- Department of Neurological Surgery, The University of Texas Southwestern Medical Center, Dallas, Texas, United States.
- Department of Pathology The University of Texas Southwestern Medical Center, Dallas, Texas, United States.
- Department of Neurology, The University of Texas Southwestern Medical Center, Dallas, Texas, United States.
Correspondence Address:
Kalil G. Abdullah, Department of Neurological Surgery, University of Texas Southwestern Medical Center, Dallas, Texas, United States.
DOI:10.25259/SNI_224_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Cylaina E. Bird1, Jeffrey I. Traylor1, Jenna Thomas1, James P. Caruso1, Benjamin Kafka1, Flavia Rosado2, Kyle M. Blackburn3, Kimmo J. Hatanpaa2, Kalil G. Abdullah1. Primary peripheral T-cell central nervous system lymphoma. 13-Sep-2021;12:465
How to cite this URL: Cylaina E. Bird1, Jeffrey I. Traylor1, Jenna Thomas1, James P. Caruso1, Benjamin Kafka1, Flavia Rosado2, Kyle M. Blackburn3, Kimmo J. Hatanpaa2, Kalil G. Abdullah1. Primary peripheral T-cell central nervous system lymphoma. 13-Sep-2021;12:465. Available from: https://surgicalneurologyint.com/surgicalint-articles/11105/
Abstract
Background: Primary peripheral T-cell central nervous system lymphoma (PCNSL) is a rare, aggressive tumor that arises in the craniospinal axis and has an increased risk in individuals who are immunocompromised. This lesion often mimics other benign and malignant processes on radiographic imaging, leading to misdiagnosis and delays in treatment. We present a case of a patient with a history of Sjögren’s syndrome and progressive neurologic symptoms who underwent craniotomy for diagnosis.
