Falls Predict Acute Hospitalization in Parkinson’s Disease
Abstract
Background:
There is a need for identifying risk factors for hospitalization in Parkinson’s disease (PD) and also interventions to reduce acute hospital admission.
Objective:
To analyze the frequency, causes, and predictors of acute hospitalization (AH) in PD patients from a Spanish cohort.
Methods:
PD patients recruited from 35 centers of Spain from the COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015) cohort from January 2016 to November 2017, were included in the study. In order to identify predictors of AH, Kaplan-Meier estimates of factors considered as potential predictors were obtained and Cox regression performed on time to hospital encounter 1-year after the baseline visit.
Results:
Thirty-five out of 605 (5.8%) PD patients (62.5±8.9 years old; 59.8% males) presented an AH during the 1-year follow-up after the baseline visit. Traumatic falls represented the most frequent cause of admission, being 23.7% of all acute hospitalizations. To suffer from motor fluctuations (HR [hazard ratio] 2.461; 95% CI, 1.065–5.678; p = 0.035), a very severe non-motor symptoms burden (HR [hazard ratio] 2.828; 95% CI, 1.319–6.063; p = 0.008), falls (HR 3.966; 95% CI 1.757–8.470; p = 0.001), and dysphagia (HR 2.356; 95% CI 1.124–4.941; p = 0.023) was associated with AH after adjustment to age, gender, disease duration, levodopa equivalent daily dose, total number of non-antiparkinsonian drugs, and UPDRS-IIIOFF. Of the previous variables, only falls (HR 2.998; 95% CI 1.080–8.322; p = 0.035) was an independent predictor of AH.
Conclusion:
Falls is an independent predictor of AH in PD patients.
INTRODUCTION
Motor and non-motor symptoms (NMS) in Parkinson’s disease (PD) progress as the disease advances, which leads to complications such as falls, fractures, or infections. As a consequence, PD patients are reported to have 1.44 times more hospital admissions when compared to age and sex-matched peers [1, 2]. These admissions are associated with prolonged length-of-stay and increased morbidity and mortality [3–5]. Once PD patients are admitted to hospital, they have prolonged inpatient stays [6], poor motor outcomes, infections, prescription errors, and increased postoperative mortality [7–10]. Moreover, following a first hospital encounter, the rate of a second encounter increased to approximately 50% when patients were followed into a second year [11]. Previous studies reported a significant economic burden from PD hospitalization as well [12, 13]. There is a need for identifying risk factors for hospitalization in PD and also interventions to reduce acute PD hospital admission [14].
The aim of the present study was (1) to analyze the frequency and causes of acute hospitalization in PD patients from a Spanish cohort followed for 1-year and (2) to identify predictors of acute hospitalization.
MATERIAL AND METHODS
PD patients recruited from 35 centers of Spain from the COPPADIS cohort [15], from January 2016 to November 2017, were included in the study. Methodology about COPPADIS-2015 study can be consulted in https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-016-0548-9. This is a multi-center, observational, longitudinal-prospective, 5-year follow-up study designed to analyze disease progression in a Spanish population of PD patients. All patients included were diagnosed according to UK PD Brain Bank criteria. Exclusion criteria were: atypical parkinsonism, dementia (Mini-Mental State Examination [MMSE] < 26), age < 18 or > 75 years, inability to read or understand the questionnaires, to be receiving any advanced therapy (continuous infusion of levodopa or apomorphine, and/or with deep brain stimulation at baseline), and the presence of comorbidity, sequelae, or any disorder that could interfere with the assessment.
The data for the present study was obtained from the baseline evaluation [15]. Patient baseline evaluation included staging of severity of disease (modified Hoehn &Yah [H&Y] scale), motor assessment (Unified Parkinson’s Disease Rating Scale [UPDRS] part III and part IV, Freezing of Gait Questionnaire [FOG-Q]), non-motor symptoms (Non-Motor Symptoms Scale [NMSS], Parkinson’s Disease Sleep Scale [PDSS], Visual Analog Scale-Pain [VAS-Pain], Visual Analog Fatigue Scale [VAFS]), cognition (MMSE, Parkinson’s Disease Cognitive Rating Scale [PD-CRS], completing a simple 16-piece puzzle), mood and neuropsychiatric symptoms (Beck Depression Inventory-II [BDI-II], Neuropsychiatric Inventory [NPI], Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale [QUIP-RS]), disability (Schwab &England Activities of Daily Living Scale [S&E]), health related quality of life (QoL) (the 39-item Parkinson’s disease Questionnaire [PDQ-39SI]), and global QoL (PQ-10, EUROHIS-QOL 8-item index [EUROHIS-QOL8]) [16]. Interviews were conducted to determine the presence of falls according to the definition for falls proposed by the Kellogg International Work Group on the prevention of falls by the elderly [17]: a fall is an unintentional or unexpected event, results in the person coming to rest on the ground or another lower level and is not the result of a major intrinsic event (such as a loss of consciousness) or an overwhelming external force. The event was considered when it was not something that happened for a specific reason some time ago but was a repeated abnormal phenomenon. A time frame was not defined but it was specifically asked about the presence and number of falls in the last month. Moreover, falls diary, calendar, or postcard were not used. In patients with motor fluctuations, the motor assessment was conducted during the OFF state (without medication in the last 12 hours) and during the ON state. However, in patients without motor fluctuations, the assessment was only performed without medication (first hour in the morning without taking medication in the previous 12 hours).
