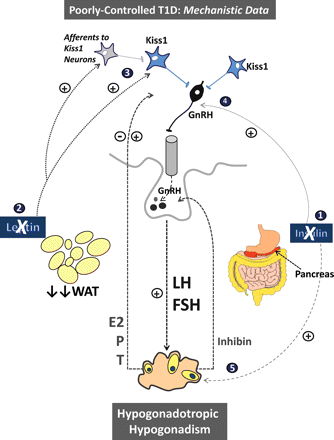
In STZ-treated male and female rats, uncontrolled diabetes is linked to suppressed hypothalamic expression of the Kiss1 gene (Castellano et al., 2006, 2009). Notably, acute kisspeptin administration is sufficient to normalize gonadotrophin secretion in diabetic rats, and testosterone levels in diabetic males (Castellano et al., 2006, 2009). Similarly, chronic treatment with kisspeptin-10 significantly ameliorates several long-term reproductive deficits in diabetic male rats (Castellano et al., 2006). These findings suggest that defective Kiss1 tone in the hypothalamus is a major contributing factor to the hypogonadotropic hypogonadism state frequently observed in poorly controlled T1D (Fig. 2).
A tentative model of the pathophysiological alterations in the HPG axis that involve the hypothalamic Kiss1 system, as elucidated by mechanistic studies in preclinical models of uncontrolled T1D. A putative sequence of major perturbations observed in this condition is provided. (1) T1D is associated with severely decreased insulin, which induces a catabolic/negative energy balance state that results in medium- and long-term decreases in body weight and a state of hypoleptinaemia (2). Decreased leptin suppresses, either directly or indirectly, the hypothalamic Kiss1/kisspeptin tone (3), which in turn decreases GnRH/gonadotrophin secretion. These conditions define a state of hypogonadotropism that ultimately hampers proper gonadal function (hypogonadism). In addition, the lack of direct insulin action on GnRH neurons may (moderately) contribute to the GnRH secretory disruption in T1D (4). Similarly, the absence of direct trophic insulin action at the ovarian level may participate in the hypogonadism state observed in models/patients with uncontrolled T1D (5). For further details, see Section ‘Metabolic sensing and reproduction: roles of kisspeptins and leptin in T1D and other conditions of metabolic stress’.
Pharmacological studies of (Fig. 1) central insulin or leptin infusion in long-term, STZ-induced diabetic rats have searched for the metabolic signals responsible for altered Kiss1 expression and/or function in T1D (Castellano et al., 2006). Leptin administration was justified given the profound hypoleptinaemia of diabetic rats and the putative stimulatory actions of leptin on hypothalamic Kiss1 expression. These analyses revealed that central infusion of leptin, but not insulin, restores defective hypothalamic Kiss1 gene expression and ameliorates or normalizes various reproductive parameters, including LH and sex steroid levels, in STZ-treated rats (Castellano et al., 2006). These observations suggest that, while defective insulin levels are responsible for the metabolic perturbations in this T1D model, the hypoleptinaemia linked to persistent negative energy balance is seemingly the ultimate cause of suppressed Kiss1 expression and hypogonadotropism in STZ-treated rats. Exogenous kisspeptin administration is apparently sufficient to normalize gonadal steroidogenic function in STZ-treated male rats, which strongly suggests a dominant central component to the reproductive failure mechanisms observed in preclinical models of uncontrolled T1D (Castellano et al., 2006).
The molecular mechanism whereby leptin regulates Kiss1 expression may involve the mammalian target of rapamycin (mTOR), a ubiquitous cellular energy sensor. At the ARC, mTOR signalling is thought to transduce leptin's effects on feeding and energy homeostasis. Central mTOR signalling also regulates Kiss1 expression and may contribute to the functional coupling between energy balance and gonadal activation and function. Thus, the permissive effects of leptin on puberty onset are blocked by central inhibition of mTOR, which also results in defective Kiss1 expression and low gonadotrophin levels. This finding suggests a tenable leptin-mTOR-kisspeptin pathway that directly or indirectly regulates the GnRH axis (Roa et al., 2009). In addition, interactions between Kiss1 and NPY or POMC neurons may be involved in integrating metabolism and the gonadal axis, as illustrated by studies in rodents and sheep (Luque et al., 2007; Backholer et al., 2010b; Fu and van den Pol, 2010; Kim et al., 2010). Some of the pathophysiological mechanisms mentioned above are integrated into Fig. 2.
T1D and ovarian function: insights from clinical studies
Pathophysiology of the reproductive axis in patients with T1D
Pituitary-hypothalamic function
Similar to animal studies, hypogonadotropic hypogonadism is present in women with uncontrolled T1D (Fig. 3) (Djursing et al., 1985b; Griffin et al., 1994). Studies performed in the 1980s showed that patients with primary or secondary amenorrhea and insufficient metabolic control exhibit low LH, FSH and estradiol levels (Fig. 3) (La Marca et al., 1999; Djursing et al., 1982; Djursing et al., 1983) that are frequently associated with a lack of residual insulin secretion (Prelevic et al., 1989). These perturbations are explained by the poor metabolic control observed in some of these patients (Arrais and Dib, 2006). The hypogonadotropic hypogonadism observed in amenorrheic T1D women is similar to that linked to other forms of metabolic stress, such as anorexia nervosa and strenuous exercise (Griffin et al., 1994). This hypothesis has been confirmed by studies in preclinical models (summarized in Section ‘Mechanistic studies on the metabolic control of reproduction: implications for T1D’). However, O'Hare et al. (1987) have described a group of patients with T1D and secondary amenorrhea due to hypogonadotropic hypogonadism who did not recover after improvement of metabolic control, thus suggesting that there is a particular group of T1D patients that is prone to hypogonadism.
Pathophysiology of the reproductive axis in patients with T1D. Modified from Codner and Cassorla. Puberty and ovarian function in girls with T1D mellitus. Horm Res 2009;71:12–21 (Codner and Cassorla, 2009)]. †Catabolism and leptin deficiency secondary to severe insulin deficiency has been observed in patients with ketoacidosis (Fluck et al., 1999; Soliman et al., 2002) *Findings that have been demonstrated in animals.
The hypothalamic origin of the decreased gonadotrophin levels observed in patients with T1D and amenorrhea has been demonstrated. Abnormalities in the GnRH pulse generator have been postulated based on studies of LH pulses, which are an indicator of the secretory activity of GnRH neurons. These studies have shown a decreased number of LH pulses, a decreased pulse amplitude and wider pulses in T1D patients with amenorrhea compared with those with normal menstrual cycles (Djursing et al., 1985a; South et al., 1993).
Most studies have shown that the pituitary in T1D patients responds normally to exogenous administration of GnRH, further suggesting that the hypogonadism is secondary to a hypothalamic disruption (South et al., 1993; La Marca et al., 1999). South et al. (1993) have found a greater total and incremental LH response to GnRH stimuli in T1D patients compared with healthy controls. Some boys with poor metabolic control show changes in their biological-to-immunological LH ratios (Nishimura et al., 2007). However, other studies have shown some degree of decreased LH response to GnRH stimuli (Distiller et al., 1975; Djursing et al., 1983) to be associated with higher fasting glucose (Distiller et al., 1975) or more severe insulin deficiency, as shown by the presence of negative C-peptide levels (Volpi et al., 1998).
A toxic effect of hyperglycaemia on the hypothalamic neurons has also been suggested by observations of the diminished LH response to GnRH stimuli with increasing diabetes duration (Volpi et al., 1998) and by abnormalities in GnRH secretion and increased apoptosis in an immortalized GnRH cell line exposed to hyperglycaemia (450 mg/dl; Pal et al., 2007).
