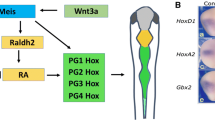
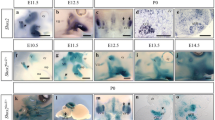
Abstract
Respiration is a rhythmic motor behavior that appears in the fetus and acquires a vital importance at birth. It is generated within central pattern-generating neuronal networks of the hindbrain. This region of the brain is of particular interest since it is the most understood part with respect to the cellular and molecular mechanisms that underlie its development. Hox paralogs and Hox-regulating genes kreisler/mafB and Krox20 are required for the normal formation of rhombomeres in vertebrate embryos. From studies of rhombomeres r3 and r4, the authors review mechanisms whereby these developmental genes may govern the early embryonic development of para-facial neuronal networks and specify patterns of motor activities operating throughout life. A model whereby the regional identity of progenitor cells can be abnormally specified in r3 and r4 after a mutation of these genes is proposed. Novel neuronal circuits may develop from some of these misspecified progenitors while others are eliminated, eventually affecting respiration and survival after birth.
Similar content being viewed by others
References
Lumsden T. L. (1923) Observation on the respiratory centres in the cat. J. Physiol. 57, 153–160.
Harper R. M., Kinney H. C., Fleming P. J., and Thach B. T. (2000) Sleep influences on homeostatic functions: implications for sudden infant death syndrome. Respir. Physiol. 119(2–3), 123–132.
Kobayashi S., Nishimura M., Yamamoto M., Akiyama Y., Kishi F., and Kawakami Y. (1993) Dyspnea sensation and chemical control of breathing in adult twins. Am. Rev. Respir. Dis. 139, 1192–1198.
Redline S. R. and Tishler P. V. (2000) The genetics of sleep apnea. Sleep Med. Rev. 4, 583–602.
Tankersley C. G., Fitzgerald R. S., Levitt R. C., Mitzner W. A., Ewart S. L., and Kleeberger S. R. (1997) Genetic control of differential baseline breathing pattern. J. Appl. Physiol. 82, 874–881.
Mortola J. P. (1987) Dynamics of breathing in newborn mammals. Physiol. Rev. 67, 187–234.
Lumsden A. and Keynes R. (1989) Segmental patterns of neuronal development in the chick hindbrain. Nature 337, 424–428.
Lumsden A. (1990) The cellular basis of segmentation in the developing hindbrain. TINS 13, 329–335.
Lumsden A. and Krumlauf R. (1996) Patterning the vertebrate neuraxis. Science 274, 1109–1115.
Rijli F. M., Gavalas A., and Chambon P. (1998) Segmentation and specification in the branchial region of the head: the role of the Hox selector genes. Int. J. Dev. Biol. 42, 393–401.
Dupe V., Davenne M., Brocard J., Dolle P., Mark M., Dierich A., Chambon P., and Rijli F. M. (1997) In vivo functional analysis of the Hoxa-1 3′ retinoic acid response element (3′RARE). Development 124(2), 399–410.
Studer M., Gavalas A., Marshall H., Ariza-McNaughton L., Rijli F. M., Chambon P., and Krumlauf R. (1998) Genetic interactions between Hoxa1 and Hoxb1 reveal new roles in regulation of early hindbrain patterning. Development 125, 1025–1036.
Gavalas A. and Krumlauf R. (2000) Retinoid signalling and hindbrain patterning. Curr. Opin. Genet. Dev. 10(4), 380–386.
Graham A., Maden M., and Krumlauf R. (1991). The murine Hox-2 genes display dynamic dorsoventral patterns of expression during central nervous system development. Development 112(1), 255–264.
Tiret L., Le Mouellic H., Maury M., and Brulet P. (1998) Increased apoptosis of motoneurons and altered somatotopic maps in the brachial spinal cord of Hoxc-8 deficient mice. Development 125, 279–291.
Davenne M., Maconochie M. K., Neun R., Pattyn A., Chambon P., Krumlauf R., and Rijli F. M. (1999) Hoxa2 and Hoxb2 control dorsoventral patterns of neuronal development in the rostral hindbrain. Neuron 22, 677–691.