The patients were followed for 1-year after the baseline visit and information about hospital admission was collected. Specifically, the following hospitalization-related data were recorded: number of hospitalizations, hospital stay days, and reasons for hospital admission. Those patients with at least one acute hospitalization during the follow-up were defined as Patients with acute hospitalization, whereas those with non-acute hospitalization during the follow-up was defined as Patients without acute hospitalization. Therefore, those patients with one or more programmed admission but without any acute hospitalization were considered as Patients without acute hospitalization. Rehospitalization was not considered for the analysis. The reasons for hospital admissions were divides in three groups [18]: 1) Direct PD-related morbidity: motor complications, psychiatric symptoms, autonomic dysfunction, sensory symptoms, sleep disorders, and side effects of anti-parkinsonian drugs; 2) Indirect PD-related morbidity: traumas, pneumonia, and intestinal obstruction / gastroparesis; 3) Non-PD related causes.
Data analysis
Data were processed using SPSS 20.0 for Windows. For comparisons between patients with and without an acute hospitalization, the Student’s t-test, Mann-Whitney-Wilcoxon test, Chi-square test, or Fisher test, as appropriate, were used (distribution for variables was verified by one-sample Kolmogorov-Smirnov test). Kaplan-Meier estimates were obtained to determine the risk of acute hospitalization with regards to the presence of different PD symptoms and/or complications: motor fluctuations; dyskinesia; very severe NMS (NMSS total score > 70) [19]; major depression [16]; cognitive impairment [20]; freezing of gait (FOG); falls; dysphagia. Self-reported FOG was defined regarding the FOG-Q as presenting with a FOG-Q item-3 score > 0. Functional dependency was defined as a S&E score less than 80% (80% = completely independent; 70% = not completely independent) [21]. Patients with a NMSS item-19 score > 0 were considered as suffering from dysphagia. Cox proportional hazards models were applied to identify independent predictors of acute hospitalization during the 1-year follow-up. Factors included as potential predictors were motor severity, motor complications, axial symptoms and related complications (FOG; falls; dysphagia), NMS burden, mood, cognition, neuropsychiatric symptoms, and disability for activities of daily living (Supplementary Table 1). Age, gender, disease duration, LEDD (levodopa equivalent daily dose [22]), and total number of non-antiparkinsonian drugs (as a marker of comorbidity [23]) were included as covariates (Supplementary Table 1). Values of p < 0.05 were considered significant.