These data suggest that chronic hyperglycaemia may induce glucotoxicity in GnRH neurons. However, central nervous system mediators, such as increased dopaminergic tone (Djursing et al., 1983), opioidergic activity (O'Hare et al., 1987; Volpi et al., 1998) and catecholamine levels (Christensen, 1970), may also be involved in the pathophysiology of hypogonadism in T1D patients (Arrais and Dib, 2006).
Ovarian function
With the advent of modern intensive T1D treatment, a decrease in the prevalence of hypogonadism has occurred, as shown by the decrease in the prevalence of amenorrhea (Table I), from >20 to <10%, and the delay of menarche, from several years to some months (discussed later). Unfortunately, an increased incidence of reproductive abnormalities due to insulin excess, especially hyperandrogenism, polycystic ovaries and excessive weight gain, has taken place. When the pancreas secretes insulin into the portal circulation under physiological conditions, the liver is the organ exposed to the highest insulin concentrations, and it eliminates an important fraction of the secreted insulin (Polonsky et al., 1988). In T1D patients, insulin administered to the subcutaneous tissue is absorbed into the systemic circulation, omitting this hepatic first-pass step (Rizza et al., 1980; Kryshak et al., 1990; Shishko et al., 1992; Bolli, 2001) and exposing the peripheral tissues to supraphysiological insulin levels (Rizza et al., 1980; Shishko et al., 1992).
Menstrual irregularities in T1D women.
| Author, country, year published . | T1D Patients (n) . | Age at study (years) . | Prevalence of menstrual irregularities (%) . | Oligomenorrhea (%) . | Secondary amenorrhea (%) . | Polymenorrhea (%) . | Factors associated with menstrual abnormalities . |
|---|---|---|---|---|---|---|---|
| Adolescence | |||||||
| Adcock, UK, 1994 | 24 | 12–20 | 54 | 21 | Metabolic control, higher BMI, lower SHBG | ||
| Yeshaya, Israel, 1995 | 100 | 32 | Prepubertal onset of T1D, late menarche | ||||
| Snajderova, Czech Republic, 1999 | 43 | 13–19 | 28 | 15 | 0.5 | 15 | Presence of certain types of ovarian autoantibodies and autoinmune thyroiditis. Metabolic control |
| Schroeder, USA, 2000 | 46 | 10–18 | 19 | 15 | 2.1 | Metabolic control | |
| Escobar-Morreale, Spain, 2000 | 85 | 17–28 | 18.8 | Prepubertal onset of T1D | |||
| Strotmeyer, USA, 2003 | 143 | <20 | 78.7 | 24.8 | NR | ||
| Codner, Chile, 2006 | 42 | 22–24 | 19 | Intensive insulin treatment | |||
| Gaete, Chile, 2010 | 56 | 13–17 | 81 | 58.9 | 10.7 | 39.3 | Metabolic control, higher insulin doses |
| Deltsidou, Greece, 2010 | 100 | 12–18 | 49.3 | 37 | Metabolic control (higher HbA1c and higher frequency of hypoglycemia) | ||
| Bizarri, Italy, 2011 | 54 | 15–25 | 11.1 | Metabolic control, intensive treatment | |||
| Adult women | |||||||
| Bergqvist, Sweden, 1954 | 62 | 20–39 | 30.6 | 9.7 | 19.4 | Prepubertal onset of T1D | |
| Kjær, Denmark, 1992 | 245 | 18–49 | 21.6 | 10.6 | 8–10 | 7.3 | Prepubertal onset of T1D |
| Strotmeyer, USA, 2003 | 143 | 30–39 | 67.5 | 11.9 | NR | ||
| Snell-Bergeon, USA, 2008 | 293 | 19–55 | 30.5 | 22 | 16.6 | NR | |
| Author, country, year published . | T1D Patients (n) . | Age at study (years) . | Prevalence of menstrual irregularities (%) . | Oligomenorrhea (%) . | Secondary amenorrhea (%) . | Polymenorrhea (%) . | Factors associated with menstrual abnormalities . |
|---|---|---|---|---|---|---|---|
| Adolescence | |||||||
| Adcock, UK, 1994 | 24 | 12–20 | 54 | 21 | Metabolic control, higher BMI, lower SHBG | ||
| Yeshaya, Israel, 1995 | 100 | 32 | Prepubertal onset of T1D, late menarche | ||||
| Snajderova, Czech Republic, 1999 | 43 | 13–19 | 28 | 15 | 0.5 | 15 | Presence of certain types of ovarian autoantibodies and autoinmune thyroiditis. Metabolic control |
| Schroeder, USA, 2000 | 46 | 10–18 | 19 | 15 | 2.1 | Metabolic control | |
| Escobar-Morreale, Spain, 2000 | 85 | 17–28 | 18.8 | Prepubertal onset of T1D | |||
| Strotmeyer, USA, 2003 | 143 | <20 | 78.7 | 24.8 | NR | ||
| Codner, Chile, 2006 | 42 | 22–24 | 19 | Intensive insulin treatment | |||
| Gaete, Chile, 2010 | 56 | 13–17 | 81 | 58.9 | 10.7 | 39.3 | Metabolic control, higher insulin doses |
| Deltsidou, Greece, 2010 | 100 | 12–18 | 49.3 | 37 | Metabolic control (higher HbA1c and higher frequency of hypoglycemia) | ||
| Bizarri, Italy, 2011 | 54 | 15–25 | 11.1 | Metabolic control, intensive treatment | |||
| Adult women | |||||||
| Bergqvist, Sweden, 1954 | 62 | 20–39 | 30.6 | 9.7 | 19.4 | Prepubertal onset of T1D | |
| Kjær, Denmark, 1992 | 245 | 18–49 | 21.6 | 10.6 | 8–10 | 7.3 | Prepubertal onset of T1D |
| Strotmeyer, USA, 2003 | 143 | 30–39 | 67.5 | 11.9 | NR | ||
| Snell-Bergeon, USA, 2008 | 293 | 19–55 | 30.5 | 22 | 16.6 | NR | |
NR, not reported.
Menstrual irregularities in T1D women.
