Abstract
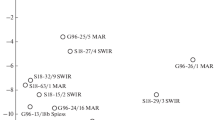
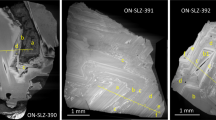
Here we present data on nitrogen and argon isotopic compositions and He–Ar–N–C(CO2) elemental ratios obtained during stepwise crushing of fresh basaltic glasses of the N-MORB family from Mid-Atlantic ridge rift valley at 16°07′–17°11′ N. The bulk nitrogen isotopic composition in the samples varies from δ15N(total) = –5.2 ± 0.2‰ (i.e., typical for MORB glasses) to δ15N(total) = +4.6 ± 0.3‰ (pointing to the presence of organic nitrogen). The δ15N variations in the crushing steps are wider and range from –13.8 to +8.3‰. The 40Ar/36Ar ratios in the crushing steps vary from the value close to that in the atmosphere (~296) up to 11 100 ± 590 (the bulk values cover a range from 355 ± 11 to 2799 ± 159). Correlations between argon and nitrogen isotopic and elemental ratios imply mixing between an N-MORB type mantle component and a surface-derived component enriched in 15N. Carbon (CO2)—nitrogen systematic suggests that the most plausible source for isotopically heavy nitrogen is the organic matter brought into the fluid source. Strong relationships between Ar and N isotopic compositions and Cl, H2O, and K, as well as Ar–N2, N2–CO2 and Ar–He–CO2 systematics, indicate that melt degassing and contamination with atmospheric Аr and organic nitrogen are the two dominant processes responsible for elemental and isotopic variation. The contamination of magmatic melts with surface related noble gases and organic nitrogen occurred through their interaction with high salinity hydrothermal brines. We propose that this contamination mechanism may be universal and largely responsible for the observed variations in the isotopic composition for a number of volatile elements in MORB glasses. However larger set of samples from the hydrothermal fields’ related areas has to be studied for better understanding how common is the established contamination mechanism.













Similar content being viewed by others
REFERENCES
M. Alletti, D. R. Baker, B. Scaillet, A. Aiuppa, R. Moretti, and L. Ottolini, “Chlorine partitioning between a basaltic melt and H2O–CO2 fluids at Mount Etna,” Chem. Geol. 263, 37–50 (2009).
C. J. Ballentine and D. N. Barfod, “The origin of air-like noble gases in MORB and OIB,” Earth Planet. Sci. Lett. 180 (1), 39–48 (2000).
H. Bougault, L. Dmitriev, J. G. Schilling, A. Sobolev, J. L. Joron, and H. D. Needham, “Mantle heterogeneity from trace elements, MAR triple junction near 14° N,” Earth Planet. Sci. Lett. 88 (1), 27–36 (1988).
A. I. Buikin, A. B. Verchovsky, V. A. Grinenko, S. A. Silantyev, V. S. Sevastyanov, Yu A. Nevinny, and E. P. Smirnova, “C, N, He, and Ar isotope and element ratios in fluid inclusions from MORB chilled glasses, Stepwise crushing data,” Geochem. Int. 51 (4), 338–343 (2013).
A. I. Buikin, N. A. Migdisova, J. Hopp, E. V. Korochantseva, and M. Trieloff, “He, Ne, Ar stepwise crushing data on basalt glasses from different segments of Bouvet Triple Junction. Geochem. Int. 55 (11), 977–987 (2017).
A. I. Buikin, A. B. Verchovsky, and N. A. Migdisova, “N–C–Ar–He isotopic systematics of quenched tholeiitic glasses from the Bouvet Triple Junction area,” Geochem. Int. 56 (13), 1368–1383 (2018).
A. I. Buikin, O. V. Kuznetsova, T. A. Velivetskaya, V. S. Sevastyanov, and A. V. Ignatiev, “An isotope ratio mass spectrometry-based method for hydrogen isotopic analysis in sub-microliter volumes of water, Application for multi-isotope investigations of gases extracted from fluid inclusions,” Rapid Comm. Mass Spectrom. 34 (22) (2020).