Standard protocol approvals, registrations, and patient consents
For this study, we received approval from the Comité de Ética de la Investigación Clínica de Galicia from Spain (2014/534; 02/DEC/2014). Written informed consents from all participants in this study were obtained before the start of the study. COPPADIS-2015 was classified by the AEMPS (Agencia Española del Medicamento y Productos Sanitarios) as a Post-authorization Prospective Follow-up study with the code COH-PAK-2014-01.
Data availability
The protocol and the statistical analysis plan are available on request. De-identified participant data are not available for legal and ethical reasons.
RESULTS
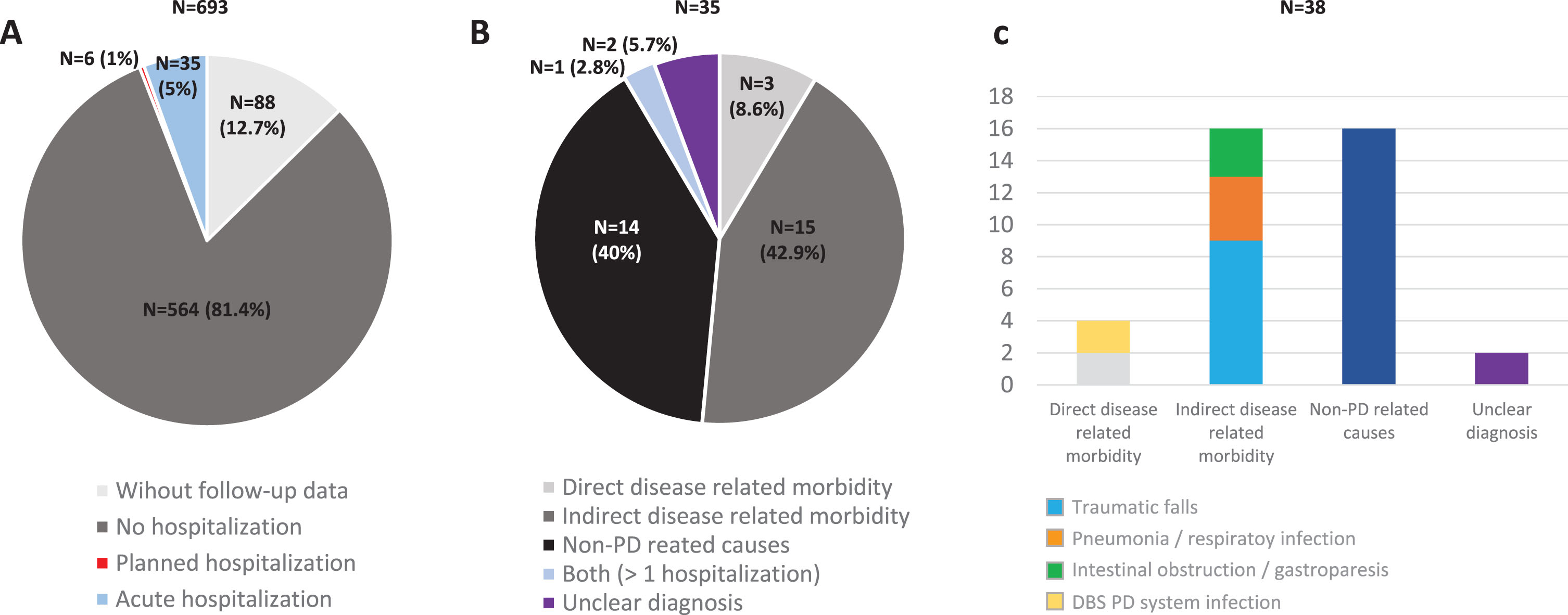
A total of 605 (62.5±8.9 years old; 59.8% males) from the 694 patients diagnosed with PD at baseline from the COPPADIS cohort were included in the analysis (87.2%). Figure 1 shows the reasons for the lack of follow-up of 89 patients. In 64 cases (9.2%) no data was recorded. The mean disease duration (N = 605) at baseline was 5.5±4.4 years. Forty-one out of 605 PD patients (6.8%) presented at least one hospitalization during the 1-year follow-up after the baseline visit, being 35 of them (85.4%) with at least one acute hospitalization. From the initial PD cohort (N = 693; 1 case excluded due to change in diagnosis), 5% of the patients presented an acute hospitalization (Fig. 2A). In 35 out of 41 patients (85.4%) hospital admission was reported once, twice in 4 patients (9.8%), and three times in 2 patients (4.8%). The mean hospital stay days was 8.4±8.3 (range 1–39). A total of 49 admissions were reported, being 38 acute and 11 programmed hospitalizations (Table 1). With regards to the acute admissions, 3 patients out of 35 (8.6%) were hospitalized due to a direct PD-related morbidity, 15 (42.9%) due to an indirect PD-related morbidity, and 14 (40%) due to non-PD related causes (Fig. 2B). Traumatic falls (9 events) represented the most frequent cause of admission, being 56.3% of all indirect PD-related morbidity causes, 23.7% all acute hospitilizations (38 events), and 18.4% of all hospitalizations (49 events) (Table 1 and Fig. 2C). No deaths were reported, neither in patients who presented a hospitalization nor in those who did not.
Fig. 1