| Author, country, year published . | T1D Patients (n) . | Age at study (years) . | Prevalence of menstrual irregularities (%) . | Oligomenorrhea (%) . | Secondary amenorrhea (%) . | Polymenorrhea (%) . | Factors associated with menstrual abnormalities . |
|---|---|---|---|---|---|---|---|
| Adolescence | |||||||
| Adcock, UK, 1994 | 24 | 12–20 | 54 | 21 | Metabolic control, higher BMI, lower SHBG | ||
| Yeshaya, Israel, 1995 | 100 | 32 | Prepubertal onset of T1D, late menarche | ||||
| Snajderova, Czech Republic, 1999 | 43 | 13–19 | 28 | 15 | 0.5 | 15 | Presence of certain types of ovarian autoantibodies and autoinmune thyroiditis. Metabolic control |
| Schroeder, USA, 2000 | 46 | 10–18 | 19 | 15 | 2.1 | Metabolic control | |
| Escobar-Morreale, Spain, 2000 | 85 | 17–28 | 18.8 | Prepubertal onset of T1D | |||
| Strotmeyer, USA, 2003 | 143 | <20 | 78.7 | 24.8 | NR | ||
| Codner, Chile, 2006 | 42 | 22–24 | 19 | Intensive insulin treatment | |||
| Gaete, Chile, 2010 | 56 | 13–17 | 81 | 58.9 | 10.7 | 39.3 | Metabolic control, higher insulin doses |
| Deltsidou, Greece, 2010 | 100 | 12–18 | 49.3 | 37 | Metabolic control (higher HbA1c and higher frequency of hypoglycemia) | ||
| Bizarri, Italy, 2011 | 54 | 15–25 | 11.1 | Metabolic control, intensive treatment | |||
| Adult women | |||||||
| Bergqvist, Sweden, 1954 | 62 | 20–39 | 30.6 | 9.7 | 19.4 | Prepubertal onset of T1D | |
| Kjær, Denmark, 1992 | 245 | 18–49 | 21.6 | 10.6 | 8–10 | 7.3 | Prepubertal onset of T1D |
| Strotmeyer, USA, 2003 | 143 | 30–39 | 67.5 | 11.9 | NR | ||
| Snell-Bergeon, USA, 2008 | 293 | 19–55 | 30.5 | 22 | 16.6 | NR | |
| Author, country, year published . | T1D Patients (n) . | Age at study (years) . | Prevalence of menstrual irregularities (%) . | Oligomenorrhea (%) . | Secondary amenorrhea (%) . | Polymenorrhea (%) . | Factors associated with menstrual abnormalities . |
|---|---|---|---|---|---|---|---|
| Adolescence | |||||||
| Adcock, UK, 1994 | 24 | 12–20 | 54 | 21 | Metabolic control, higher BMI, lower SHBG | ||
| Yeshaya, Israel, 1995 | 100 | 32 | Prepubertal onset of T1D, late menarche | ||||
| Snajderova, Czech Republic, 1999 | 43 | 13–19 | 28 | 15 | 0.5 | 15 | Presence of certain types of ovarian autoantibodies and autoinmune thyroiditis. Metabolic control |
| Schroeder, USA, 2000 | 46 | 10–18 | 19 | 15 | 2.1 | Metabolic control | |
| Escobar-Morreale, Spain, 2000 | 85 | 17–28 | 18.8 | Prepubertal onset of T1D | |||
| Strotmeyer, USA, 2003 | 143 | <20 | 78.7 | 24.8 | NR | ||
| Codner, Chile, 2006 | 42 | 22–24 | 19 | Intensive insulin treatment | |||
| Gaete, Chile, 2010 | 56 | 13–17 | 81 | 58.9 | 10.7 | 39.3 | Metabolic control, higher insulin doses |
| Deltsidou, Greece, 2010 | 100 | 12–18 | 49.3 | 37 | Metabolic control (higher HbA1c and higher frequency of hypoglycemia) | ||
| Bizarri, Italy, 2011 | 54 | 15–25 | 11.1 | Metabolic control, intensive treatment | |||
| Adult women | |||||||
| Bergqvist, Sweden, 1954 | 62 | 20–39 | 30.6 | 9.7 | 19.4 | Prepubertal onset of T1D | |
| Kjær, Denmark, 1992 | 245 | 18–49 | 21.6 | 10.6 | 8–10 | 7.3 | Prepubertal onset of T1D |
| Strotmeyer, USA, 2003 | 143 | 30–39 | 67.5 | 11.9 | NR | ||
| Snell-Bergeon, USA, 2008 | 293 | 19–55 | 30.5 | 22 | 16.6 | NR | |
NR, not reported.
The importance of insulin action on reproductive function in humans is highlighted by insulin receptor expression in most tissues, including the hypothalamus, pituitary, uterus and ovaries (Poretsky and Kalin, 1987; Poretsky et al., 1999). Insulin binds the insulin and insulin-like growth factor-I (IGF-I) receptors in the ovary, including on theca, granulosa and stromal cells, and acts mainly through the tyrosine kinase signalling pathway (Poretsky and Kalin, 1987; Bergh et al., 1993; Poretsky et al., 1999). Insulin stimulates androgen secretion via theca cells and increases the activity of several steroidogenic enzymes (Poretsky and Kalin, 1987; Cara and Rosenfield, 1988; Poretsky et al., 1999; Codner and Escobar-Morreale, 2007). This response is greatly enhanced when the cells are simultaneously exposed to LH and insulin, which indicates that insulin may act as a co-gonadotrophin (Poretsky and Kalin, 1987).
Insulin enhances follicular development and ovarian steroidogenesis, via insulin receptors in granulosa cells (Poretsky et al., 1999; Sirotkin, 2011). Insulin potentiates FSH-stimulated steroid secretion, as shown by increased estrogen secretion in granulosa cells simultaneously exposed to insulin and FSH (Willis et al., 1996). In addition, the gonadotropic effect of insulin on folliculogenesis enhances the recruitment and growth of pre-ovulatory follicles (Poretsky et al., 1999), suppresses apoptosis and atresia in ovarian follicles and promotes follicle maturation, ovarian growth and eventual cyst formation (Poretsky et al., 1992; Hsueh et al., 1994; Poretsky et al., 1999; Kezele et al., 2002). These numerous actions are the basis for the potential impact of disturbed insulin secretion (from null levels to hyperinsulinaemia) on ovarian development and function.
Folliculogenesis in T1D patients has been evaluated by determining anti-Müllerian hormone (AMH) levels, which correlate with the number of small follicles and may be used as an index of ovarian reserve. AMH is elevated in prepubertal girls with T1D, suggesting that insulin stimulates the growth of small follicles (Codner et al., 2011b). The growth of these small follicles, usually observed in the prepubertal ovary, depends on the presence of local factors that act through autocrine and paracrine mechanisms (Knight and Glister, 2001). The elevated AMH observed in prepubertal girls with T1D suggests that more small follicles are present in their ovaries, likely in response to insulin treatment.
The effect of T1D on folliculogenesis changes with the pubertal activation of the gonadal axis. After puberty, AMH levels in women with T1D are similar to those of healthy women (Codner et al., 2007, 2011b). The first phase of ovarian folliculogenesis, involving the non-cyclic recruitment of primordial follicles up to the small antral stage (∼2–5 mm), is gonadotrophin independent. After the onset of puberty, the second phase of folliculogenesis, the cyclic recruitment stage, occurs under the control of gonadotrophins and other metabolic signals. Insulin acts as a co-gonadotrophin, stimulating the recruitment and growth of larger follicles (Poretsky et al., 1992; Gougeon, 1996; Fulghesu et al., 1997), which only secrete a small amount of AMH. Therefore, we postulated that before puberty, insulin stimulates the growth of small follicles, but with the presence of pubertal or adult levels of gonadotrophins, insulin may act as a co-gonadotrophin and enhance the maturation of large follicles, which produce less AMH (Codner et al., 2007, 2011b).
Hyperglycaemia is another factor that may affect reproductive function in T1D patients. Elevated blood glucose induces peripheral insulin resistance (Amiel et al., 1986), which is a process known as glucose toxicity (Rossetti et al., 1990; Vuorinen-Markkola et al., 1992). The existing hyperinsulinaemia and insulin resistance, as observed in T1D girls (Szadkowska et al., 2008), lead to polycystic ovaries more frequently than either condition alone (Poretsky et al., 1992). Hyperglycaemia may also affect ovarian function through the presence of advanced glycation receptors and products. These receptors have been detected in the granulosa and theca cells of healthy women (Diamanti-Kandarakis et al., 2007b).
In summary, several factors may be involved in altering ovarian function in T1D patients. Insulin deficiency may lead to lower gonadotrophin levels due to decreased GnRH secretion. In addition, hyperglycaemia may affect the ovary, both directly and through inducing insulin resistance. Finally, higher serum insulin may lead to overstimulation of the insulin and IGF-1 receptors in the ovary, increasing androgen secretion and fostering the development of PCOS.