M. Cannat and J. F. Casey, “An ultramafic lift at the Mid-Atlantic Ridge, Successive stages of magmatism in serpentinized peridotites from the 15 N Region,” In, Mantle and Lower Crust Exposed in Oceanic Ridges and in Ophiolites, Ed. by R. L. M. Vissers (Kluwer Academic Publishers, 1995), pp. 5–34.
P. Cartigny and B. Marty, “Nitrogen Isotopes and Mantle Geodynamics, The Emergence of Life and the Atmosphere-Crust-Mantle Connection,” Elements 9 (5), 359–366 (2013).
P. Cartigny, N. Jendrzejewski, F. Pineau, E. Petit, and M. Javoy, “Volatile (C, N, Ar) variability in MORB and the respective roles of mantle source heterogeneity and degassing, the case of the Southwest Indian Ridge,” Earth Planet. Sci. Lett. 194, 241–257 (2001).
D. Chavrit, R. Burgess, H. Sumino, D. A. H. Teagle, G. Droop, A. Shimizu, and C. J. Ballentine, “The contribution of hydrothermally altered ocean crust to the mantle halogen and noble gas cycles,” Geochim. Cosmochim. Acta 183, 106–124 (2016).
N. Dauphas and B. Marty, “Heavy nitrogen in carbonatites of the Kola Peninsula”: A possible signature of the deep mantle,” Science 286 (5449), 2488 (1999).
L. Dosso, B. B. Hanan, H. Bougault, J. -G. Schilling, and J. -L. Joron, “Sr-Nd-Pb geochemical morphology between 10° and 17°N on the Mid-Atlantic Ridge: a new MORB isotope signature,” Earth Planet. Sci. Lett. 106 (1), 29–43 (1991).
T. P. Fischer, P. Burnard, B. Marty, D. R. Hilton, E. Füri, F. Palhol, Z. D. Sharp, and F. Mangasini, “Upper-mantle volatile chemistry at Oldoinyo Lengai volcano and the origin of carbonatites,” Nature 459 (7243), 77–80 (2009).
D. Harrison, P. Burnard, and G. Turner, “Noble gas behaviour and composition in the mantle, constraints from the Iceland Plume,” Earth Planet. Sci. Lett. 171 (2), 199–207 (1999).
A. W. Hofmann, “Mantle geochemistry: The message from oceanic volcanism,” Nature 385, 219–229 (1997).
G. Holland and C. J. Ballentine, “Seawater subduction controls the heavy noble gas composition of the mantle,” Nature 441(7090), 186–191 (2006).
A. Jambon, H. Weber, and O. Braun, “Solubility of He, Ne, Ar, Kr and Xe in a basalt melt in the range 1250–1600°C. Geochemical implications,” Geochim. Cosmochim. Acta 50 (3), 401– 408 (1986).
M. A. Kendrick, M. Scambelluri, M. Honda, and D. Phillips, “High abundances of noble gas and chlorine delivered to the mantle by serpentinite subduction,” Nature Geosci. 4 (11), 807–812 (2011).
M. A. Kendrick, R. Arculus, P. Burnard, and M. Honda, “Quantifying brine assimilation by submarine magmas: Examples from the Galápagos Spreading Centre and Lau Basin,” Geochim. Cosmochim. Acta 123, 150–165 (2013).
M. A. Kendrick, C. Hémond, V. S. Kamenetsky, L. Danyushevsky, C. W. Devey, T. Rodemann, M. G. Jackson, and M. R. Perfit, “Seawater cycled throughout Earth’s mantle in partially serpentinized lithosphere,” Nature Geosci. 10 (3), 222–228 (2017).
M. A. Kendrick, M. Scambelluri, J. Hermann, and J. A. Padr’on–Navarta, “Halogens and noble gases in serpentinites and secondary peridotites: Implications for seawater subduction and the origin of mantle neon,” Geochim. Cosmochim. Acta 235, 285–304 (2018).
A. J. R. Kent, M. D. Norman, I. D. Hutcheon, and E. M. Stolper, “Assimilation of seawater- derived components in an oceanic volcano, evidence from matrix glasses and glass inclusions from Loihi seamount, Hawaii,” Chem. Geol. 156 (1–4), 299–319 (1999).