Flowchart about PD patients from the COPPADIS cohort participating in the present study. Of 594 patients, 1 patient was excluded due to change in the diagnosis (from PD to MSA) and 88 for other reasons. Of 605 patients included in the analysis, 41 (6.8%) presented at least one hospitalization (35 acute and 6 planned hospitalization). MSA, multiple system atrophy; PD, Parkinson’s disease.
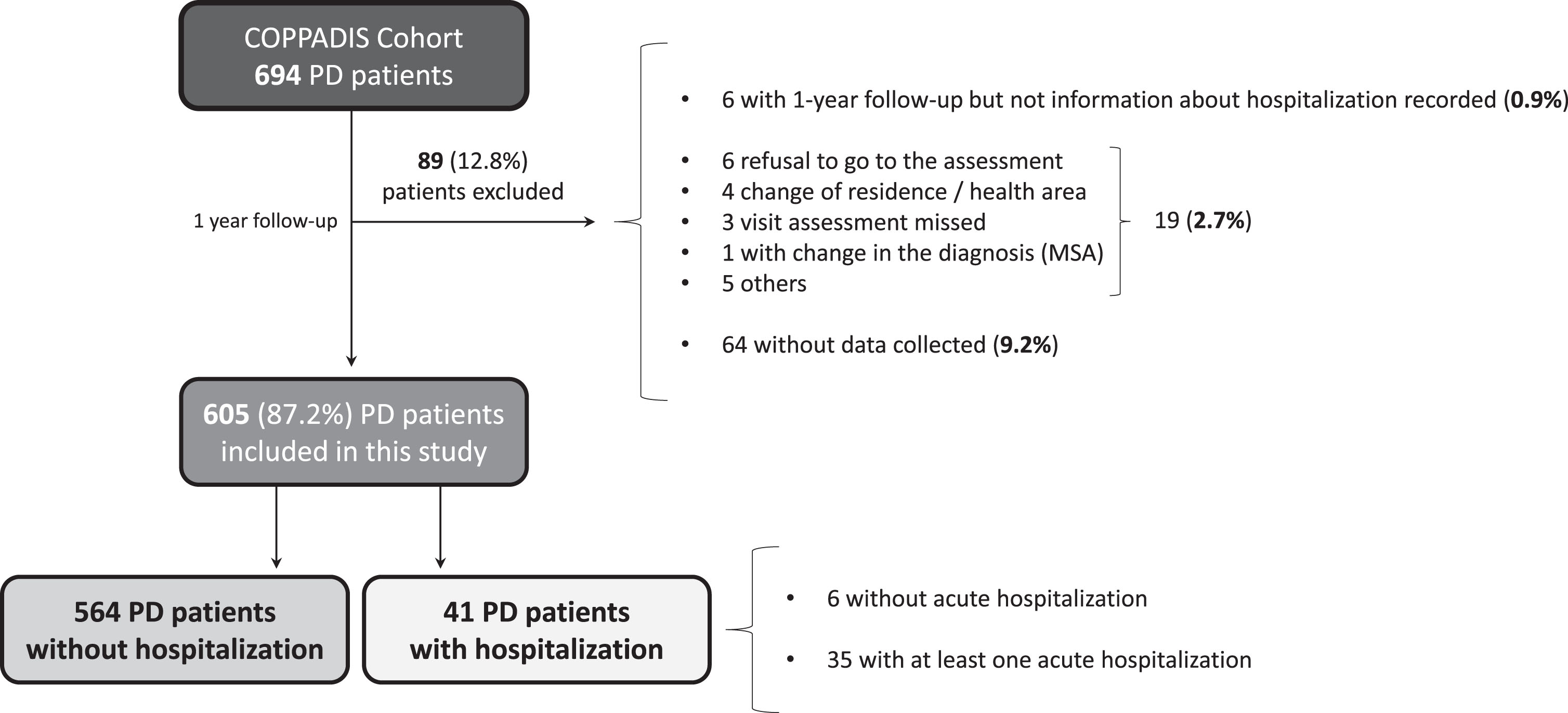
Table 1
Causes of hospitalization during the 1 year follow-up after the baseline visit: 49events in 41 PD patients
| Acute unplanned hospitalizations (38 events) |
| Direct disease related morbidity (4 events) |
| -DBS PD system infection (2 events) |
| -Motor impairment(1 event) |
| -Orthostatic hypotension (1 event) |
| Indirect disease related morbidity (16 events) |
| -Traumatic falls (9 events) |
| -Pneumonia and/or respiratory infection (4 events) |
| -Intestinal obstruction and/orgastroparesia (3 events) |
| Non-PD related causes (16 events) |
| -Other infections (6 events) |
| -Cardiac ischemic attack (3 events) |
| -Urinary system complications (3 events) |
| -Cardiac insufficiency (1 event) |
| -Syncope secondary to a orticstenosis (1 event) |
| -Stroke (1 event) |
| -Esophagitis (1 event) |
| Unclear diagnosis (2 events) |
| Programmed hospitalizations (11 events) |
| -Lumbar canal stenosis surgery (2 events) |
| -Deep brain stimulation surgery (2events) |
| -Carotid bypass surgery (1 event) |
| -Aortic valve replacement (1 event) |
| -knee replacement surgery (1 event) |
| -Transurethral resection of the prostate (1 event) |
| -Urinary incontinence surgery (1 event) |
| -Carcinoma pulmonary resection (1 event) |
| -Apomorphine test (1 event) |
Fig. 2
A) Percentage of PD patients from the COPPADIS cohort (N = 693; 1 excluded due to change in the diagnosis) without follow-up data (N = 88; 12.7%), without hospitalization (N = 564; 81.4%), with planned hospitalization (N = 6; 1%), and with acute hospitalization (N = 35; 5%) during the 1-year follow-up after the baseline visit. B) Percentage of patients with at least one acute hospitalization (N = 35) regarding the reason of admission: Direct PD-related morbidity (N = 3; 8.6%); Indirect PD-related morbidity (N = 15; 42.9%); Non-PD related causes (N = 40; 40%); More than 1 hospitalization with both reasons (N = 1; 2.8%); Unclear diagnosis (N = 2; 5.7%). C) Reasons for hospital admissions (38 events) in the subgroup of patients with acute hospitalization: 1) Direct PD-related morbidity (4 events; 10.5%); 2) Indirect PD-related morbidity: traumas, pneumonia, and intestinal obstruction / gastroparesis (16 events; 42.1%); 3. Non-PD related causes (16 events; 42.1%). PD, Parkinson’s disease.
