
Mediastinal staging for non-small cell lung cancer
Introduction
The correct evaluation of mediastinal and hilar lymph nodes (Ns) is critical for choosing the best option for the management and treatment of patients with non-small cell lung cancer (NSCLC) who are potential candidates for curative therapeutic strategies, including surgery, radiotherapy, chemotherapy, and multimodal treatments (1-7). For preoperative mediastinal staging the current guidelines state that it is important to confirm the absence of neoplastic mediastinal involvement with the highest level of certainty possible before performing surgical resection (1,3). Computed tomography (CT) imaging or positron emission tomography (PET) combined with CT (PET-CT) should be used for mediastinal exploration. Moreover, based on the recommendations and imaging results, both the European Society of Thoracic Surgeons (ESTS) and American Thoracic Society (ATS) agree that a mediastinal examination should be performed in every potentially resectable case except for patients with primary tumours with a diameter of 3 cm or less with no evidence of nodal involvement according to these imaging techniques (1,3). In addition, these societies also agree that the techniques used should be as little invasive as possible while also maximising accuracy.
Thus, mediastinal examination should begin by using endoscopic techniques which are less invasive than surgical techniques (1-8). The most diagnostically accurate endoscopic techniques currently available are endobronchial or oesophageal ultrasound-guided techniques. Therefore, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), or their combination known as mediastinal endosonography (1-8), have overtaken endoscopic blind puncture techniques in terms of diagnostic accuracy. However, to make the results of mediastinal endosonography reliable, this technique should adhere to international standards (9). There are factors that can produce false negatives (FNs) in EBUS-TBNA such as the extensiveness of satisfactory node sampling or the presence of nodes which cannot be reached, especially tumours located on the left side of the body (10). Other factors that, when combined, increase the risk of mediastinal malignancy include the tumour characteristics [histological type, consolidation/tumour ratio, tumour size, and maximum standard uptake value (SUV)], clinical parameters [serum carcinoembryonic antigen (CEA) level and patient age], and methylation biomarkers (7,11).
Surgical techniques including cervical mediastinoscopy or more extensive surgical procedures are used to validate negative results obtained by mediastinal endosonography. However, the accuracy of endosonography is similar to mediastinoscopy and therefore, in every patient should also be assessed by an expert multidisciplinary lung cancer committee in order to predict their risk of malignancy in terms of nodal involvement. Postoperative pathological staging performed during surgery by combining imaging techniques and sampling procedures is also crucial in planning therapeutic plans for patients. Nonetheless, there is still no consensus regarding the extent of nodal resection required for adequate staging (4,12,13) although the International Association of Lung Cancer Study (IASLC) recommends the exploration and sampling of at least six lymph node areas (4).
Several studies have reported that systematic lymph node dissection (SND) decreases the rate of undetected malignant lymph node involvement (4). It is important that patients be diagnosed as accurately as possible because the incomplete removal of lymph nodes during surgery could result in a FN N status diagnosis meaning that the patient is not put on adjuvant therapy. Thus, the recommendations for inter-operative sampling designed to avoid downstaging errors is another issue which remains open (4). The recommendations for restaging after induction therapy are also difficult to define because of a high rate of false-positive or FN results (between 20% and 30%), despite the variety of imaging and sampling techniques used for primary staging (4-6). Therefore, the best approach in these cases is the case-by-case assessment of patients by an expert multidisciplinary lung cancer committee.
This review summarises the best approach for mediastinal staging and restaging of NSCLC based on the latest available evidence. It also includes a critical review of the issues that remain open to discussion based on disagreements between different recommendations and emphasises individual discussion as well as the expertise of multidisciplinary lung cancer committees.
Imaging techniques
The clinical diagnosis of N1, N2, or N3 cancer corresponds to different disease subgroups defined by the nodal zone affected and also considers the primary tumour (T) location. The anatomical area defined by the nodal zone includes one or more nodal stations located in the same area; the mediastinal oncological midline is located in the left paratracheal margin. Therefore, tumours on the left or right side of the body involve pretracheal or subcarinal lymph nodes and are considered N3 or N2, respectively (14). CT imaging is the best tool for defining anatomical margins and nodal zones and for measuring lymph node size.