Case Description: A 61-year-old woman with a history of Sjögren’s syndrome, progressive aphasia, left facial droop, and right-sided paresthesias for 4 months presented for evaluation and management. An enhancing, infiltrative lesion in the left frontal lobe with underlying vasogenic edema was appreciated and suggestive of a primary or metastatic neoplasm. The patient underwent an open biopsy for further evaluation of the lesion. Extensive histopathologic evaluation revealed a diagnosis of T-cell PCNSL. The patient was started on induction methotrexate and temozolomide followed by consolidative radiotherapy.
Conclusion: Autoimmune conditions are a risk factor for T-cell PCNSL development. T-cell PCNSL has radiographic and gross histologic features that are consistent with a broad differential, including gliomas and inflammatory processes. Prompt diagnosis and extensive histopathological evaluation is essential to ensure appropriate treatment.
Keywords: Autoimmune disease, Glioma, Primary central nervous system lymphoma, Sjögren’s syndrome
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is a rare lymphoma that accounts for 2–6% of all primary brain tumors.[
CASE REPORT
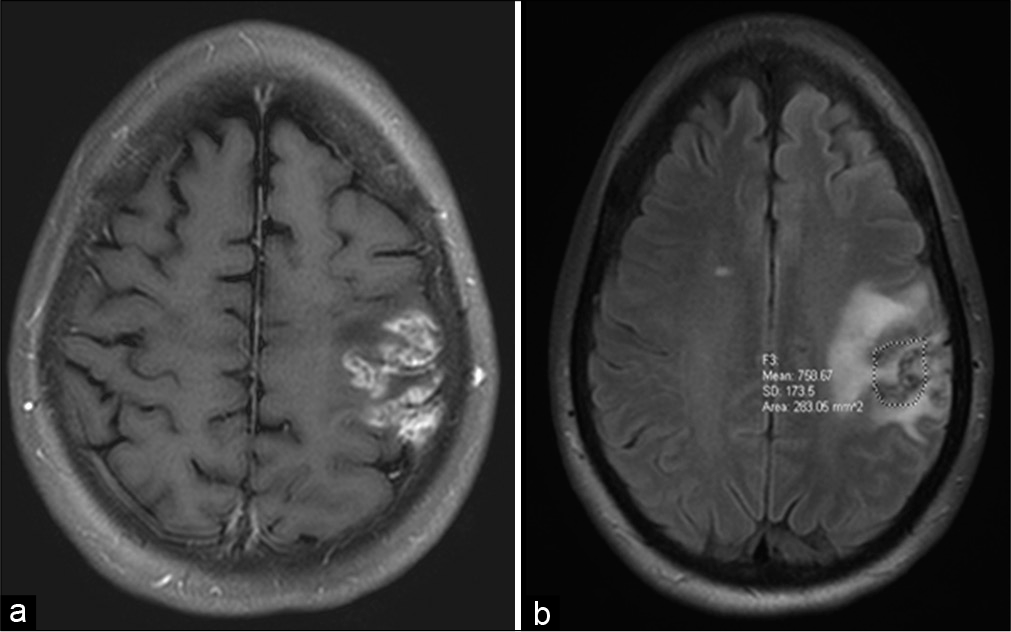
A 61-year-old woman with a medical history of Sjögren’s syndrome and a long history of treatment with immunomodulatory therapy presented to clinic after recurrent and fluctuating stroke-like symptoms including aphasia, right-sided paresthesia, and left facial droop over a 4-month period. Her symptoms were initially attributed to an acute thromboembolic left middle cerebral artery stroke. However, examination revealed progressive expressive and receptive aphasia, right-sided House-Brackmann II facial droop, and decreased strength in the right upper extremity. Magnetic resonance (MR) imaging revealed an enhancing, infiltrative lesion in the left frontal lobe with progressive vasogenic edema in the left pre- and postcentral gyri [
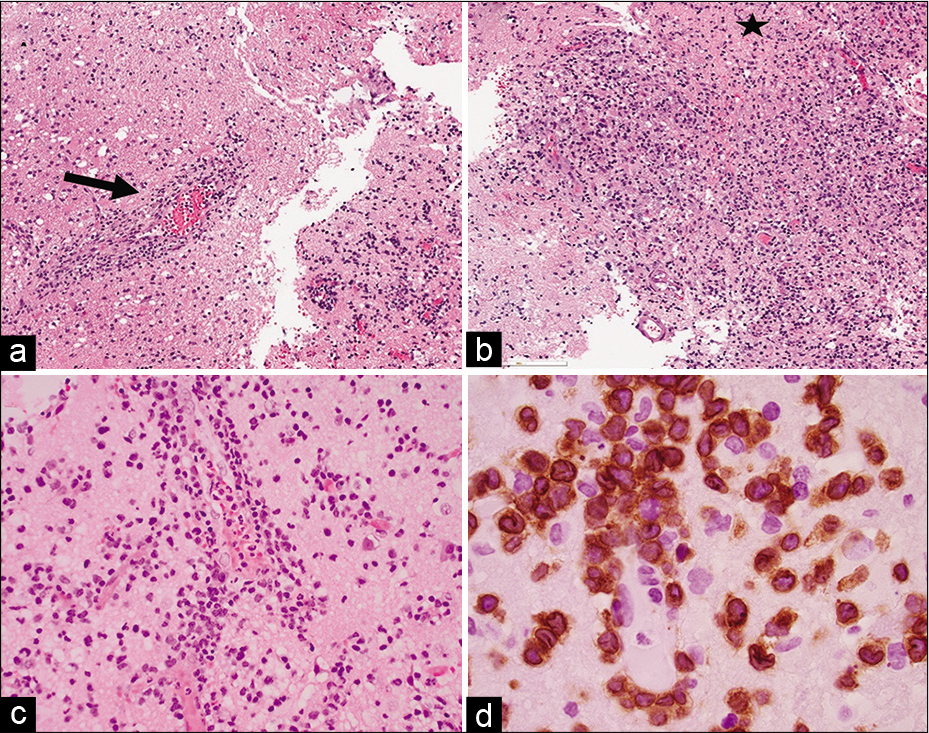