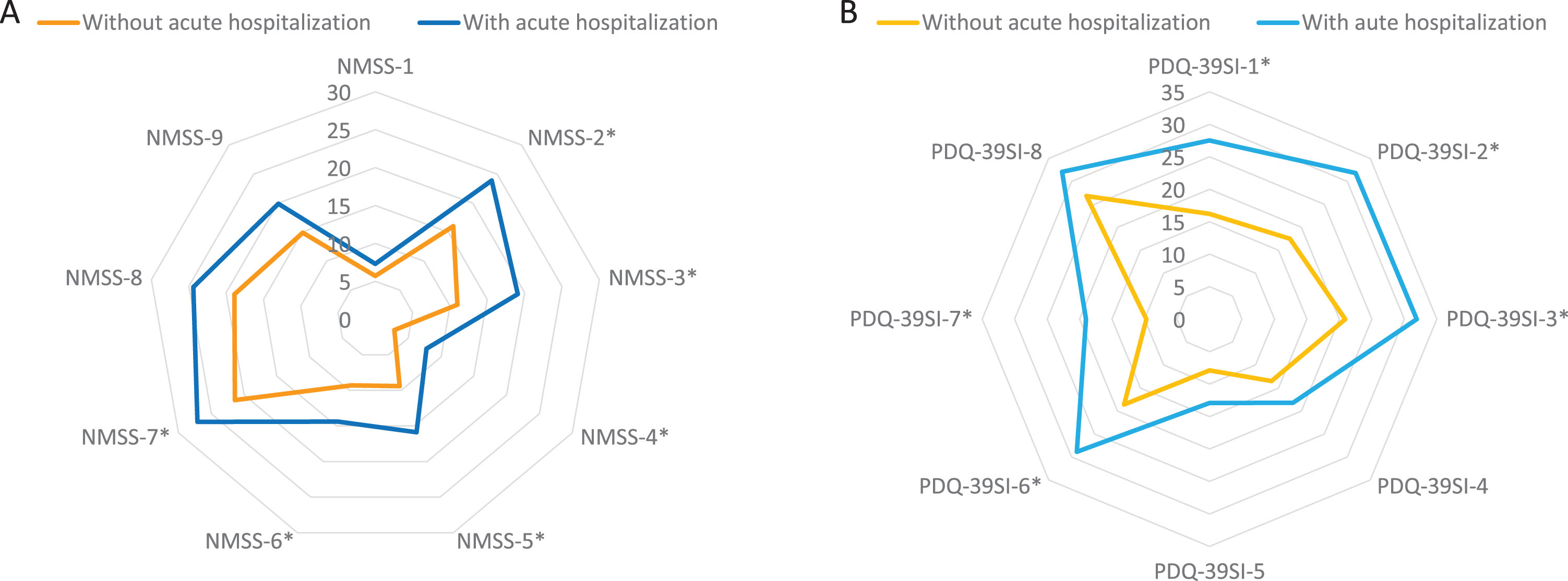
There were no differences between patients who presented an acute hospitalization compared to those who did not in terms of age, gender, disease duration, motor phenotype, and H&Y motor stage (Table 2). However, patients who suffered from an acute hospitalization presented a worse motor (UPDRS-III-OFF and UPDRS-IV) and non-motor status (PD-CRS, NMSS, BDI-II, NPI, and PDSS) at baseline (Table 2). Motor fluctuations, dyskinesia, falls, dysphagia, a severe and/or very severe NMS burden, and pain were more frequent in patients who presented an acute hospitalization compared to those who did not (Table 2). Specifically, the NMSS total score (66.1±42.8 vs 41.9±35; p < 0.0001) and the score on domains 2 (sleep/fatigue), 3 (mood/apathy), 4 (perceptual problems/hallucinations), 5 (attention/memory), 6 (gastrointestinal symptoms), and 7 (urinary symptoms) of the NMSS indicating a greater severe burden were higher in patients with acute hospitalization (Fig. 3A and Supplementary Table 2). Moreover, QoL and disability were worse in this group of patients with acute hospitalization, presenting a higher score at baseline on the PDQ-39SI (26.6±16 vs 15.9±13.3; p < 0.0001) and a lower score on the EUROHIS-QOL8 (3.6±0.6 vs 3.8±0.5; p = 0.038) and S&E score (83.4±11.4 vs 87.9±10.7; p = 0.048) when compared with patients without acute hospitalization (Table 2). With regards to the domains of the PDQ-39SI, patients with acute hospitalizations presented significantly higher scores in all domains indicating a worse QoL except on domains 4 (stigma), 5 (social support), and 8 (pain/discomfort) (Fig. 3B and Supplementary Table 2).
Table 2
Disease related characteristics, motor and non-motor symptoms, autonomy for activities of daily living and quality of life in PD patients with and without acute hospitalization during the year after the baseline visit (n = 605)
| All sample | Without acute hospitalization | With acute hospitalization | p | |
| (N = 605) | (N = 570) | (N = 35) | ||
| Age | 62.5±8.9 | 62.5±8.9 | 63.5±7.9 | 0.646 |
| Males (%) | 59.8 | 71.4 | 59.3 | 0.106 |
| Disease duration (years) | 5.5±4.4 | 5.5±4.3 | 5.8±4.4 | 0.656 |
| Number of non anti parkinsonian drugs | 2.6±2.4 | 2.6±2.4 | 2.6±2.5 | 0.980 |
| L-dopa eq. daily dose (mg) | 563.9±420.5 | 555.8±416.6 | 703.7±468.4 | 0.063 |
| Motor phenotype (%) | 0.621 | |||
| - Tremoricdominant | 47.3 | 48.4 | 40 | |
| - PIGD | 38.8 | 37.8 | 45.7 | |
| - Indeterminate | 13.9 | 13.8 | 14.3 | |
| Hoehn&Yahr | 2 [2, 2] | 2 [2, 2] | 2 [2, 2] | 0.720 |
| - Stage from 3 to 5(%) | 10.3 | 9.9 | 13.3 | 0.373 |
| UPDRS-III | 21.6±10.4 | 20.7±9.9 | 27.8±11.8 | 0.001 |
| UPDRS-IV | 2±2.4 | 1.8±3.3 | 3.3±3 | 0.001 |
| - Motor fluctuations (%) | 32.7 | 29.3 | 57.1 | 0.001 |
| - Dyskinesia (%) | 18.1 | 15.6 | 36.4 | 0.006 |
| FOG-Q | 3.5±4.6 | 3.4±4.5 | 4.7±4.7 | 0.006 |
| - Patients with FOG (%) | 33.8 | 32.5 | 42.9 | 0.154 |
| - Patients with falls (%) | 17.5 | 15.5 | 31.4 | 0.020 |
| PD-CRS | 91.6±15.3 | 92.2±15.3 | 86.8±13.9 | 0.049 |
| - Cognitive impairment (PD-CRS≤84) (%) | 28 | 26.6 | 38.2 | 0.114 |
| NMSS | 44.8±36.7 | 41.9±35 | 66.1±42.8 | < 0.0001 |
| - Severe and/or NMS burden (NMSS > 40) (%) | 40.7 | 37 | 67.6 | 0.001 |
| - Very severe NMS burden (NMSS > 70) (%) | 21.1 | 18.3 | 41.2 | 0.004 |
| - Dysphagia | 21.1 | 17.5 | 47.1 | < 0.0001 |
| BDI-II | 8.9±7.7 | 8.4±7.5 | 12.1±8.5 | 0.007 |
| - Major depression (%) | 17.1 | 15.4 | 28.6 | 0.051 |
| NPI | 6.7±8.4 | 6.2±8.1 | 10.1±9.4 | 0.005 |
| QUIP-RS | 4.3±8.1 | 3.4±7.1 | 4.5±8 | 0.227 |
| PDSS | 118.1±23.9 | 119.2±23.7 | 110.9±24.3 | 0.014 |
| VAS-PAIN | 2.8±3 | 2.8±3.1 | 3.4±2.4 | 0.133 |
| - Patients with pain (%) | 61.2 | 58.9 | 77.1 | 0.027 |
| VASF –physical | 2.9±2.8 | 2.9±2.8 | 3.3±2.8 | 0.342 |
| VASF –mental | 2.1±2.6 | 2.1±2.5 | 2.3±2.9 | 0.668 |
| ADLS | 87.5±10.9 | 87.9±10.7 | 84.3±11.4 | 0.048 |
| - Patients with functional dependency (%) | 12.1 | 11.4 | 17.1 | 0.233 |