Childhood
Childhood is characterized by a quiescent reproductive axis, with low gonadotrophin levels and the predominance of FSH over LH secretion. In children aged 6–8 years, adrenarche occurs due to maturation of the reticularis zone of the adrenal gland (Auchus, 2011). This process leads to adrenal androgen secretion (Williams et al., 2011). Excessive androgen secretion by the adrenal glands and increased AMH levels during childhood have been described in patients at risk for developing PCOS later in life (Ibanez et al., 1993, 1997, 1998; Sir-Petermann et al., 2006; Maliqueo et al., 2009).
Few studies have evaluated ovarian function during childhood in girls with T1D. To determine whether these patients exhibit a similar endocrine profile to other groups of girls at risk for PCOS, we studied sexual steroid, gonadotrophin, AMH and inhibin-B levels in 20 prepubertal girls with T1D and 24 healthy controls aged 5–7 years (Codner et al., 2011b). The girls with T1D had higher levels of AMH and inhibin B. The prepubertal T1D patients had higher AMH levels than other groups of children at risk for developing PCOS later in life (Ibanez et al., 2000; Sir-Petermann et al., 2006, 2007). The gonadotrophin, estradiol and testosterone levels were similar in both groups.
Although the precocious appearance of pubic hair has not been reported in girls with T1D, adrenal steroids, either in plasma or as urinary metabolites, are elevated in TD1 patients during childhood. Remer et al. (2006) studied urinary steroid metabolites in a group of prepubertal children and pubertal girls with T1D and found elevated levels of total adrenal androgens, dehydroepiandrosterone (DHEA)/dehydroepiandrosterone sulphate (DHEAS) and androstenedione in T1D girls during childhood and puberty compared with the control group. Similarly, we have reported that prepubertal girls with T1D have significantly higher DHEAS and almost significantly higher levels of androstenedione than normal girls. The above data suggest that some degree of elevated adrenal androgen is present during the prepubertal period but not enough to be associated with an increased incidence of precocious pubic hair, and that the effects of T1D on the gonads and adrenal reticularis begin during childhood and that these girls exhibit an endocrine profile similar to that of other groups at risk for PCOS.
Puberty
An in-depth review of the mechanisms of puberty and age of menarche have recently been published (Codner and Cassorla, 2009; Codner et al., 2012), and a brief summary of these topics will be presented here. Several decades ago, girls with T1D frequently exhibited severe pubertal delay associated with poor metabolic control (Mauriac, 1930; Bergqvist, 1954). Studies performed in the 1980s and 1990s, at a time when children were treated with the conventional insulin protocol of two daily doses, found that severe pubertal delay was rare but that a delay of 6 months to 1 year in the onset of breast development was described (Clarson et al., 1985; Salardi et al., 1987; Du Caju et al., 1995). Other series that evaluated pubertal development during the same period showed no delay, although they compared the timing of puberty with that of historical controls published in 1969 by Marshall and Tanner (Salerno et al., 1997; Ahmed et al., 1998).
Two studies evaluating pubertal development in girls with T1D treated with modern insulin therapy have been published in the last two decades [reviewed in (Codner and Cassorla, 2009)]. Our group observed a similar age of puberty onset in girls with T1D and the control group, but one and a half years earlier than Chilean historical controls. We concluded that both groups followed the secular trend towards earlier onset of puberty, which has also been described in the general US population (Parent et al., 2003). Rohrer et al. found that thelarche in girls with T1D occurred 6 months later than in historical controls studied in the 1980s, although the average age was within the normal range for both groups. These data suggest that the onset of puberty in girls with T1D treated with multiple daily insulin doses occurs within a normal age, especially in those with lower HbA1c (Rohrer et al., 2007), and follows the secular trend towards an earlier onset that has been observed in the general population (Codner et al., 2004; Codner and Cassorla, 2009). Similarly, only a delay of 2–6 months in reaching the final stages of breast development (Codner et al., 2004; Codner and Cassorla, 2009) and pubic hair growth (Codner et al., 2004; Rohrer et al., 2007; Codner and Cassorla, 2009) has recently been reported.
In contrast to the few studies evaluating the final stages of development, several publications have reported the effect of T1D on the age of menarche. A significant menarche delay was described during the first half of the 20th century. In the 1940s and 1950s, menarche occurred 2 years later in girls with T1D than in the general population, and a significant proportion of the T1D patients exhibited primary amenorrhea into their late teens (Bergqvist, 1954; Tattersall and Pyke, 1973). Girls diagnosed with T1D in the 1970s or 1980s displayed a 1-year delay in the average age of menarche compared with controls (Schriock et al., 1984; Kjaer et al., 1992a; Strotmeyer et al., 2003; Schweiger et al., 2010). With the advent of intensive insulin therapy in the 1990s, only a mild delay in menarche in girls with T1D, ranging from 2 to 9 months, has been reported in countries in Europe and North and South America (Strotmeyer et al., 2003; Codner et al., 2004; Danielson et al., 2005; Picardi et al., 2008; Rohrer et al., 2008; Lombardo et al., 2009; Deltsidou, 2010; Schweiger et al., 2010). The clinical significance of the delay in menarche depends on its magnitude. Late menarche is associated with irregular menses and other gynaecological disturbances in T1D women (Kjaer et al., 1992a; Adcock et al., 1994; Yeshaya et al., 1995; Danielson et al., 2005). The association of late menarche, amenorrhea and menstrual irregularities with estrogen deficiency could play a role in the cardiovascular complications observed in women with T1D (Codner, 2008).
The hormonal mechanisms involved in delayed puberty have not been studied thoroughly, but several pathophysiological mechanisms may be involved. The first is related to a delay in the activation of gonadotrophin secretion. Lower insulin doses have been associated with delayed menarche in T1D (Rohrer et al., 2008), which may be mediated by the action of insulin on the central nervous system and therefore on the activation of gonadotrophin secretion. However, no longitudinal studies have examined whether this process is retarded in T1D.
Another hormonal finding that may explain abnormal puberty in T1D patients is the appearance of hyperandrogenism at the final stages of pubertal development (Meyer et al., 2000; Remer et al., 2006). We have studied androgen levels and ovarian responses to a GnRH agonist in pubertal girls with T1D; we found a higher proportion of abnormally located hair, suggesting some degree of hirsutism, and increasing free androgen levels throughout puberty in the girls with T1D. In addition, the girls had an ovarian response to GnRH that suggested the presence of ovarian hyperandrogenism by the end of puberty, together with larger ovaries and increased LH/FSH ratios, which may be associated with PCOS (Codner et al., 2005).
Other mechanisms that may play some role in the pubertal delay of girls with T1D are the occasional presence of ovarian antibodies (Snajderova et al., 1999), increased advanced glycation end products (Berg et al., 1997), exacerbation of the insulin resistance of puberty (Szadkowska et al., 2008) and higher SHBG at the onset of puberty, leading to decreased steroid bioavailability (Codner et al., 2005; Codner and Cassorla, 2009).
Adolescence
After menarche, girls with T1D have greater risks for several metabolic and reproductive complications than do boys with T1D. Excessive weight gain, deteriorating metabolic control, menstrual irregularities, unplanned pregnancies and the appearance of microvascular complications make this a difficult period for young women with T1D (Du Caju et al., 1995; Danne et al., 1997; Ferrante et al., 1999; Riihimaa et al., 2000; Bryden et al., 2001; Codner et al., 2004; Codner, 2008; Iniguez et al., 2008; Codner and Cassorla, 2009). The abnormal insulin sensitivity pattern and deteriorating metabolic control during adolescence may contribute to the abnormalities of reproductive function observed in girls with T1D. Difficulties in glycemic controls frequently observed during puberty intensify at the end of the growth period, especially in girls (Tylleskar et al., 2001; Codner et al., 2004). Whereas non-diabetic girls become more insulin sensitive during puberty than boys, T1D girls become more insulin resistant than boys (Arslanian et al., 1991; Szadkowska et al., 2008). Furthermore, whereas in healthy girls the progression of insulin resistance during puberty is attenuated after they complete growth, this insulin resistance continues to increase with age in girls with T1D (Moran et al., 2008; Szadkowska et al., 2008).