Gaufo G. O., Flodby P., and Capecchi M. R. (2000) Hoxb1 controls effectors of sonic hedgehog and Mash1 signaling pathways. Development 127, 5343–5354.
Mark M., Lufkin T., Vonesh J. L., et al. (1993) Two rhombomeres are altered in Hoxa-1 mutant mice. Development 119, 319–338.
Carpenter E. M., Goddard J. M., Chisaka O., Manley N. R., and Capecchi M. R. (1993) Loss of Hoxa1 (Hox-1.6) function results in the reorganization of the murine hindbrain. Development 118, 1063–1075.
Studer M., Lumsden A., Ariza-McNaughton L., Bradley A., and Krumlauf R. (1996) Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature 384, 630–634.
Goddard J. M., Rossel M., Manley N. R., and Capecchi M. R. (1996) Mice with targeted disruption of Hoxb-1 fail to form the motor nucleus of the VIIth nerve. Development 122, 3217–3228.
Barrow J. R. and Capecchi M. R. (1996) Targeted disruption of the Hoxb-2 locus in mice interferes with expression of Hoxb-1 and Hoxb-4. Development 122(12), 3817–3828.
Gavalas A., Davenne M., Lumsden A., Chambon P., and Rijli F. M. (1997) Role of Hoxa-2 in axon pathfinding and rostral hindbrain patterning. Development 124, 3683–3691.
Gavalas A., Studer M., Lumsden A., Rijli F. M., Krumlauf R., and Chambon P. (1998) Hoxa1 and Hoxb1 synergize in patterning the hindbrain, cranial nerves and second pharyngeal arch. Development 125, 1123–1136.
Helmbacher F., Pujades C., Desmarquet C., Frain M., Rijli F. M., Chambon P., and Charnay P. (1998) Hoxa1 and Krox-20 synergize to control the development of rhombomere 3. Development 125, 4739–4748.
Rossel M. and Capecchi M. R. (1999) Mice mutant for both Hoxa1 and Hoxb1 show extensive remodeling of the hindbrain and defects in craniofacial development. Development 126, 5027–5040.
Jungbluth S., Bell E., and Lumsden A. (1999) Specification of distinct motor neuron identities by the singular activities of individual Hox genes. Development 126, 2751–2758.
Bell E., Wingate R. J., and Lumsden A. (1999) Homeotic transformation of rhombomere identity after localized Hoxb1 misexpression. Science 284, 2168–2171.
Barrow J. R., Stadler H. S., and Capecchi M. R. (2000) Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse. Development 127(5), 933–944.
Pasqualetti M., Neun R., Davenne M., and Rijli F. M. (2001) Retinoic acid rescues inner ear defects in Hoxa1 deficient mice. Nat Genet. 29(1), 34–39.
Pasqualetti M. and Rijli F. M. (2001) Homeobox gene mutations and brain-stem developmental disorders: learning from knockout mice. Curr Opin Neurol. 14(2), 177–184.
Pattyn A., Vallstedt A., Dias J. M., Samad O. A., Krumlauf R., Rijli F. M., Brunet J. F., and Ericson J. (2003) Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 17(6), 729–737.
Maves L., Jackman W., and Kimmel C. B. (2002) FGF3 and FGF8 mediate a rhombomere 4 signaling activity in the zebrafish hindbrain. Development 129, 3825–3837.
Walshe J., Maroon H., McGonnell IM., Dickson C., and Mason I. (2002) Establishment of hindbrain segmental identity requires signaling by FGF3 and FGF8. Curr. Biol. 12, 1117–1123.
Marin F. and Charnay P. (2000) Hindbrain patterning: FGFs regulate Krox20 and mafB/kr expression in the otic/preotic region. Development 127, 4925–4935.
Waskiewicz A. J., Rikhof H. A., and Moens C. B. (2002) Eliminating zebrafish PBX proteins reveals a hindbrain ground state. Dev. Cell 3, 723–733.
Hertwig P. (1942) Sechs neue Mutationen bei der Hausmaus in ihrer Bedeutung für allgemeine Vererbungsfragen. YX. Menschl. Vererbgslehre 26, 1–21.
Hertwig P. (1944) Die Genese der Hirn- und Gehörorganmißbildungen bei röntgenmutierten Kreisler-Maüsen. Zeit. Konstlehre 28, 327–354.