Given the broad differential diagnosis and the progression of symptoms, the patient underwent a craniotomy with asleep motor mapping for open biopsy of the lesion. Intraoperatively, the lesion appeared ashen and gray with some areas of mineralization under the arachnoid. Histopathologic evaluation of the biopsy specimen demonstrated a small focus of perivascular lymphocytic infiltrate composed of predominately medium-sized atypical lymphoid cells in a background of histiocytes and small lymphocytes [
Figure 2:
Histopathologic examination of the left frontal lobe lesion brain biopsy (a) and (b) hematoxylin-eosin (H&E, ×2) staining shows perivascular inflammatory infiltrates (arrow) and incomplete coagulation necrosis (star), (c) (H&E, ×10): perivascular inflammatory infiltrates of atypical medium-sized lymphoid cells and (d) (CD3 immunohistochemical stain, ×100 oil): the positive CD3 highlights the nuclear irregularities of the atypical cells and indicates the T-cell lineage of this process.
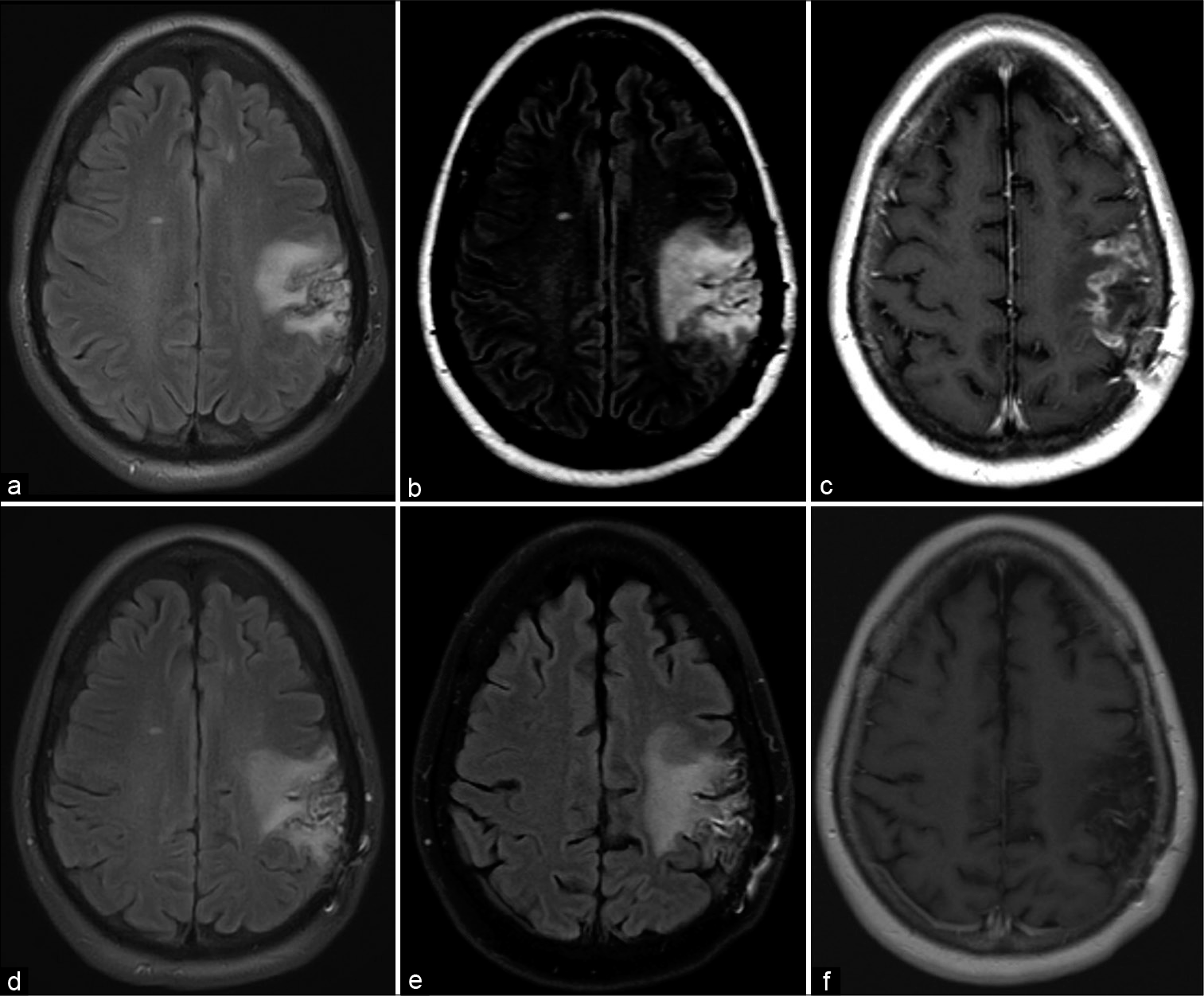
Figure 3:
Follow-up neuroradiologic images. One-month postoperative representative axial T2-weighted FLAIR image (a), 2-month postoperative representative axial T2-weighted FLAIR image (b), 2-month postoperative representative axial T1-weighted postgadolinium contrast image (c), 4-month postoperative representative axial T2-weighted FLAIR image (d), 1-year postoperative representative axial T2-weighted FLAIR image with appreciated stable FLAIR signal (e), 1-year postoperative representative axial T1-weighted postgadolinium contrast image with no appreciated contrast-enhanced disease (f).
DISCUSSION
T-cell PCNSL is an uncommon extranodal non-Hodgkin lymphoma in the craniospinal axis. It has an incidence of about 2% in Western countries, but is more commonly diagnosed in the east.[
[
Gadolinium-enhanced MR imaging is the preferred radiographic study for PCNSL lesions.[