The presence of lymph nodes whose size exceed 10 mm on the short axis should be considered a predictor of malignancy. However, results from studies focused in the accuracy of lymph node size for malignancy prediction have demonstrated that size alone is insufficient to confirm or discard metastatic infiltration of lymph nodes because of the low sensitivity (55%) reported for this technique (1-9,15). Additionally, the clinical relevance of nodal enlargement is limited because metastases have been found in about 20% of lymph nodes with dimensions under 1 cm on the short axis (6).
Other signs are based on morphological criteria such as necrosis or disruption of the capsule, but are also not enough for a diagnosis of malignancy. Despite these limitations, CT scans still represent the basis of pre-treatment staging but should be combined with PET scans to improve the adequate planning of biopsies. PET determines the metabolic behaviour of tissues in order to establish the probability of malignancy. The combination of PET-CT may allow malignant lymph nodes to be identified with better accuracy because it combines both structural and metabolic information. The accuracy of PET-CT is also influenced by the clinical probability of malignancy. In addition, lymph nodes smaller than 1 cm may be PET negative despite being malignant (FNs). Nonetheless, PET-CT has been shown to be 80–90% more sensitive than CT, especially for peripheral tumours (1-6,9,16,17).
The main problem with using PET scans to evaluate mediastinal nodes is the lack of standardised criteria for defining positive findings, although a SUV value exceeding 2.5 is currently used to define peripherical masses. In addition, there are also non-neoplastic processes such as granulomas and other inflammatory diseases or infections that can lead to positive PET findings (1). Another concern is that PET imaging is not as good as CT techniques for defining mediastinal anatomical structures, including lymph nodes sizes. Therefore, integrated PET-CT is the most recommended technique for complete imaging mediastinal staging to detect occult Ns and to guide mediastinal histology sampling with non-invasive techniques.
Furthermore, fusion PET-CT with simultaneous radiological contrast CT in patients with nodules, lung mass(es), or mediastinal widening could save time in the diagnostic staging process. Mediastinum-positive PET-CT scans will require mandatory sampling; special PET-CT-positive cases with bulky lymph nodes (short axis exceeding 2 cm) should also be sampled. However, if the probability of malignancy is high and requires surgical staging, the diagnosis of malignancy can be assumed after case-by-case discussion by an expert multidisciplinary lung cancer committee.
In a meta-analysis including 779 NSCLC patients with T1–2, N0, PET-CT imaging negative cases demonstrated a high negative predictive value (NPV) (94%) in tumours less than 3 cm compared to less than 90% for tumours whose size exceeded 3 cm (16,17). In another study, the rate of unforeseen N2s after surgery was significantly lower in peripheral tumours compared with those with a central location (2.9% vs. 21.6%), thus indicating that the latter had a substantial risk of having occult nodal disease (3-9). In addition, adenocarcinomas also tend to have increased SUVs (4,18,19) and for tumours larger than 3 cm, especially adenocarcinomas with high SUVs, further histological mediastinal techniques should be considered.
Non-surgical techniques
The non-surgical techniques used are bronchoscopy or oesophagus endoscopy sampling. When properly performed, blind TBNA is sometimes adequate when the lymph nodes exceed 2 cm in size. The results reported for its accuracy widely vary between centres and it is also critically dependent on the prevalence of mediastinal metastasis (20). However, TBNA usually has a low sensitivity and high FN rate (78% and 28%, respectively). Thus, this technique cannot be established as a definitive mediastinal staging method (20).
EBUS-TBNA and EUS-FNA are endoscopy techniques that have shown a high diagnostic value for mediastinal staging in patients with NSCLC. Compared to mediastinoscopy—the current gold-standard for mediastinal lymph node staging—mediastinal lymph node sampling with these procedures is associated with reduced morbidity (21). EBUS-TBNA can access lymph nodes in paratracheal (stations 2 and 4), subcarinal (station 7), and hilar (stations 10, 11, and 12) areas. While EUS-FNA is useful in the study of lymph nodes in the subcarinal (station 7) and paraesophageal (stations 8 and 9) areas.