Deol M. S. (1964) The abnormalities of the inner ear in kreilser mice. J. Embryol. Exp. Morphol. 12, 475–490.
Manzanares M., Cordes S., Kwan C. T., Sham M. H., Barsh G. S., and Krumlauf R. (1997) Segmental regulation of Hoxb3 by kreisler. Nature 387, 191–195.
Manzanares M., Cordes S., Ariza-McNaughton L., Sadl V., Maruthainar K., Barsh G. S., and Krumlauf R. (1999) Conserved and distinct roles of kreisler in regulation of the paralogous Hoxa3 and Hoxb3 genes. Development 726(4), 759–769.
Wilkinson D. G., Bhatt S., Chavrier P., Bravo R., and Charnay P. (1989) Segment-specific expression of a zinc finger gene in the developing nervous system of the mouse. Nature 337, 461–464.
Sham M. H., Vesque C., Nonchev S., et al. (1993) The zinc finger gene Krox20 regulates Hoxb-2 (Hox 2.8) during hindbrain segmentation. Cell 72(2), 183–196.
Nonchev S., Maconochie M., Vesque C., et al. (1996) The conserved role of Krox20 in directing Hox gene expression during vertebrate hindbrain segmentation. Proc. Natl. Acad. Sci. USA 93(18), 9339–9345.
Giudicelli F., Taillebourg E., Charnay P., and Gilardi-Hebenstreit P. (2001) Krox20 patterns the hindbrain through both cell-autonomous and non cell-autonomous mechanisms. Genes Dev. 15(5), 567–580.
Schneider-Maunoury S., Topilko P., Seitandou T., et al. (1993) Disruption of Krox20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell 75(6), 1199–1214.
Schneider-Maunoury S., Seitanidou T., Charnay P., and Lumsden A. (1997) Segmental and neuronal architecture of the hindbrain of Krox-20 mouse mutants. Development 124, 1215–1226.
Swiatek P. J. and Gridley T. (1993) Perinatal lethality and defects in hindbrain development in Krox20 −/− mice for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 7(11), 2071–2084.
Rovainen C. M. (1996) Feeding and breathing in lampreys. Brain Behav. Evol. 48(5), 297–305.
Wild J. M. (1993) The avian nucleus retroambigualis: a nucleus for breathing, singing and calling. Brain Res. 606, 319–324.
Pack A. I. (1993) In: Respiratory Control (Speck, D. F., Dekin, M. S., Revelette, W. R. and Frazier, D. T., eds). Univ. Press of Kentucky, Lexington, pp. 52–57.
McLean H. A., Perry S. F., and Remmers J. E. (1995) Two regions in the isolated brainstem of the frog that modulate respiratory-related activity. J. Comp. Physiol. 177(2), 135–144.
Wilson R. J., Vasilakos K., Harris M. B., Straus C., and Remmers J. E. (2002) Evidence that ventilatory rhythmogenesis in the frog involves two distinct neuronal oscillators. J. Physiol. 540(2), 557–570.
Takeda R., Remmers J. E., Baker J. P., and Farber J. P. (1986) Postsynaptic potentials of bulbar respiratory neurons of the turtle. Respir. Physiol. 64, 149–160.
Fortin G., Foutz A. S., and Champagnat J. (1994) Respiratory rhythm generation in chick hindbrain: effects of MK-801 and vagotomy. NeuroReport 5(9), 1137–1140.
Milsom W. K. (1991) Intermittent breathing in vertebrates. Annu. Rev. Physiol. 53, 87–105.
Perry S. F., Wilson R. J., Straus C., Harris M. B., and Remmers J. E. (2001) Which came first, the lung or the breath? Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 129(1), 37–47.
Bianchi A. L., Denavit-Saubié M., and Champagnat J. (1995) Central control of breathing in mammals: neuronal circuitry, membrane properties and neurotransmitters. Physiol. Rev. 75, 1–45.
Richter D. W. and Spyer K. M. (2001) Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 24(8), 464–472.
Suzue T. (1984) Respiratory rhythm generation in the in vitro brainstem-spinal cord preparation of the neonatal rat. J. Physiol. 354, 173–183.