Given the radiographic findings and clinical presentation, the differential diagnosis of our patient included gliomas, metastasis, subacute infarction, demyelinating diseases, and space-occupying infectious or parasitic lesions. The presentation of the patient may have been complicated by her history of intermittent high-dose corticosteroid use to help manage her autoimmune symptoms. Corticosteroids are a known lymphocytotoxic drug and can interfere with diagnosis when administered before biopsy.[
Diagnosis of PCNSL neoplasms is challenging, often requiring integration of clinical, radiologic, and histopathologic findings.[
T-cell PCNSL has a poor prognosis.[
HD-MTX followed by radiotherapy is first-line treatment for PCNSL.[
CONCLUSION
T-cell PCNSL is a rare, aggressive tumor in the craniospinal axis. The presentation of this lesion is variable and often includes progressive neurologic symptoms. As the radiographic and histologic features are often nonspecific and mimic other lesions, including inflammatory processes and high-grade gliomas, it is a challenging tumor to diagnosis. For patients with preexisting inflammatory conditions who present with progressive neurologic symptoms and inconclusive radiographic findings, T-cell PCNSL should be considered and a biopsy performed with subsequent immunohistochemical and molecular analysis of the specimen.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
This research was funded by the Eugene P. Frenkel, M.D. Scholar in Clinical Medicine Endowment (KGA). The University of Texas Southwestern Medical Center – Dallas holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund (CEB).
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
This research was funded by the Eugene P. Frenkel, M.D. Scholar in Clinical Medicine Endowment (KGA). The University of Texas Southwestern Medical Center – Dallas holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund (CEB).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We wish to acknowledge Dr. Seema Jabbar, MD for her valuable contributions in the histopathologic evaluation of diagnosis in the case presented.