Menstrual irregularities are a prevalent problem during adolescence for individuals with T1D and should be included in the list of critical problems for adolescent girls with T1D (Table I). T1D, especially when associated with insufficient metabolic control, leads to longer menstrual cycles, making oligomenorrhoea the most prevalent menstrual cycle abnormality observed in T1D adolescents. The prevalence of menstrual irregularities varies among series and depends on the criteria used to define these abnormalities. As menstrual periods are longer during adolescence, a normal menstrual cycle interval has been defined as 21–45 days in the first 5 years following menarche (American Academy of Pediatrics et al., 2006; American College of Obstetrics and Gynecology, 2006). Studies that apply the adult criteria for menstrual irregularities to adolescence have found a prevalence ranging from 20–30% (Snajderova et al., 1999; Schroeder et al., 2000) to 50% (Adcock et al., 1994; Deltsidou et al., 2010) and even 80% (Strotmeyer et al., 2003). We have recently applied the adolescent criteria for menstrual irregularities to girls with T1D and have observed longer menstrual cycles compared with the control group (48 and 32 days in the T1D and control groups, respectively). Sixty percent of the T1D patients experienced at least one episode of oligomenorrhoea during the 6-month observation period, which was significantly higher than the 20% observed in the control girls (odds ratio = 5.9; Gaete et al., 2010). Despite the presence of menstrual irregularities, adolescents with T1D and healthy girls have similar rates of ovulation (Codner et al., 2011a; discussed further in Section ‘Young adult women’).
Secondary amenorrhea has become increasingly infrequent during recent decades and is currently observed in only 5–10% of T1D patients, which is nonetheless higher than in the general population (Snajderova et al., 1999; Gaete et al., 2010). Prolonged or heavy bleeding and polymenorrhea are not prevalent problems in adolescents with T1D (Strotmeyer et al., 2003) and have been only rarely described (Snajderova et al., 1999). Teens with T1D can show increased variability in their menstrual cycles (Gaete et al., 2010).
Metabolic control is the most important determinant of menstrual irregularities in adolescents with T1D (Adcock et al., 1994; Schroeder et al., 2000; Deltsidou et al., 2010; Gaete et al., 2010). We have found HbA1c to be the only factor significantly associated with menstrual cycle length in T1D patients; a regression analysis demonstrated that menstrual cycle duration was prolonged by 5.1 days for each one percent increase in HbA1c (Gaete et al., 2010). Deltsidou et al. (2010) have shown that for each one percent increase in HbA1c, the risk of oligomenorrhoea increased by 4.8-fold. Moreover, Gaete et al. found that girls with optimal metabolic control (HbA1c lower than 7.6%) had a prevalence of oligomenorrhoea twice that of controls (OR = 4.7). These data suggest that even if menstrual irregularities are increasingly frequent with HbA1c above 10% (Adcock et al., 1994; Schroeder et al., 2000), they are still observed in patients with optimal or suboptimal metabolic control (Gaete et al., 2010).
Hyperandrogenism may be another factor explaining the presence of menstrual cycle abnormalities in patients with good metabolic control. Adcock subsequently showed that almost 80% of adolescents with irregular menstrual cycles had polycystic ovaries, decreased SHBG and an elevated LH/FSH ratio, which are frequent findings in PCOS (Adcock et al., 1994). Virdis et al. (1997) studied ovarian function in girls with T1D and oligomenorrhoea and found elements of ovarian hyperandrogenism in 50% of them. Recently, a French group studied adolescents with T1D and irregular menstrual cycles and found that oligomenorrhoea was associated with hyperandrogenism and with higher testosterone, androstenedione, LH and free androgen levels compared with the T1D group without menstrual irregularities (Samara-Boustani et al., 2012). However, there is scarce information about the prevalence and severity of hyperandrogenism in adolescents with T1D, irrespective of their menstrual status.
Young adult women
Menstrual cycles
Although the prevalence of menstrual irregularities is lower than in adolescents, a significant proportion (20–40%, Table I) of adult women with T1D still experience these problems (Kjaer et al., 1992a; Yeshaya et al., 1995; Escobar-Morreale et al., 2000; Strotmeyer et al., 2003; Codner et al., 2006; Codner and Escobar-Morreale, 2007; Snell-Bergeon et al., 2008). Menstrual irregularities in non-diabetic women are linked to increased cardiovascular and metabolic dysfunction (Weiss et al., 1994; Solomon et al., 2001; Solomon et al., 2002), which are also common in women with T1D (Snell-Bergeon et al., 2008). Snell-Bergeon et al. (2008) have shown that T1D women with a history of menstrual irregularities had increased coronary artery calcification progression, suggesting that menstrual irregularities may represent a marker for cardiovascular risk in these patients just as in healthy women. These authors suggested that the increased cardiovascular risk observed in T1D women with menstrual dysfunction may have been explained by underlying hypo-estrogenism (Snell-Bergeon et al., 2008), which has been observed in T1D women with amenorrhea (Djursing et al., 1985b) and with regular menstrual cycles (Salonia et al., 2006; Codner, 2008).
Fluctuations in plasma glucose associated with the menstrual cycle are a prevalent complaint in T1D. Only certain women are prone to this abnormality; although the patients have heterogeneous blood glucose profiles during their menstrual cycles, a pattern that is reproducible from cycle to cycle tends to occur in each woman (Goldner et al., 2004). The most prevalent complication is hyperglycaemia during the luteal phase or bleeding period, which is observed in 40–70% of the patients in some series (Widom et al., 1992; Cawood et al., 1993; Lunt and Brown, 1996) and which is still observed in some women using oral contraceptives (Lunt and Brown, 1996). Some reports have observed that hypoglycaemia may be associated with the bleeding period.
Hyperglycaemia associated with the menstrual cycle is frequently managed with self-adjustments in insulin treatment protocols, but cases of extreme difficulty in controlling glycaemia at specific stages of the cycle have been described. In these ‘catamenial’ stages, extreme hyperglycaemia and recurrent ketoacidosis occur monthly in association with menstruation (Walsh and Malins, 1977; Letterie and Fredlund, 1994; Herring and Gearhart, 1996; Li Voon Chong, 2010; Sennik et al., 2010). Walsh et al. (1977) described an increased prevalence of ketoacidosis in women during the perimenstrual period. These catamenial reactions were treated with GnRH analogues, increased insulin, or low doses of combined oral contraceptives (Sacerdote and Bleicher, 1982; Letterie and Fredlund, 1994; Sennik et al., 2010).
Variations in glucose levels during the menstrual cycles of T1D patients may be related to diminished insulin sensitivity or excessive craving for sweets as part of the premenstrual cycle syndrome. Widom et al. (1992) have shown that women who had hyperglycaemia in the premenstrual period had decreased insulin sensitivity associated with higher estrogen during the luteal phase, when compared with the follicular phase. However, other studies have shown non-significant decreases in luteal phase insulin sensitivity (Scott et al., 1990; Moberg et al., 1995).
Premenstrual syndrome is not a frequent complaint in T1D patients, but glucose irregularities have been observed more frequently in patients with these symptoms than in patients without them (Cawood et al., 1993), which may be explained by a craving for sweets that contributes to the hyperglycaemia associated with menstruation.