In a systematic review analysing data from 2,756 patients undergoing EBUS-TBNA, the median sensitivity was 89% (range, 46–97%) and the median NPV was 91% (1) while for EUS-FNA, the sensitivity was similar and the NPV was a little lower (86%). The use of both these techniques ensures access to the most mediastinal lymph node areas, with the exception of stations 5 and 6. Moreover, experienced teams recommend the systematic exploration of all accessible mediastinal nodes (22-24). Although the mediastinal area (region 5) lymph nodes are not normally accessible for puncture, depending on their anatomical position they can sometimes be accessed and punctured via the oesophagus, as shown in Figure 1. In this case, a median sensitivity and specificity of 91% and 100% and a NPV of 96% have been reported (25-27).
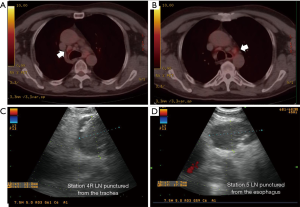
To answer the key question of whether validation of negative EBUS/EUS results by surgical examination of the mediastinum is necessary, some studies have shown that both tests have a similar diagnostic accuracy (24). A randomised study performed by Sharples et al. comparing endosonography staging followed by surgery to surgical staging alone found that the former group had greater sensitivity for negative results (85% vs. 79%) and also resulted in fewer unnecessary thoracotomies (28). Thus, these two techniques could be complementary to each other. Indeed, the ASTER randomised controlled trial which analysed both strategies from both a clinical and cost-effectiveness viewpoint found that the median sensitivity for the endosonography strategy was 94% (85–98%) compared to 79% (66–88%) for the surgical arm, corresponding a NPV of 93% (84–97%) and 86% (76–92%), respectively. The rate of unnecessary thoracotomies in the surgical arm was 7%, a significantly higher value than reported in the endosonography arm (P=0.002). Thus, the cost of the endosonography strategy was significantly lower and the resulting patient quality of life significantly better (28).
The ASTER trial also demonstrated equal 5-year survival rates of 35% in both arms (29). Thus, endosonography was unquestionably valuable in decision making processes, particularly when the results were positive. A wider range of variables had to be considered in cases with negative results, including those related with lymph nodes such as site, echographic features, size, and PET uptake, tumour characteristics such as type, size, stage, and location, performance of the procedure under the optimal conditions (number of puncture passes, number and location of station sampled, and type of sedation used), the experience of endoscopists and pathologists, as well as the sample viability for tumour cell analysis, especially when necrosis or blood contamination was also present (8-10). Thus, according as current guideline recommendations, multidisciplinary discussion of individual cases could help in decision-making processes in the case of negative endosonography results (26).
The consensus of three previous meta-analyses was that the probability of having mediastinal metastatic disease after receiving negative endosonography results was 13–15%, which is not sufficiently low to avoid the need for surgical confirmation. According to these reports, mediastinal staging should start with endosonography-guided puncture followed by subsequent surgical procedures only in the case of negative results. A metanalysis which included 42 studies and a total of 3,248 patients, the rate of unforeseen N2s after negative endosonography was similar in patients who underwent surgical resection with or without prior surgical mediastinal staging (30). Thus, there is sufficient evidence to recommend surgical resection alone for early stage tumours. In addition, the future MEDIASTrial will study the additional value and cost-effectiveness of performing mediastinoscopy in endosonography- negative patients with NSCLC (31).
International guidelines agree that EBUS-TBNA and/or EUS-FNA should be employed after PET-CT for optimal staging of intrathoracic lymph nodes. A systematic approach should be applied, starting by sampling N3 nodes, followed N2, and then N1 lymph nodes, puncturing any lymph node measuring in excess of 5 mm on the short axis. The SCORE study showed that endosonography systematic nodal sampling (EBUS-TBNA and/or EUS-FNA) was more sensitive in the detection of N2/N3 compared to sampling based on the appearance of nodes in PET-CT images (32). Indeed, this former approach is required in order to calculate the probability occult N2-3 disease in cases of PET-CT N2/3 negative disease (1-10). In contrast, a significantly higher risk of pathological N2s has been reported for PET-CT N0 patients with central versus peripheral tumours, (21.6% vs. 2.9%) (4,17-19). Thus, endosonography is recommended for patients with normal PET-CT mediastinum and centrally located tumours.