References
1. Aaroe AE, Nevel KS. Central nervous system involvement of natural killer and T cell neoplasms. Curr Oncol Rep. 2019. 21: 40
2. Ahn SY, Kwon SY, Jung SH, Ahn JS, Yoo SW, Min JJ. Prognostic significance of interim 11C-methionine PET/CT in primary central nervous system lymphoma. Clin Nucl Med. 2018. 43: e259-64
3. Bednar MM, Salerni A, Flanagan ME, Pendlebury WW. Primary central nervous system T-cell lymphoma. Case report. J Neurosurg. 1991. 74: 668-72
4. Behbahani M, Lyons M. Primary central nervous system T-cell lymphoma of the brain. Open Neurosurg J. 2011. 4: 62-5
5. Bellinzona M, Roser F, Ostertag H, Gaab RM, Saini M. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: A series of 33 cases. Eur J Surg Oncol. 2005. 31: 100-5
6. Bhagavathi S, Wilson JD. Primary central nervous system lymphoma. Arch Pathol Lab Med. 2008. 132: 1830-4
7. Cheng G, Zhang J. Imaging features (CT, MRI, MRS, and PET/CT) of primary central nervous system lymphoma in immunocompetent patients. Neurol Sci. 2019. 40: 535-42
8. Chukwueke UN, Nayak L. Central nervous system lymphoma. Hematol Oncol Clin North Am. 2019. 33: 597-611
9. Clark AJ, Lee K, Broaddus WC, Martin MJ, Ghatak NR, Grossman CE. Primary brain T-cell lymphoma of the lymphoblastic type presenting as altered mental status. Acta Neurochir (Wien). 2010. 152: 163-8
10. Comes PC, André A, Nguyen-Khac F, Carpentier A, Bielle F, Amelot A. Intracranial cell lymphomas that mimic meningiomas: Case report to understand complex genetic, radiologic, and histopathologic entities. World Neurosurg. 2019. 125: 339-42
11. Corns R, Crocker M, Kumar A, Salisbury J, Tolias C, Sadler G. Low grade cerebellar T-cell lymphoma: A novel response to treatment; a case report. Acta Neurochir (Wien). 2010. 152: 1075-7
12. Demetriades A, McEvoy A, Galloway M, Kitchen N. Peripheral T-cell lymphoma presenting as a solitary vermian mass. Br J Neurosurg. 2003. 17: 473-5
13. Dulai MS, Park CY, Howell WD, Smyth LT, Desai M, Carter DM. CNS T-cell lymphoma: An under-recognized entity?. Acta Neuropathol. 2008. 115: 345-56
14. Ferreri AJ, Marturano E. Primary CNS lymphoma. Best Pract Res Clin Haematol. 2012. 25: 119-30
15. Fuentes-Raspall R, Solans M, Auñón-Sanz C, Sáez M, MarcosGragera R. Incidence and survival of primary central nervous system lymphoma (PCNSL): Results from the Girona cancer registry (199T 2013). Clin Transl Oncol. 2018. 20: 1628-30
16. Goldbrunner R, Warmuth-Metz M, Tonn JC, Vince GH, Roosen K. Primary Ki-1-positive T-cell lymphoma of the brain-an aggressive subtype of lymphoma: Case report and review of the literature. Surg Neurol. 1996. 46: 37-41
17. Gualco G, Wludarski S, Hayashi-Silva L, Filho PM, Veras G, Bacchi CE. Primary central nervous system peripheral T-cell lymphoma in a child. Fetal Pediatr Pathol. 2010. 29: 224-30
18. Gupta N, Nasim M, Spitzer SG, Zhang X. Primary central nervous system T-cell lymphoma with aberrant expression of CD20 and CD79a: A diagnostic pitfall. Int J Surg Pathol. 2017. 25: 599-603
19. Harder A, Dudel C, Anagnostopoulos I, Hummel M, Brück W. Molecular genetic diagnosis of a primary central nervous system T cell lymphoma. Acta Neuropathol. 2003. 105: 65-8
20. Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Rudà R. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European association for neuro-oncology. Lancet Oncol. 2015. 16: e322-32
21. Illerhaus G. III Current concepts in primary central nervous lymphoma. Hematol Oncol. 2015. 33: 25-8
22. Inoue M, Kawaguchi T, Yokoyama H, Shimada M. Primary T-cell lymphoma with myelopathy associated with HTLV-1. Neurosurgery. 1990. 27: 148-51
23. Jellinger KA, Paulus W. Primary central nervous system lymphomas-new pathological developments. J Neurooncol. 1995. 24: 33-6
24. Karschnia P, Batchelor TT, Jordan JT, Shaw B, Winter SF, Barbiero FJ. Primary dural lymphomas: Clinical presentation, management, and outcome. Cancer. 2020. 126: 2811-20
25. Kato Y, Hayashi T, Kawai-Masaoka A, Ichimura T, Sasaki A, Uchino A. Primary central nervous system cytotoxic T-cell lymphoma mimicking demyelinating disease. Intern Med. 2014. 53: 1197-200
26. Kleopa K, Becker G, Roggendorf W, Reichmann H. Primary T-cell lymphoma of the cerebellum. J Neurooncol. 1996. 27: 225-30
27. Knorr JR, Ragland RL, Stone BB, Woda BA, Gelber ND. Cerebellar T-cell lymphoma: An unusual primary intracranial neoplasm. Neuroradiology. 1992. 35: 79-81
28. Lai A, Wierzbicki A, Norman P. Immunocytological diagnosis of primary cerebral non-Hodgkin’s lymphoma. J Clin Pathol. 1991. 44: 251-3
29. Lin TK, Yeh TH, Hsu PW, Chuang CC, Tu PH, Chen PY. Primary central nervous system lymphomas of the brain: A retrospective analysis in a single institution. World Neurosurg. 2017. 103: 550-6
30. Liu XH, Jin F, Zhang M, Liu MX, Wang T, Pan BJ. Peripheral T cell lymphoma after chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS): A case report. BMC Neurol. 2019. 19: 266
31. Long H, Li S, Zhang Y, Li R, Fong T, Yang C. Primary central nervous system T-cell lymphoma: An analysis from the surveillance, epidemiology, and end results program. J Clin Neurosci. 2020. 79: 74-9
32. Lotan I, Khlebtovsky A, Inbar E, Strenov J, Djaldetti R, Steiner I. Primary brain T-cell lymphoma in an HTLV-1 serologically positive male. J Neurol Sci. 2012. 314: 163-5
33. Löw S, Han CH, Batchelor TT. Primary central nervous system lymphoma. Ther Adv Neurol Disord. 2018. 11: 1-16
34. Malikova H. Primary central nervous system lymphoma: Is whole-body CT and FDG PET/CT for initial imaging reasonable?. Quant Imaging Med Surg. 2019. 9: 1615-8
35. Manenti G, di Giuliano F, Bindi A, Liberto V, Funel V, Garaci FG. A case of primary T-cell central nervous system lymphoma: MR imaging and MR spectroscopy assessment. Case Rep Radiol. 2013. 2013: 916348
36. McCue MP, Sandrock AW, Lee JM, Harris NL, Hedley-Whyte ET. Primary T-cell lymphoma of the brainstem. Neurology. 1993. 43: 377-81
37. Menon MP, Nicolae A, Meeker H, Raffeld M, Xi L, Jegalian AG. Primary CNS T-cell Lymphomas: A clinical, morphologic, immunophenotypic, and molecular analysis. Am J Surg Pathol. 2015. 39: 1719-29
38. Mineura K, Sawataishi J, Sasajima T, Kowada M, Sugawara A, Ebina K. Primary central nervous system involvement of the so called ‘peripheral T-cell lymphoma’. Report of a case and review of the literature. J Neurooncol. 1993. 16: 235-42