Ovulation and fertility
Few studies have evaluated ovulatory function in women with T1D. More than 50 years ago, Bergqvist showed that adult women with T1D displayed signs of ovulation, such as variations in basal temperature, despite menstrual irregularities (Bergqvist, 1954). Steel (1984) showed a delay in ovulation in 11 adult women with T1D who were trying to become pregnant, suggesting a longer follicular phase. The only thorough prospective study of ovulation was recently reported by our group. We comparatively followed a group of non-hyperandrogenic adolescents with T1D (n = 31) and a group of healthy girls (n = 52; Codner et al., 2011a). Each girl was followed for an average of five cycles, and ovulation was assessed by measuring salivary progesterone. Ovulation was not decreased in the girls with T1D. The fraction of ovulatory cycles was similar in the T1D and control groups (34.5 and 36.3%, respectively). Metabolic control had a slight effect on the ovulation rate. A higher percentage of ovulatory cycles and an increased rate of ovulation every 100 days were observed in the T1D girls with optimal metabolic control than in the T1D girls with insufficient metabolic control. However, some of the girls with high HbA1c levels still had a considerable number of ovulatory cycles.
Despite these data suggesting preserved ovulation, most series have found fewer pregnancies and live births in women with T1D, which may be associated with the presence of diabetes-related complications or with a voluntary choice by T1D women to have fewer children (Kjaer et al., 1992b; Pedersen et al., 1994; Jonasson et al., 2007; Soto et al., 2009). Whitworth et al. (2011) studied a large cohort of Norwegian women (221 women with T1D) and showed that fecundability (the probability of conception in one menstrual cycle) was decreased by 24% compared with the general population and was similar in women with and without menstrual irregularities.
One factor involved in the decreased fecundability of some T1D women may be sexual dysfunction. Enzlin et al. (2002, 2003) found that 27% of the women with T1D they studied had sexual dysfunction, especially decreased desire, dyspareunia, and alterations of the arousal phase, which was associated with marital problems and the presence of depressive symptoms. Salonia et al. (2006) found decreased sexual function and increased sexual distress during the luteal, but not the follicular, phase in women with T1D compared with controls. Trials with sildenafil have reported some degree of success in treating sexual arousal dysfunction in women with T1D (Caruso et al., 2006a, b).
Despite decreased fecundability and sexual function, T1D is a rare cause of consultation in infertility clinics (Thonneau et al., 1991; Healy et al., 1994; Hargreave and Mills, 1998). Involuntary infertility has been observed in 17% of T1D women, a rate similar to that of healthy controls (Strotmeyer et al., 2003). Studies evaluating fertility treatments in women with T1D agree that the major factor in attaining a successful pregnancy is achieving optimal metabolic control before the use of more invasive techniques (Taylor, 2002; Livshits and Seidman, 2009). In vitro fertilization treatments in women with T1D show results similar to those in women without this condition only when optimal metabolic control is attained (Dicker et al., 1992; Hovav et al., 1995).
Polycystic ovarian syndrome and hyperandrogenism in T1D
Although hyperandrogenism has been classically associated with T2D, increasing evidence shows that T1D women may also exhibit this abnormality (Codner and Escobar-Morreale, 2007). Djursing et al. (1985b) reported that adult women with T1D but without amenorrhea had high androgen levels, suggesting an ovarian origin of their androgen excess. O'Hare et al. (1987) showed that intensification of insulin treatment in amenorrheic women led to elevated testosterone. Subsequently, a 40% prevalence of clinical or biochemical hyperandrogenism was found by Escobar-Morreale and Codner in Spain and Chile, respectively (Escobar-Morreale et al., 2000; Codner et al., 2006) and 25% prevalence in Italy (Bizzarri et al., 2011).
The most frequent hyperandrogenic symptom in these women was hirsutism, which is present in 20–30% of the young adult women with T1D (Escobar-Morreale et al., 2000; Codner et al., 2006; Bizzarri et al., 2011). This prevalence is much higher than that of the Spanish and Chilean general populations (7.1 and 3.0%, respectively) (Tellez and Frenkel, 1995; Asuncion et al., 2000). Biochemical hyperandrogenism was present in 20% of the young adult women in the three series that have compared androgens in T1D patients to androgens in healthy women (Escobar-Morreale et al., 2000; Codner et al., 2006; Bizzarri et al., 2011), with testosterone and androstenedione levels being increased in all three of the studies. The prevalence of PCOS in women with T1D varies depending on the diagnostic criteria employed and on the ethnicity of the population being studied. Using the NIH criteria for PCOS, 12 and 18.8% PCOS prevalence has been observed in T1D women from Chile and Spain, respectively, which are much higher than the 6.5% observed by the same authors in the Spanish general population (Asuncion et al., 2000; Escobar-Morreale et al., 2000; Codner et al., 2006). When the Rotterdam criteria for PCOS (which include polycystic ovarian morphology) were employed, the prevalence of PCOS increased to 40.5%, as the combination of hyperandrogenism and PCOS is especially common in these women (Codner et al., 2006). However, an Italian group reported a much lower prevalence of PCOS (7.5%) when using the Rotterdam criteria, even though the patients in their study had even higher androgen levels than the Spanish and Chilean subjects (Bizzarri et al., 2011). Finally, applying the AES criteria, which require the presence of hyperandrogenism, the prevalence of PCOS was 31% in Chilean T1D women (Codner and Escobar-Morreale, 2007).
The ultrasonographic appearance of polycystic ovaries, also known as polycystic ovarian morphology, is frequently observed in women with PCOS and has been explained by the effects of insulin on folliculogenesis (Codner et al., 2006). Early studies reported ultrasonographic polycystic ovaries in as many as 80% of adolescents with T1D (Adcock et al., 1994). Increased ovarian volume and numbers of follicles have been observed, resulting in polycystic ovaries in half of the adult women with T1D, when compared with only 13% of age-matched non-diabetic controls (Codner et al., 2006).
Women with PCOS and T1D exhibit phenotype and hormonal profile differences from patients with PCOS alone (Table II). Hirsutism is usually mild in T1D women, which may explain why this sign is frequently overlooked in general clinical practice (Codner et al., 2006). Biochemical hyperandrogenism and ultrasonographic polycystic ovaries may be underdiagnosed if only a clinical evaluation is performed (Roldan et al., 2001; Codner et al., 2006). Adult women with T1D and PCOS usually display a milder form of hyperandrogenism than do non-diabetic PCOS women, and the classical PCOS phenotype, oligomenorrhoea and hyperandrogenism, is less frequent in diabetic PCOS than in non-diabetic PCOS patients (30 versus 90%; Codner et al., 2007).
Comparison of clinical, laboratory and physiopathology characteristics of polycystic ovarian syndrome in women with T1D and PCOS vs. patients with PCOS without T1D.