Smith J. C., Ellenberger H. H., Ballanyi K., Richter D. W., and Feldman J. L. (1991) Pre-Bötzinger complex: a region that may generate respiratory rhythm in mammals. Science 254, 726–729.
Di Pasquale E., Monteau R., and Hilaire G. (1992) In vitro study of central respiratory-like activity of the fetal rat. Exp. Brain Res. 89, 459–464.
Onimaru H. and Homma I. (1987) Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 403(2), 380–384.
Gray P. A., Rekling J. C., Bocchiaro C. M., and Feldman J. L. (1999) Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 286, 1566–1568.
Lieske S. P., Thoby-Brisson M., Telgkmap P., and Ramirez J. M. (2000) Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat. Neurosci. 3(6), 600–607.
Borday V., Kato F., and Champagnat J. (1997) A ventral pontine pathway promotes rhythmic activity in the medulla of neonate mice. NeuroReport 8, 3679–3683.
Onimaru H. and Homma I. (2003) A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J. Neurosci. 23(4), 1478–1486.
Denavit-Saubie M., Champagnat J., and Zieglgansberger W. (1978) Effects of opiates and methionine-enkephalin on pontine and bulbar respiratory neurones of the cat. Brain Res. 155(1), 55–67.
Morin-Surun M.-P., Boudinot E., Gacel G., Champagnat J., Roques B. P., and Denavit-Saubie M. (1984) Different effects of mu and delta opiate agonists on respiration. Eur. J. Pharmacol. 98(2), 235–240.
Morin-Surun M.-P., Boudinot E., Dubois C., et al. (2001) Respiratory function in adult mice lacking the mu-opioid receptor: role of deltareceptors. Eur. J. Neurosci. 13(9), 1703–1710.
Mellen N. M., Janczewski W. A., Bocchiaro C. M., and Feldman J. L. (2003) Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37(5), 821–826.
Morin-Surun M. P., Boudinot E., Kato F., Foutz A. S., and Denavit-Saubié M. (1995) Involvement of NMDA receptors in the respiratory phase transition is different in the adult guinea pig in vivo and in the isolated brainstem preparation. J. Neurophysiol. 74, 770–778.
Blanco C. E. (1994) Maturation of fetal breathing activity. Biol. Neonate 65, 182–188.
Jansen A. H. and Chernick V. (1983) Development of respiratory control. Physiol. Rev. 63, 437–483.
Ramirez J. M., Quellmalz U. J., and Richter D. W. (1996) Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J. Physiol. 491, 799–812.
DeVries J. I. P., Visser G. H. A., and Prechtl H. F. R. (1982) The emergence of behavior. I. Qualitative aspects. Early Hum. Dev. 7, 301–322.
Suzue T. (1994) Mouse fetuses in late gestation maintained in vitro by transplacental. Neurosci. Res. 21, 173–176.
Greer J. J., Smith J. C., and Feldman J.. (1992) Respiratory and locomotor patterns generated in the fetal rat brainstem-spinal cord in vitro. J. Neurophysiol. 67, 996–999.
Fortin G., Champagnat J., and Lumsden A. (1994) Onset and maturation of branchiomotor activities in the chick hindbrain. NeuroReport 5(9), 1149–1152.
Fortin G., Kato F., Lumsden A., and Champagnat J. (1995) Rhythm generation in the segmented hindbrain of chick embryos. J. Physiol. 486(3), 735–744.
Clarke J. D. and Lumsden A. (1993) Segmental repetition of neuronal phenotype sets in the chick embryo hindbrain. Development 118, 151–162.
Lumsden A., Clarke J. D. W., Keynes R., and Fraser S. (1994) Early phenotypic choices by neuronal precursors, revealed by clonal analysis of chick embryo hindbrain. Development 120(6), 1581–1589.
Domínguez del Toro E., Borday V., Davenne M., Neun R., Rijli F. M., and Champagnat J. (2001) Generation of a novel functional neuronal circuit in Hoxa1 mutant mice. J. Neurosci. 21(15), 5637–5642.
Gavalas A., Trainor P., Ariza-McNaughton L., and Krumlauf R. (2001) Synergy between Hoxa1 and Hoxb1: the relationship between arch patterning and the generation of cranial neural crest. Development 128(15), 3017–3027.