E. M. Klein and C. H. Langmuir, “Global correlations of ocean ridge basalt chemistry with axial depth and crustal thickness,” J. Geophys. Res. 92 (B8), 8089 (1987).
H. Kumagai and I. Kaneoka, “Relationship between submarine MORB glass textures and atmospheric component of MORBs,” Chem. Geol. 200 (1), 1–24 (2003).
A. Mallik, Y. Li, and M. Wiedenbeck, “Nitrogen evolution within the Earth’s atmosphere– mantle system assessed by recycling in subduction zones,” Earth Planet. Sci. Lett. 482, 556–566 (2018).
B. Marty and F. Humbert, “Nitrogen and argon isotopes in oceanic basalts,” Earth Planet. Sci. Lett. 152,101–112 (1997).
B. Marty and L. Zimmermann, “Volatiles (H, C, N, Ar) in Mid-Ocean ridge basalts: assessment of shallow-level fractionation and characterization of source composition,” Geochim. Cosmochim. Acta. 63, 3619–3633 (1999).
B. Marty, S. Zashu, and M. Ozima, “Two noble gas components in a Mid-Atlantic Ridge basalt,” Nature, 302 (5905), 238–240 (1983).
S. M. McLennan, S. R. Taylor, M. T. McCulloch, and J. B. Maynard, “Geochemical and Nd-Sr isotopic composition of deep-sea turbidites: Crustal evolution and plate tectonic associations. Geochim. Cosmochim. Acta 54(7), 2015–2050 (1990).
P. Michael and J. -G. Schilling, “Chlorine in mid-ocean ridge magmas, Evidence for assimilation of seawater-influenced components,” Geochim. Cosmochim. Acta 53, 3131–3143 (1989).
R. K. Mohapatra and S. V.S. Murty, “Origin of air-like noble gases in oceanic basalts,” Geophys. Res. Lett. 27 (11), 1583–1586 (2000).
S. Mukhopadhyay, “Early differentiation and volatile accretion recorded in deep–mantle neon and xenon,” Nature 486 (7401), 101–104 (2012).
M. Ozima and F. A. Podosek, Noble Gas Geochemistry (Cambridge University Press, 2002).
R. Parai, S. Mukhopadhyay, J. M. Tucker, and M. K. Pet˝o, “The emerging portrait of an ancient, heterogeneous and continuously evolving mantle plume source,” Lithos 346, 105153 (2019).
D. B. Patterson, M. Honda, and I. Mcdougall, “Atmospheric Contamination - a Possible Source for Heavy Noble-Gases in Basalts from Loihi Seamount, Hawaii,” Geophys. Res. Lett. 17 (6), 705–708 (1990).
K. E. Peters, R. E. Sweeney, and L. R. Kaplan, “Correlation of carbon and nitrogen stabel isotope ratios in sedimentary organic matter,” Limnol. Oceanogr. 23, 598–604 (1978).
F. Pineau and M. Javoy, “Carbon isotopes and concentrations in mid-oceanic ridge basalts,” Earth Planet. Sci. Lett. 62 (2), 239–257 (1983).
D. L. Pinti and K. Hashizume, “15N-depleted nitrogen in Early Archean kerogens, clues on ancient marine chemosyntetic-based ecosystems? A comment to V. Beaumont and F. Robert, Precambrian Res. 96, 62–82,” Precambrian Res. 105, 85–88 (2001).
D. C. Presnall and J. D. Hoover, 1987. “High pressure phase equilibrium constraints on the origin of mid-ocean ridge basalts,” In: Magmatic processes: Physicochemical Principles, Ed. by B. O.Mysen, Geochem. Soc. Spec. Publ., No. 1, 75–89 (1987)
P. Sarda, M. Moreira, and T. Staudacher, “Argon-lead isotopic correlation in Mid-Atlantic Ridge basalts,” Science, 283 (5402), 666–668 (1999).
H. Shinohara, “A missing link between volcanic degassing and experimental studies on chloride partitioning,” Chem. Geol. 263 (1), 51–59 (2009).
Shipboard Scientific Party, “Leg 209 Preliminary Report,” (2003).
Shipboard Scientific Party, “SERPENTINE. Scientific Cruise Report (Iferemer, Centre de Brest) (2007).