| . | T1D + PCOS . | PCOS . |
|---|---|---|
| Childhood | ↑AMH/Adrenal Androgens | ↑AMH/adrenal androgens |
| Precocious Pubarche | Not Reported | Associated |
| Puberty | Normal or Mild delay | Normal or early |
| Onset of hyperandrogenism | Late | Peri-menarcheal |
| Onset of weight gain or increase in adipose tissue | Begins during puberty/adolescence | Begins during childhood |
| Source of hyperinsulinemia | Systemic circulation | Pancreas/portal vein |
| Insulin resistance | Secondary to glucose toxicity | Primary/obesity related |
| Tissue that are exposed to higher insulin concentration | Muscle, adipose tissue | Liver |
| Degree of Hirsutism | Mild | More severe |
| Most prevalent phenotype | Hyperandrogenism clinical or biochemical | Oligomenorrhea + Hyperandrogenism + PCOM |
| Total testosterone/androstenedione | ↑ | ↑ |
| Free testosterone | N or ↑ | ↑↑↑ |
| SHBG levels | N or ↑ | ↓ |
| LH levels | N | ↑ |
| AMH levels | N | ↑ |
| Anovulation | ? | Yes |
| PCOM on ultrasonography | Yes | Yes |
| . | T1D + PCOS . | PCOS . |
|---|---|---|
| Childhood | ↑AMH/Adrenal Androgens | ↑AMH/adrenal androgens |
| Precocious Pubarche | Not Reported | Associated |
| Puberty | Normal or Mild delay | Normal or early |
| Onset of hyperandrogenism | Late | Peri-menarcheal |
| Onset of weight gain or increase in adipose tissue | Begins during puberty/adolescence | Begins during childhood |
| Source of hyperinsulinemia | Systemic circulation | Pancreas/portal vein |
| Insulin resistance | Secondary to glucose toxicity | Primary/obesity related |
| Tissue that are exposed to higher insulin concentration | Muscle, adipose tissue | Liver |
| Degree of Hirsutism | Mild | More severe |
| Most prevalent phenotype | Hyperandrogenism clinical or biochemical | Oligomenorrhea + Hyperandrogenism + PCOM |
| Total testosterone/androstenedione | ↑ | ↑ |
| Free testosterone | N or ↑ | ↑↑↑ |
| SHBG levels | N or ↑ | ↓ |
| LH levels | N | ↑ |
| AMH levels | N | ↑ |
| Anovulation | ? | Yes |
| PCOM on ultrasonography | Yes | Yes |
N, Normal; ↑, Elevated; ↑↑↑, Very elevated; ↓, Diminished; ?, Unknown.
Comparison of clinical, laboratory and physiopathology characteristics of polycystic ovarian syndrome in women with T1D and PCOS vs. patients with PCOS without T1D.
| . | T1D + PCOS . | PCOS . |
|---|---|---|
| Childhood | ↑AMH/Adrenal Androgens | ↑AMH/adrenal androgens |
| Precocious Pubarche | Not Reported | Associated |
| Puberty | Normal or Mild delay | Normal or early |
| Onset of hyperandrogenism | Late | Peri-menarcheal |
| Onset of weight gain or increase in adipose tissue | Begins during puberty/adolescence | Begins during childhood |
| Source of hyperinsulinemia | Systemic circulation | Pancreas/portal vein |
| Insulin resistance | Secondary to glucose toxicity | Primary/obesity related |
| Tissue that are exposed to higher insulin concentration | Muscle, adipose tissue | Liver |
| Degree of Hirsutism | Mild | More severe |
| Most prevalent phenotype | Hyperandrogenism clinical or biochemical | Oligomenorrhea + Hyperandrogenism + PCOM |
| Total testosterone/androstenedione | ↑ | ↑ |
| Free testosterone | N or ↑ | ↑↑↑ |
| SHBG levels | N or ↑ | ↓ |
| LH levels | N | ↑ |
| AMH levels | N | ↑ |
| Anovulation | ? | Yes |
| PCOM on ultrasonography | Yes | Yes |
| . | T1D + PCOS . | PCOS . |
|---|---|---|
| Childhood | ↑AMH/Adrenal Androgens | ↑AMH/adrenal androgens |
| Precocious Pubarche | Not Reported | Associated |
| Puberty | Normal or Mild delay | Normal or early |
| Onset of hyperandrogenism | Late | Peri-menarcheal |
| Onset of weight gain or increase in adipose tissue | Begins during puberty/adolescence | Begins during childhood |
| Source of hyperinsulinemia | Systemic circulation | Pancreas/portal vein |
| Insulin resistance | Secondary to glucose toxicity | Primary/obesity related |
| Tissue that are exposed to higher insulin concentration | Muscle, adipose tissue | Liver |
| Degree of Hirsutism | Mild | More severe |
| Most prevalent phenotype | Hyperandrogenism clinical or biochemical | Oligomenorrhea + Hyperandrogenism + PCOM |
| Total testosterone/androstenedione | ↑ | ↑ |
| Free testosterone | N or ↑ | ↑↑↑ |
| SHBG levels | N or ↑ | ↓ |
| LH levels | N | ↑ |
| AMH levels | N | ↑ |
| Anovulation | ? | Yes |
| PCOM on ultrasonography | Yes | Yes |
N, Normal; ↑, Elevated; ↑↑↑, Very elevated; ↓, Diminished; ?, Unknown.
The hormone profiles of T1D patients presenting with PCOS are different from those observed in non-diabetic hyperandrogenic women (Roldan et al., 2001; Codner et al., 2007). Serum testosterone is similarly increased in PCOS patients with and without T1D, but free androgens are lower in T1D patients with PCOS than in non-diabetic PCOS patients, which may be explained by normal sex hormone-binding globulin (SHBG) levels. Decreased SHBG, a characteristic of PCOS without T1D, has not been described in patients with PCOS and T1D. The normal SHBG levels in women with T1D and PCOS may be related to insulin concentration at the portal vein being the main regulator of SHBG (Yki-Jarvinen et al., 1995); in women with T1D, insulin is subcutaneously administered to the systemic circulation and may not result in increased portal levels, even when supraphysiological doses are given. The normal SHBG levels in patients with PCOS and T1D increase the binding of sex steroids to this protein and may explain why free androgens are not as elevated (Codner et al., 2007) and why hirsutism is less severe in PCOS patients with T1D than in those without T1D.
Elevated serum AMH is a typical feature of non-T1D patients with PCOS. As discussed in Section ‘Pathophysiology of the reproductive axis in patients with T1D’, AMH is normal in patients with PCOS and T1D, despite an elevated number of 2–9 mm follicles. An explanation for this finding may be that the increased follicle number observed by ultrasonography in T1D patients corresponds mostly to follicles >5 mm, which produce limited amounts of AMH (Codner et al., 2007, 2011b). These data suggest that not all hyperandrogenic disorders exhibit the same abnormalities in follicular development and that some features may be observed exclusively in PCOS.
Androgen excess in T1D women appears to be mostly of ovarian origin, given that the responses of their adrenal androgen precursors to an ACTH stimulation test are similar to those of healthy women (Roldan et al., 2001). An ovarian origin is also supported by the increased 17-hydroxyprogesterone responses to GnRH agonists found in a significant proportion of T1D adolescents (Virdis et al., 1997; Codner et al., 2005). Similarly, LH and FSH levels are normal in patients with T1D and PCOS, which suggests that the pituitary–gonadal axis is not central to the pathophysiology of androgen excess.
Intensive conventional insulin therapy has been associated with PCOS in T1D women. We recently reported that 75% of the T1D women on intensive insulin therapy had either PCOS or asymptomatic polycystic ovarian morphology on ultrasound scans, when compared with only 33% of the patients on a more conservative conventional regimen of two daily insulin injections (Codner et al., 2006). Recently, Bizzarri et al. (2011) have reported that a high body mass index and low birthweight were related to testosterone and androstenedione levels in young women with T1D. However, the mean daily insulin dose received, diabetes duration and degree of metabolic control are not significantly associated with hyperandrogenism.
Apparently, the onset of hyperandrogenism occurs later in life in patients with PCOS and T1D than in non-diabetics (Adcock et al., 1994; Meyer et al., 2000; Codner et al., 2005; Sir-Petermann et al., 2009). Escobar-Morreale et al. found that developing PCOS was associated with the onset of diabetes before menarche in T1D patients, which led the authors to hypothesise that exogenous hyperinsulinism at the onset of ovarian function during puberty re-programs ovarian function towards increased androgen secretion, leading to hyperandrogenism and PCOS later in life (Escobar-Morreale et al., 2000; Codner and Escobar-Morreale, 2007).