| PDQ-39SI | 17.2±14.1 | 15.9±13.3 | 26.6±16 | < 0.0001 |
| PQ-10 | 7.2±1.6 | 7.3±1.6 | 6.8±1.7 | 0.119 |
| EUROHIS-QOL8 | 3.8±0.5 | 3.8±0.5 | 3.6±0.6 | 0.038 |
The results represent percentages, mean±SD or median [p25, p75].Chi-squared and Mann-Whitney-Wilcoxon test were applied for comparisons between Non acute hospitalization (N = 570) and Acute unplanned hospitalization (N = 35) patients. Data about H&Y and UPDRS-III are during the OFF state (first thing in the morning without taking medication in the previous 12 hours). ADLS, Schwab and England Activities of daily living Scale); BDI, Beck Depression Inventory-II; NMSS, Non-Motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD, Parkinson’s disease; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; PDSS, Parkinson’s Disease Sleep Scale; PIGD, Postural Inestability Gait Difficulty; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale; VAFS, Visual Analog Fatigue Scale; VAS-Pain, Visual Analog Scale-Pain.
Fig. 3
A) Comparison in PD patients with vs without acute hospitalization of mean NMSS score on each domain of the scale at baseline; NMSS-1, Cardiovascular (p = 0.892); 2) NMSS-2, Sleep/fatigue (p = 0.003); NMSS-3, Depression/apathy (p = 0.003); NMSS-4, Perceptual problems/hallucinations (p = 0.001); NMSS-5, Attention/memory (p = 0.001); NMSS-6, Gastrointestinal tract (p = 0.041); NMSS-7, Urinary symptoms (p = 0.040); NMSS-8, Sexual dysfunction (p = 0.152); NMSS-9, Miscellaneous (p = 0.106). B) Comparison in PD patients with vs without acute hospitalization of mean PDQ-39SI score on each domain of the scale: PDQ-39SI-1, Mobility (p = 0.001); PDQ-39SI-2, Activities of daily living (p = 0.002); PDQ-39SI-3, Emotional well-being (p = 0.002); PDQ-39SI-4, Stigma (p = 0.153); PDQ-39S-5, Social support (p = 0.259); PDQ-39SI-6, Cognition (p = 0.001); PDQ-39SI-7, Communication (p < 0.0001); PDQ-39SI-8, Pain and discomfort (p = 0.133). NMS, Non-motor symptoms; PD, Parkinson’s disease.
In Kaplan-Meier analysis, the presence at baseline of motor fluctuations (p = 0.004), dyskinesia (p = 0.003), very severe NMS burden (p = 0.001), major depression (p = 0.037), falls (p = 0.001), and dysphagia (p = 0.001) was related to a higher risk of acute hospitalization (Fig. 4). Specifically, to suffer from motor fluctuations (HR [hazard ratio] 2.461; 95% CI, 1.065–5.678; p = 0.035), a very severe NMS burden (HR [hazard ratio] 2.828; 95% CI, 1.319–6.063; p = 0.008), falls (HR 3.966; 95% CI 1.757–8.470; p = 0.001), and dysphagia (HR 2.356; 95% CI 1.124–4.941; p = 0.023) was associated with acute hospitalization after adjustment to age, gender, disease duration, LEDD, total number of non-antiparkinsonian drugs, and UPDRS-III-OFF (Table 3). Patients who presented a score on the UPDRS-III-OFF > 20 at baseline had a significantly higher risk of acute hospitalization after adjustment to the same covariates as well (HR 3.644; 95% CI 1.430–9.284; p = 0.007). Although unadjusted HR associated with dyskinesia (HR 2.792; 95% CI 1.365–5.711; p = 0.005) and major depression (HR 2.159; 95% CI 1.033–4.515; p = 0.041) were significant (Table 3), the effect was not after adjustment to the previous commented covariates. Of the previous variables, only falls (HR 2.998; 95% CI 1.080–8.322; p = 0.035) was an independent predictor of acute hospitalization when different covariates as potential predictors of hospitalization were included in an “a priori” well-planned model (Table 4). In the model, having falls triples the probability of acute hospital admission regardless of other variables, and the frequency of falls at baseline was double in those patients who were admitted during the 1-year follow-up compared to those who did not (31.4% vs 15.5%; p = 0.020). Specifically, having falls during the previous month to the baseline visit was associated with acute admission during the 1-year follow-up (Supplementary Figure 1).