S. A. Silantyev, L. V. Danyushevsky, A. A. Plechova, L. Dosso, B. A. Bazylev, and V. E. Bel’tenev, “Geochemical and isotopic signatures of magmatic products in the MAR rift valley at 12°49′– 17°23′ N and 29°59′–33°41′ N: evidence of Two contrasting sources of the parental melts,” Petrology 16 (1), 36–62 (2008).
J. Stelling, R. E. Botcharnikov, O. Beermann, and M. Nowak, “Solubility of H2O- and chlorine-bearing fluids in basaltic melt of Mount Etna at T = 1050–1250°C and P = 200 MPa,” 256 (3-4), 102–110 (2008).
N. A. Stroncik and S. Niedermann, “Atmospheric contamination of the primary Ne and Ar signal in mid-ocean ridge basalts and its implications for ocean crust formation,” Geochim. Cosmochim. Acta 172, 306–321 (2016).
H. Sumino, R. Burgess, T. Mizukami, S. R. Wallis, G. Holland, and C. J. Ballentine, “Seawater-derived noble gases and halogens preserved in exhumed mantle wedge peridotite,” Earth Planet. Sci. Lett. 294 (1), 163–172 (2010).
S. S. Sun and W. F. McDonough, “Chemical and isotopic systematics of oceanic basalts, implications for mantle composition and processes,” Magmatism in the Ocean Basins, Ed. by A. D. Saunders & M. J. Norry, Geol. Soc. Spec. Publ. 42, 313–345 (1989).
I. N. Tolstikhin, J. D. Kramers, and A. W. Hofmann, “A chemical Earth model with whole mantle convection, The importance of a core–mantle boundary layer (D″) and its early formation,” Chem. Geol. 226 (3–4), 79–99 (2006).
M. Trieloff, M. Falter, and E. K. Jessberger, “The distribution of mantle and atmospheric argon in oceanic basalt glasses,” Geochim. Cosmochim. Acta 67 (6), 1237–1253 (2003).
A. B. Verchovsky, M. A. Sephton, I. P. Wright, and C. T. Pillinger, “Separation of planetary noble gas carrier from bulk carbon in enstatite chondrites during stepped combustion,” Earth Planet. Sci. Lett. 199 (3), 243–255 (2002).
A. B. Verchovsky, “Origin of isotopically light nitrogen in meteorites,” Geochem. Int. 55 (11), 957–970 (2017).
A. B. Verchovsky, N. A. Goltsin, E. M. Prasolov, and K. I. Lokhov, “Nitrogen isotopic composition of shungite from the Onega Structure, Russia, and the origin of the organic matter,” Geochem. Int. 56 (13), 1341–1353 (2018).
M. Wilson, Igneous Petrogenesis (Unwin Hyman, London, 1989).
F. Witham, J. Blundy, S. C. Kohn, P. Lesne, J. Dixon, S. V. Churakov, and R. Botcharnikov, “SolEx, a model for mixed COHSCl-volatile solubilities and exsolved gas compositions in basalt,” Comp. Geosci. 45, 87–97 (2012).
A. Y. Yang, C. H. Langmuir, Y. Cai, P. Michael, S. L. Goldstein, and Z. Chen, “A subduction influence on ocean ridge basalts outside the Pacific subduction shield,” Nature Commun. 12 (1), 4757 (2021).
A. Zindler and S. R. Hart, “Chemical geodynamics,” Ann. Rev. Earth Planet. Sci. 14, 493–571 (1986).
ACKNOWLEDGMENTS
The authors acknowledge reviewers O. Lukanin and M. Gannibal for constructive reviews that improved the manuscript.
Funding
The work was partially supported by Russian Science Foundation grant no. 22-27-00815.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Buikin, A.I., Silantyev, S.A. & Verchovsky, A.B. N–Ar–He–CO2 Systematics Combined with H2O, Cl, K Abundances in MORB Glasses Demonstrate Interaction of Magmatic and Hydrothermal Systems: a Case for MAR at 16°07′–17°11′ N. Geochem. Int. 60, 1068–1086 (2022). https://doi.org/10.1134/S0016702922110027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702922110027