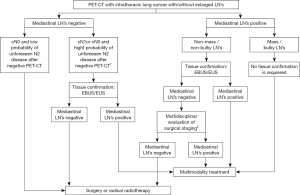
Another meta-analysis showed that the NPV for tumours larger than 3 cm was significantly lower than for those smaller than this threshold (89% vs. 94%) (16) and so endosonography sampling is also recommended in the former cases (3). The ESTS and ATS guidelines disagree in this respect because the ATS does not recommend carrying out a complete mediastinal study with EBUS/EUS and instead recommend that patients undergo surgical resection directly (1,3). For patients with PET-CT evidence of N1 disease, the probability of occult N2 or N3 ranges between 20–30% and so the ATS recommend this group of patients undergo a mediastinal endosonography study. In conclusion, cases with negative PET-CT scans and with clinical factors indicative of a low probability of unforeseen N metastasis (not a central tumour and N size of 3 cm or less) can be referred for surgical resection, while mediastinal endosonography study is recommended in cases of N1, N2, or N3 disease (Figure 2) (1,3).
Surgical staging
Current international guidelines for preoperative mediastinal nodal staging recommend performing histological procedures for staging in all cases except in patients with tumours sized less than 3 cm in diameter and with no evidence of nodal involvement in the PET-CT images. Surgical mediastinal staging should be performed after negative endosonography staging or when the mediastinal zone is only accessible by surgical methods. Surgical staging is still considered the gold standard and has a higher NPV (1-9). Indeed, surgical techniques should be used to validate negative endosonography results. Therefore, with the exception mentioned above, surgical techniques for mediastinal staging should be discussed in the preoperative algorithm for all patients diagnosed with lung cancer who are potential candidates for surgery. Moreover, we recommend that these decisions be multidisciplinary and consider the probability of lymph node malignancy.
Cervical mediastinoscopy
Cervical mediastinoscopy is the gold standard technique for complete invasive staging of the upper mediastinum. According to the IASLC lymph node map (14), mediastinoscopy can evaluate the upper and lower paratracheal stations (stations 2 and 4), pretracheal station (station 3), and subcarinal (station 7) areas. However, neither endosonography nor mediastinoscopy can be used to access stations 5, 6, 8, or 9, although EUS-FNA can be used to access the latter two zones and previously published data indicate a sensitivity and NPV of 78% and 91% for these, respectively (1).
Most FN cases were due to the inaccessibility of lymph nodes or limited node dissection or sampling. The intensity of this procedure depends on its indications. If N3 is confirmed intraoperatively, further exploration is not necessary. However, a systematic exploration must be carried out to explore the nodal regions that determine later disease stages in cases with a low suspicion, with the recommendation that at least five nodal stations should be sampled. The addition of video techniques [video-assisted cervical mediastinoscopy (VACM)] has improved the quality of sampling, leading to more sensitive results (89%) and an NPV of 92% (1,7).
Video-assisted thoracic surgery
Lymph node areas corresponding to the aortopulmonary window and para-aortic zone (stations 5 and 6) can be sampled through video-assisted thoracic surgery (VATS) or extended cervical mediastinoscopy. VATS has a higher sensitivity (median 99%) and can be used to explore all the other mediastinal stations. However, it is more invasive than VACM and so is usually restricted to use for stations 5 and 6 (33).
Extended cervical mediastinoscopy
Extended cervical mediastinoscopy via a suprasternal approach lateral to the aortic arch can be used to reach nodal stations 5 and 6 in addition to those accessible by VACM; the sensitivity and NPV for this technique have been reported as 71% and 91%, respectively (34-36). Experienced centres have reported the feasibility of this technique, although it is important to consider that it requires partial resection of the costal cartilage which increases its technical complexity and the operative risks of this surgery.