Fig. 4
Proportion of patients without acute hospitalization with regards to suffering (in blue) or not (in green) from motor fluctuations (p = 0.004), dyskinesia (p = 0.003), very severe NMS burden (p = 0.001), major depression (p = 0.037), falls (p = 0.001), and dysphagia (p = 0.001). Y, survival; X, days of follow-up. NMS, Non-motor symptoms.
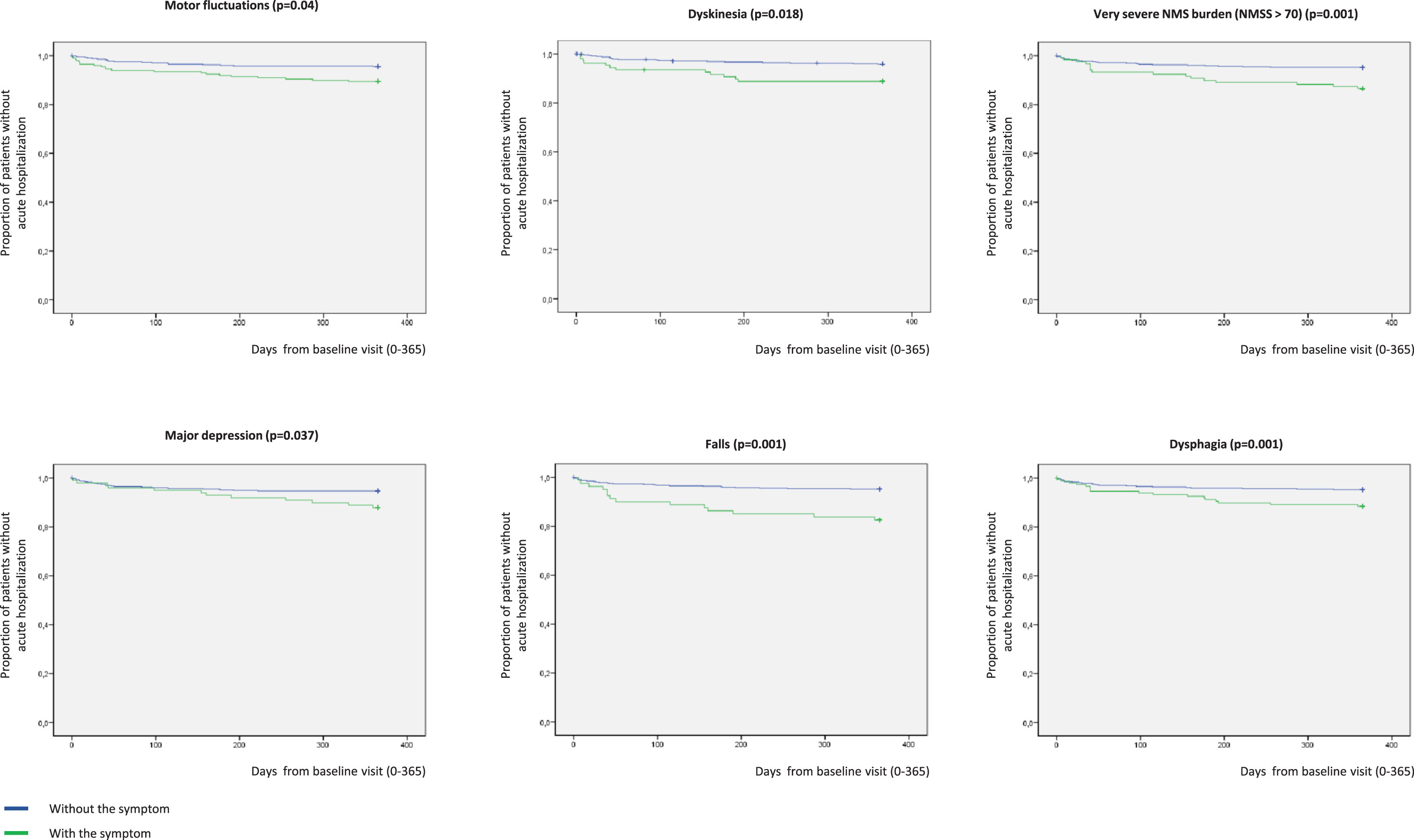
Table 3
Analysis about the risk of acute hospitalization after 1 year of follow-up with regards to having or not different complications (N = 605)
| Unadjusted HR (95% CI) | p | Adjusted HR(95% CI) | p | |
| Motor fluctuations | 2.616 (1.329–5.148) | 0.005 | 2.461 (1.065–5.678) | 0.035 |
| Dyskinesia | 2.792 (1.365–5.711) | 0.005 | 2.145 (0.905–5.085) | 0.083 |
| Severe or very severe NMSB (NMSS > 40) | 2.871 (1.400–5.890) | 0.004 | 2.645 (1.154–6.103) | 0.022 |
| Very severe NMSB (NMSS > 70) | 2.925 (1.477–5.791) | 0.002 | 2.828 (1.319–6.063) | 0.008 |
| FOG | 1.489 (0.757–2.930) | 0.249 | 1.117 (0.496–2.518) | 0.789 |
| Falls | 3.262 (1.590–6.696) | 0.001 | 3.966 (1.757–8.470) | 0.001 |
| Dysphagia | 2.843 (1.450–5.575) | 0.002 | 2.356 (1.124–4.941) | 0.023 |
| Major depression | 2.159 (1.033–4.515) | 0.041 | 1.934 (0.855–4.377) | 0.113 |
| Cognitive impairment (PD-CRS≤84) | 1.493 (0.743–3.002) | 0.260 | 1.160 (0.514–2.615) | 0.721 |
| HY-OFF from 3 to 5 | 1.331 (0.463–3.826) | 0.595 | 0.703 (0.187–1.719) | 0.602 |
| UPDRS-III-OFF > 20 | 2.800 (1.258–6.233) | 0.012 | 3.644 (1.430–9.284) | 0.007* |
| Functional dependency | 2.118 (0.877–5.116) | 0.095 | 1.894 (0.675–5.318) | 0.225 |
Age, gender, disease duration, LEDD (levodopa equivalent daily dose), total number of non-anti parkinsonian drugs (as a marker of comorbidity) and UPDRS-III-OFF were included in the model as covariates. *For this analysis the UPDRS-III-OFF was not included in the model. FOG, freezing of gait; HR, Hazard ratio; NMSB, non-motor symptoms burden; NMSS, Non-Motor Symptoms Scale; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale.
Table 4
Cox-regression model about predictors of acute hospitalization in PD patients after 1 year of follow-up (N = 605)
| HR (95% CI) | p | |
| Age | 1.002 (0.951–1.056) | 0.937 |
| Gender | 0.627 (0.267–1.469) | 0.282 |
| Disease duration | 0.838 (0.726 –0.968) | 0.016 |
| LEDD | 1.000 (0.999–1.001) | 0.614 |
| Total number of non-anti parkinsonian drugs | 0.839 (0.675–1.042) | 0.111 |
| UPDRS-III-OFF | 1.032 (0.991–1.074) | 0.128 |