The consequences of PCOS in women with T1D are unknown at present, but some data suggest that androgen excess may be associated with the renal microvascular complications of diabetes, especially with the presence of microalbuminuria (Amin et al., 2003).
The best therapeutic strategy has yet to be established, although routine screening for these conditions and subsequent treatment should be considered. Theoretically, the addition of low-dose non-androgenic oral contraceptives, metformin or both to an insulin regimen should improve hyperandrogenic symptoms in these women. Two small pilot studies, published in abstract form, have evaluated using metformin alone or in combination with flutamide in hyperandrogenic adolescents with T1D and have found beneficial effects on androgen from these treatments (Beckers et al., 2006; Codner et al., 2009).
Premenopausal period and menopause
As women with T1D approach their thirties and forties, they face an array of problems usually only observed in healthy women later in life. Cardiovascular disease, osteopenia and fractures may be observed in T1D patients during the premenopausal years, with the risk of these complications being more elevated in female than male T1D patients (Lloyd et al., 1996; Laing et al., 2003; Soedamah-Muthu et al., 2006; Secrest et al., 2010). The prevalence of cardiovascular disease in female patients with T1D during the fourth and fifth decades of life is similar to that of men of the same age (Lloyd et al., 1996; Orchard et al., 2006; Codner, 2008) and is several times greater than that of healthy women. Moreover, the relative risk of death compared with the general population is more elevated in women with T1D during the premenopausal years than in men of the same age (Secrest et al., 2010).
An earlier age of menopause, another sign of premature aging, has also been described in T1D patients (Dorman et al., 2001). Only two epidemiological studies have analysed the age of menopause in T1D patients. The first, published in 2001 studied the age of menopause in patients diagnosed at the Children's Hospital of Pittsburgh between 1950 and 1964 (n = 265). The age of menopause reported for T1D patients was 41.6 years, which was significantly lower than the ages observed in their sisters and in the controls (49.9 and 48 years, respectively). Compared with the control group, the patients with T1D had twice the risk of early menopause, as defined the last menstrual bleeding occurring before age 47. The study concluded that the reproductive period is decreased by 6 years in T1D patients due to late menarche and early menopause (Dorman et al., 2001).
However, a more recent study did not show an earlier age of menopause in T1D patients without complications (Sjoberg et al., 2011). That study evaluated a nationwide Finnish cohort of patients diagnosed between 1965 and 1979 that included only patients who did not report any condition known to affect ovarian function. The authors found the age of menopause to be 52 years, which is similar to that of the general population, and observed that the main risk factors for earlier menopause were the presence of severe microvascular complications, end-stage renal disease and proliferative retinopathy.
The mechanisms of early menopause in T1D patients have scarcely been studied. We have recently studied ovarian reserve and steroid levels in 33–45-year-old women with T1D (Soto et al., 2009). Ovarian reserve has been studied using serum AMH, which diminishes during the transition to menopause, and serum inhibin B (Sowers et al., 2008). An earlier decline in AMH and a higher proportion of AMH levels in the menopausal range were observed in women with T1D than in controls. The lower AMH and the earlier decline observed in women with T1D during the fourth decade of life suggest the presence of a precocious decline in the ovarian follicular pool in these women.
In addition, an autoimmune oophoritis has been postulated as one of the mechanisms leading to early menopause in women with T1D. Recently, Tsigkou et al. (2008) showed that measuring inhibin B may help to distinguish autoimmune premature ovarian failure from natural menopause, as the levels of this hormone are elevated in the former and diminished in the latter. Our group reported that lower inhibin B in T1D patients than in controls, even after adjusting for age (Soto et al., 2009). The low inhibin B observed by Soto et al. is more compatible with non-immune-mediated follicular loss than with the presence of autoimmune oophoritis, as serum inhibin B levels are an index of the number of antral follicles in these patients (Groome et al., 1996; Knight and Glister, 2001). Similarly, none of the larger epidemiological studies mentioned above observed an association between earlier menopause with the presence of auto-immune diseases (Dorman et al., 2001; Sjoberg et al., 2011).
Hyperandrogenism has not been reported in women with T1D approaching menopause. The only series that has compared the hormonal profiles of young adult women and those in their thirties and forties is the aforementioned study by Soto et al., which showed that hyperandrogenism is primarily observed in young women and that androgen levels are within the normal range in older women. One hypothesis explaining this observation could be that the decline in ovarian function that occurs with age in women with T1D also affects androgen production.
Conclusions
The effects of T1D on reproductive function in women have dramatically changed during the last 50 years, but despite improvements in therapy, these patients still face abnormalities in their pubertal development, menstrual cycles, fertility and age of menopause, with hyperandrogenism and oligomenorrhoea being the most prevalent problems in young adult T1D patients. Moreover, as diabetic patients approach menopause, earlier declines in their ovarian reserves pose another critical problem. In addition, insulin excess caused by more intensive therapeutic protocols has recently been associated with an increased frequency of PCOS-like symptoms among women with T1D. In summary, T1D is a state of metabolic stress that represents a multi-faceted challenge to normal reproductive function throughout life. A better understanding of the nature, evolution and underlying mechanisms of these reproductive complications will help to develop improved diagnostic and therapeutic strategies for an important set of co-morbidities affecting T1D women.
Authors' roles
E.C. wrote the section about the clinical studies, reviewed the complete manuscript and participated in several of the clinical studies that were mentioned in this review. P.M.M. performed the systematic Medline search; reviewed the literature; wrote the Abstract, Introduction and Methods section; and reviewed the final version of the manuscript. M.T.-S. wrote the mechanistic section, participated in several of the experimental studies reviewed in this manuscript and reviewed the final version of the manuscript.
Funding
This work was funded by Fondo Nacional de Ciencia y Tecnología (FONDECYT, Comisión Nacional de Investigación Científica y Tecnológica, Chile) grants 1100123 and 1050452 to E.C. and grants BFU 2008-00984 and BFU 2011-25021 to M.T-.S. (Ministerio de Ciencia e Innovación, Spain). CIBER Fisiopatología de la Obesidad y Nutrición is an initiative of Instituto de Salud Carlos III, Ministerio de Sanidad, Spain.
Conflict of interest
None declared.
Acknowledgements
We thank all of the health professionals who have collaborated with our studies, and we are especially grateful to all of the subjects for their participation.



![Pathophysiology of the reproductive axis in patients with T1D. Modified from Codner and Cassorla. Puberty and ovarian function in girls with T1D mellitus. Horm Res 2009;71:12–21 (Codner and Cassorla, 2009)]. †Catabolism and leptin deficiency secondary to severe insulin deficiency has been observed in patients with ketoacidosis (Fluck et al., 1999; Soliman et al., 2002) *Findings that have been demonstrated in animals.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/humupd/18/5/10.1093/humupd/dms024/2/m_dms02403.gif?Expires=1716314569&Signature=aA1YaZSYv84OWSey1t2P6hqbG4N-Ar4Bb6A39NPZvcGv4IILMn3OCGQB0kXD9Pf~qfm7Iqvw24nYQlXXjoyFvgpJdX6veTtdYKFVr1wmyzWXI~eSRJ7lWvh4894oNEshpMa8YZRSssWn0nyqPPjMAHUYJwptftxbS6lAECRdrseLfZg0bcb1tmD0K55UZf-M4WJ7cnNuxXWn4Z6EErcoa~WGkYLk0i4Z~ZDLXbcLf8sExXi-cM2vnScbe0RqlRCTQZ8lsEVrKVE07FTMK4NLD2jlbafxuSkQW0AjbQyq75MS8SaEfHd4q2tAw3U7FeoPEG-8LrXDuNofrOwbwXMOHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