39. Momota H, Kato S, Fujii M, Tsujiuchi T, Takahashi Y, Kojima S. Primary peripheral T-cell lymphoma, not otherwise specified, of the central nervous system in a child. Brain Tumor Pathol. 2015. 32: 281-5
40. Morisako T, Shishido-Hara Y, Inaba T, Takeuchi H, MiyagawaHayashino A, Kodama Y. Primary CNS CD45-depleted T-cell lymphoma: The first pathologically confirmed case. J Neuropathol Exp Neurol. 2020. 79: 817-20
41. Nabavizadeh SA, Vossough A, Hajmomenian M, Assadsangabi R, Mohan S. Neuroimaging in central nervous system lymphoma. Hematol Oncol Clin North Am. 2016. 30: 799-821
42. Ng HK, Lo ST, Poon CY, Poon WS. Primary cerebral T-cell lymphoma: A case report. Br J Neurosurg. 1988. 2: 523-8
43. Nigo M, Richardson S, Azizi E, Cohen JM, Shapira I. Ventriculitis caused by primary T-cell cns lymphoma in an immunocompetent patient. J Clin Oncol. 2016. 34: e145-7
44. Novak JA, Katzin WE. Primary central nervous system T-cell lymphoma with a predominant CD8 immunophenotype. Cancer. 1995. 75: 2180-5
45. Oskan F, Ganswindt U, Schwarz SB, Manapov F, Belka C, Niyazi M. Hippocampus sparing in whole-brain radiotherapy. A review. Strahlenther Onkol. 2014. 190: 337-41
46. Provinciali L, Signorino M, Ceravolo G, Pasquini U. Onset of primary brain T-lymphoma simulating a progressive leukoencephalopathy. Ital J Neurol Sci. 1988. 9: 377-81
47. Pulsoni A, Gubitosi G, Rocchi L, Iaiani G, Martino B, Carapella C. Primary T-cell lymphoma of central nervous system (PTCLCNS): A case with unusual presentation and review of the literature. Ann Oncol. 1999. 10: 1519-23
48. Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013. 70: 311-9
49. Shalabi H, Angiolillo A, Vezina G, Rubenstein JL, Pittaluga S, Raffeld M. Prolonged complete response in a pediatric patient with primary peripheral T-cell lymphoma of the central nervous system. Pediatr Hematol Oncol. 2015. 32: 529-34
50. Shenkier TN, Blay JY, O’Neill BP, Poortmans P, Thiel E, Jahnke K. Primary CNS lymphoma of T-cell origin: A descriptive analysis from the international primary CNS lymphoma collaborative group. J Clin Oncol. 2005. 23: 2233-9
51. Splavski B, Muzevic D, Ladenhauser-Palijan T, Splavski B. Primary central nervous system anaplastic large T-cell lymphoma. Med Arch. 2016. 70: 311-3
52. Suh CH, Kim HS, Park JE, Jung SC, Choi CG, Kim SJ. Primary central nervous system lymphoma: Diagnostic yield of whole-body CT and FDG PET/CT for initial systemic imaging. Radiology. 2019. 292: 440-6
53. Takeshita M, Kubo O, Tajika Y, Kawamoto T, Hori T, Takakura K. Primary central nervous system T-cell lymphoma. Case report. Neurol Med Chir (Tokyo)1999;. 39: 452-8
54. Villegas E, Villà S, López-Guillermo A, Petit J, Ribalta T, Graus F. Primary central nervous system lymphoma of T-cell origin: Description of two cases and review of the literature. J Neurooncol. 1997. 34: 157-61
55. Zhao Q, Zeng LS, Feng XL, Zhang HM. Magnetic resonance imaging characteristics of primary central nervous system T-cell lymphoma. Chin Med J (Engl). 2017. 130: 374-6