Transcervical lymphadenectomies
The new invasive techniques for mediastinal staging include video-assisted mediastinoscopic lymphadenectomy (VAMLA) and transcervical extended mediastinal lymphadenectomy (TEMLA) (7,37-39). Both these procedures aim to remove bilateral lymph nodes, including the adjacent adipose tissue. VAMLA is an endoscopic procedure that permits mediastinal lymphadenectomy by taking a cervical approach to bilaterally explore from the superior paratracheal nodes to the hilar nodes, with the removal of all the reachable mediastinal nodes. TEMLA is an open video-assisted mediastinoscope and video-thoracoscope procedure performed through a small neck incision and requiring elevation of the sternum with a special retractor (7). This allows exploration and complete removal of more lymph node stations, including supraclavicular nodes, the prevascular area, para-aortic, aortopulmonary window, and paraesophageal region, except the pulmonary ligament nodes (station 9) and most distant left paratracheal nodes (4L). The Table 1 shows the ganglionic stations that can usually be achieved with each technique.

Full table
These two methods have reduced FN results for potential micrometastases in removed nodes and have a high accuracy (between 96% and 98%) and an NPV between 97% and 99% (7,37-39). VAMLA and TEMLA are the best techniques for use when there is no evidence of N2–3 disease and when the tumour is located centrally, for cases of N1 disease located on the left side, or bilateral synchronous lung cancer, and are also useful in pre-resectional lymphadenectomy for lobectomy via VATS (7). However, data regarding the results, safety, and learning-curve time required to achieve skills to successfully implement these techniques remain limited.
Patients requiring lymph node confirmation after negative endosonography results (Figure 2) should undergo mediastinoscopy. However, VACM may be enough for these patients and VACM can be converted to VAMLA if the results remain negative. In exceptional cases with a normal mediastinum requiring surgical mediastinal confirmation, the ideal technique is VAMLA although this choice depends on the local expertise of the surgical team. Although study data remains limited, VAMLA and TEMLA have proven to be safe and useful techniques, especially for N0–1 tumours. They can also be used to perform lymphadenectomy prior to surgical tumoural resection by VATS (7).
Mediastinal staging algorithm proposal
Based on the currently available evidence, here we propose an algorithm for mediastinal staging in which the decision for surgical staging should be performed after multidisciplinary evaluation (Figure 2).
Restaging for mediastinal nodes after oncological therapy
Mediastinal restaging is based in the same imaging and sampling techniques used for mediastinal staging before treatment, although the accuracy of all these techniques is lower, probably because of tumour fibrosis and necrosis. PET-CT scans are better than CT or PET imaging alone; despite this, the false-positive and FN rates are high (25% and 20%, respectively) and require histological confirmation (40).
Although the published data is limited, the accuracy of endosonography techniques significantly decrease when used for restaging. However, these results should be interpreted with caution due to the different prevalence of N2 disease and heterogeneous approach to nodal sampling during re-evaluations. In addition, the reported NPV widely varies (20–78%), indicating that negative lymph nodes should be evaluated by invasive techniques (7,41). Nonetheless, this value was higher when the probability of N2 after treatment was low (41).
Re-mediastinoscopy has been proven as feasible but it is associated with an increased risk of complications and because of this complexity, it is performed only in selected centres. The results also greatly vary among studies, with reports of sensitivity ranging from 29% to 74% and NPV from 52% to 74% (40,42). Results from TEMLA for restaging have been reported in two reports and seem to indicate that it is more accurate than endosonography (43,44).
Pathological mediastinal staging after surgery
The accuracy of pathological staging is crucial to decide postoperative treatment strategies. Nonetheless, there is no consensus about the ideal extent of lymph node removal. The IASLC recommends that at least six lymph-node stations be removed or sampled before the confirmation of an N0 status. Controversy about whether SND or LNS should be performed remains because the results for the prevalence of N1 and N2 were similar in some published series. However, in cases with multi-station N2 involvement SND proved more useful than LNS for diagnosing N2 disease (30% vs. 12%), suggesting that complete resection of all lymphatic tissue allows the disease extent to be better defined (45). Another issue requiring exploration is whether intraoperative node staging obtained with minimally invasive surgery methods (VATS or robotic techniques) can provide similar results to thoracotomy. Currently only one published study has explored the upstaging rate for these two surgical approaches and showed a lower rate of upstaging with VATS (46).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mariano Provencio) for the series “Multimodal management of locally advanced N2 non-small cell lung cancer” published in Translational Lung Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.08). The series “Multimodal management of locally advanced N2 non-small cell lung cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e250S.