Popperl H., Bienz M., Studer M., Chan S. K., Aparicio S., Brenner S., Mann R. S., and Krumlauf R. (1995) Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell 81(7), 1031–1042.
Di Rocco G., Mavilio F., and Zappavigna V. (1997) Functional dissection of a transcriptionally active, target-specific Hox-Pbx complex. EMBO J. 16(12), 3644–3654.
Giudicelli F., Gilardi-Hebenstreit P., Mechta-Grigoriou F., Poquet C., and Charnay P. (2003) Novel activities of Mafb underlie its dual role in hindbrain segmentation and regional specification. Dev. Biol. 253, 150–162.
Manzanares M., Trainor P. A., Nonchev S., et al. (1999) The role of kreisler in segmentation during hindbrain development. Dev. Biol. 211, 220–237.
Voiculescu O., Taillebourg E., Pujades C., Kress C., Buart S., Charnay P., and Schneider-Maunoury S. (2001) Hindbrain patterning: Krox20 couples segmentation and specification of regional identity. Development 128, 4967–4978.
Chatonnet F., Domínguez del Toro E., Voiculescu O., Charnay P., and Champagnat J. (2002) Different respiratory control systems are affected in homozygous and heterozygous kreisler mutant mice. Eur. J. Neurosci. 15, 684–692.
Lund J. P., Kolta A., Westberg K. G., and Scott G. (1998) Brainstem mechanisms underlying feeding behaviors. Curr. Opin. Neurobiol. 8, 718–724.
Jacquin T. D., Borday V., Schneider-Maunoury S., Topilko P., Ghilini G., Kato F., Charnay P., and Champagnat J. (1996) Reorganisation of pontine rhythmogenic neuronal networks in Krox-20 knockout mice. Neuron 17, 747–758.
Fortin G., Jungbluth S., Lumsden A., and Champagnat J. (1999) Segmental specification of GABAergic inhibition during development of hindbrain neural networks. Nat. Neurosci. 2, 873–877.
Abadie V., Champagnat J., and Fortin F. (2000) Branchiomotor activities in mouse embryo. Neuroreport 11(1), 141–145.
Dawes G. S., Gardner W. N., Johnston B. M., and Walker D. W. (1983) Breathing in fetal lambs: the effect of brainstem section. J. Physiol. 335, 535–553.
Hanson M. A. (1988) The importance of baro- and chemoreflexes in the control of the fetal cardiovascular system. J. Dev. Physiol. 10(6), 491–511.
Kinney H. C., Filiano J. J., Sleeper L. A., Mandell F., Valdes-Dapena M., and Frost White W. (1995) Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science 269, 1446–1450.
Vert P., Aranda J. V., Catz C., and Yaffe S. (1994) Control of breathing during development, Apnea of the newborn, and in Sudden Infant Death Syndrome: In Biol. Neonate (J. P. Relier, ed.). Karger, Basel, Switzerland, pp. 133–280.
Cortez S. C. and Kinney H. C. (1996) Brainstem tegmental necrosis and olivary hypoplasia: a lethal entity associated with congenital apnea. J. Neuropathol. Exp. Neurol. 55, 841–849.
Erickson J. T., Conover J. C., Borday V., Champagnat J., and Katz D. M. (1996) Mice lacking BDNF exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J. Neurosci. 16, 5361–5371.
Gozal D. and Harper R. M. (1999) Novel insights into congenital central hypoventilation syndrome. Curr. Opin. Pulm. Med. 5(6), 335–338.
Abadie V., Champagnat J., Fortin G., and Couly G. (1999) Sucking-deglutition-respiration and brain stem development genes. Arch. Pediatr. 6, 1043–1047.
Lijowska A., Reed N., Chiodini B., and Thach B. (1995) Sequential reflex behavior leading to airway defense maneuvers and arousal from sleep in infants. Respirat. Crit. Care Med. 151, A151.
Champagnat J. and Fortin G. (1996) Primordial respiratory-like rhythm generation in the vertebrate embryo. TINS 20, 119–124.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatonnet, F., del Toro, E.D., Thoby-Brisson, M. et al. From hindbrain segmentation to breathing after birth. Mol Neurobiol 28, 277–293 (2003). https://doi.org/10.1385/MN:28:3:277
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/MN:28:3:277