- Pretreatment evaluation of non-small-cell lung cancer. The American Thoracic Society and The European Respiratory Society. Am J Respir Crit Care Med 1997;156:320-32. [Crossref] [PubMed]
- De Leyn P, Doomsb C, Kuzdzalc J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Venuta F, et al. Mediastinal staging: when and how? Gen Thorac Cardiovasc Surg 2020;68:725-32. [Crossref] [PubMed]
- Majem M, Hernández-Hernández J, Hernando-Trancho F, et al. Multidisciplinary consensus statement on the clinical management of patients with stage III non-small cell lung cancer. Clin Transl Oncol 2020;22:21-36. [Crossref] [PubMed]
- Stamatis G. Staging of lung cancer: the role of noninvasive, minimally invasive and invasive techniques. Eur Respir J 2015;46:521-31. [Crossref] [PubMed]
- Call S, Rami-Porta R. Cervical mediastinoscopy and video-assisted mediastinoscopic lymphadenectomy for the staging of non-small cell lung cancer. Mediastinum 2019;3:31. [Crossref]
- Fernández-Villar A, Mouronte-Roibás C, Botana-Rial M, et al. Ten Years of Linear Endobronchial Ultrasound: Evidence of Efficacy, Safety and Cost-effectiveness. Arch Bronconeumol 2016;52:96-102. [Crossref] [PubMed]
- Maconachie R, Mercer T, Navani N, et al. Lung cancer: diagnosis and management: summary of updated NICE guidance. BMJ 2019;364:l1049. [Crossref] [PubMed]
- Sanz-Santos J, Serra M, Gallego M, et al. Determinants of false-negative results in non-small-cell lung cancer staging by endobronchial ultrasound-guided needle aspiration. Eur J Cardiothorac Surg 2015;47:642-7. [Crossref] [PubMed]
- Leiro V, De Chiara L, Rodríguez-Girondo M, et al. Methylation Assessment for the Prediction of Malignancy in Mediastinal Adenopathies Obtained by Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Patients with Lung Cancer. Cancers (Basel) 2019;11:E1408. [Crossref] [PubMed]
- D’Andrilli A, Venuta F, Rendina EA. The role of lymphadenectomy in lung cancer surgery. Thorac Surg Clin 2012;22:227-37. [Crossref] [PubMed]
- Osarogiagbon RU, Lee YS, Faris NR, et al. Invasive mediastinal staging for resected non-small cell lung cancer in a population-based cohort. J Thorac Cardiovasc Surg 2019;158:1220-9.e2. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77
- Caupena C, Costa R, Pérez-Ochoa F, et al. Nodal size ranking as a predictor of mediastinal involvement in clinical early-stage non-small cell lung cancer. Medicine (Baltimore) 2019;98:e18208. [Crossref] [PubMed]
- Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [Crossref] [PubMed]
- Lee PC, Port LC, Korst RJ, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:177-81. [Crossref] [PubMed]
- Hishida T, Yoshida J, Nishimura M, et al. Problems in the current diagnostic standards of clinical N1 non-small cell lung cancer. Thorax 2008;63:526-31. [Crossref] [PubMed]
- Gómez-Caro A, Boada M, Cabanas M, et al. False-negative rate after positron emission tomography/computer tomography scan for mediastinal staging in clinical I stage non-small cell lung cancer. Eur J Cardiothorac Surg 2012;42:93-100. [Crossref] [PubMed]
- Holty JE, Kuschner W, Gould M. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax 2005;60:949-55. [Crossref] [PubMed]
- Monaco SE, Khalbuss WE, Pantanowitz L. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA): A Practical Approach. Karger, 2014.
- Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368-74. [Crossref] [PubMed]
- Annema JT, Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Szlubowski A, Zielínski M, Soja J, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small cell lung cancer staging-a prospective trial. Eur J Cardiothorac Surg 2010;37:1175-9. [Crossref] [PubMed]
- Herth FJ, Krasnik M, Kahn N, et al. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010;138:790-4. [Crossref] [PubMed]
- Ohnishi R, Yasuda I, Kato T, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal nodal staging of lung cancer. Endoscopy 2011;43:1082-9. [Crossref] [PubMed]
- Sharples LD, Jackson C, Wheaton E, et al. Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. Health Technol Assess 2012;16:1-75. [Crossref] [PubMed]
- Kuijvenhoven JC, Korevaar DA, Tournoy KG, et al. Five-year survival after Endosonography vs Mediastinoscopy for mediastinal nodal staging of lung Cancer. JAMA 2016;316:1110-2. [Crossref] [PubMed]
- Bousema JE, van Dorp M, Noyez VJJM, et al. Unforeseen N2 disease after negative endosonography findings with or without confirmatory mediastinoscopy in resectable non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Oncol 2019;14:979-92. [Crossref] [PubMed]
- Bousema JE, Dijkgraaf MGW, Papen-Botterhuis NE, et al. MEDIASTinal staging of non-small cell lung cancer by endobronchial and endoscopic ultrasonography with or without additional surgical mediastinoscopy (MEDIASTrial): study protocol of a multicenter randomised controlled trial. BMC Surg 2018;18:27. [Crossref] [PubMed]
- Crombag LMM, Dooms C, Stigt JA, et al. Systematic and combined endosonographic staging of lung cancer (SCORE study). Eur Respir J 2019;53:1800800. [Crossref] [PubMed]
- Sebastián-Quetglás F, Molins L, Baldó X, et al. Clinical value of video-assisted thoracoscopy for preoperative staging of non-small cell lung cancer. A prospective study of 105 patients. Lung Cancer 2003;42:297-301. [Crossref] [PubMed]
- Ginsberg RJ, Rice TW, Goldberg M, et al. Extended cervical mediastinoscopy. A single staging procedure for bronchogenic carcinoma of the left upper lobe. J Thorac Cardiovasc Surg 1987;94:673-8. [Crossref] [PubMed]
- Barendregt WB, Deleu HW, Joosten HJ, et al. The value of parasternal mediastinoscopy in staging of bronchial carcinoma. Eur J Cardiothorac Surg 1995;9:655-8. [Crossref] [PubMed]
- Nechala P, Graham AJ, McFadden SD, et al. retrospective analysis of the clinical performance of anterior mediastinotomy. Ann Thorac Surg 2006;82:2004-9. [Crossref] [PubMed]
- Hürtgen M, Friedel G, Toomes H, et al. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)—technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [Crossref] [PubMed]
- Kuzdzał J, Zieliński M, Papla B, et al. Transcervical extended mediastinal lymphadenectomy—the new operative technique and early results in lung cancer staging. Eur J Cardiothorac Surg 2005;27:384-90. [Crossref] [PubMed]
- Witte B, Wolf M, Huertgen M, et al. Video-assisted mediastinoscopic surgery: clinical feasibility and accuracy of mediastinal lymph node staging. Ann Thorac Surg 2006;82:1821-7. [Crossref] [PubMed]
- De Leyn P, Stroobants S, De Wever W, et al. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy- proven stage IIIA-N2 Non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J Clin Oncol 2006;24:3333-9. [Crossref] [PubMed]
- Szlubowski A, Zielinski M, Soja J, et al. Accurate and safe mediastinal restaging by combined endobronchial and endoscopic ultrasound-guided needle aspiration performed by single ultrasound bronchoscope. Eur J Cardiothorac Surg 2014;46:262-6. [Crossref] [PubMed]
- Stamatis G, Fechner S, Hillejan L, et al. Repeat mediastinoscopy as a restaging procedure. Pneumologie 2005;59:862-6. [Crossref] [PubMed]
- Zieliński M, Hauer L, Hauer J, et al. Non-small cell lung cancer restaging with transcervical extended mediastinal lymphadenectomy. Eur J Cardiothorac Surg 2010;37:776-80. [Crossref] [PubMed]
- Zielinski M, Szlubowski A, Kolodziej M, et al. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol 2013;8:630-6. [Crossref] [PubMed]
- Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg 2000;70:358-65. [Crossref] [PubMed]
- Kneuertz PJ, Cheufou DH, D’Souza DM, et al. Propensity-score adjusted comparison of pathologic nodal upstaging by robotic, video-assisted thoracoscopic, and open lobectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1457-66.e2. [Crossref] [PubMed]