| UPDRS-IV | 1.071 (0.909–1.260) | 0.413 |
| NMSS | 0.999 (0.989–1.009) | 0.823 |
| BDI-II | 1.016 (0.955–1.081) | 0.613 |
| PD-CRS | 0.986 (0.958–1.015) | 0.339 |
| NPI | 1.021(0.972–1.072) | 0.406 |
| Falls | 2.998 (1.080 –8.322) | 0.035 |
| Dysphagia | 1.593 (0.645–3.932) | 0.313 |
| ADLS | 1.004 (0.962–1.048) | 0.865 |
Hazard ratio; LEED, Levodopa equivalent daily dose; NMSS, Non-Motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale. The omnibus test indicated an overall significant model χ2 = 34,149 (p = 0.001).
DISCUSSION
In the present study, we observed different important findings: 1) the probability of presenting an acute hospitalization is not infrequent in patients with PD; 2) the QoL and autonomy for activities of daily living is worse and the NMS burden is greater in those PD patients who will have an acute hospitalization; 3) there are certain symptoms associated with a greater risk of acute hospitalization such as motor fluctuations, dyskinesias, severe NMS burden, major depression, falls, and dysphagia; 4) falls is an independent predictor of acute hospitalization that increases the risk by three times.
About 5% of the patients from the COPPADIS cohort [15, 16] presented an acute hospitalization after 1-year of follow-up. This percentage is low compared to other studies, ranging from 7 to 28% per year [17]. The results between studies vary in part to differences in methodology [1, 2, 24–27]. Moreover, many studies are prospective [6, 26, 28] or, more frequently, retrospective [29–34] analysis conducted only in PD patients admitted with the aim to analyze problems during hospitalization. Moreover, several studies were conducted in small samples and/or without control group: 76 patients [28]; 108 patients [26]; 130 patients [25]; 132 patients [6]; 143 patients [33]; 144 patients [24]; 173 patients [30]; 367 patients [3]. A recent study conducted in a large population from North America, the Netherlands, and Israel showed that of 4,680 PD patients followed during an average of 2 years (median 1.85, maximum 4.85 years), 2,264 patients (48.4%) had a hospital encounter after the baseline visit [35]. Compared with many other studies [6, 29, 30, 33, 34, 36, 37], the mean age of the patients from the COPPADIS cohort is lower, and, as previously reported [23, 38], our sample is not fully representative of the PD population due to inclusion and exclusion criteria at baseline (i.e., age limit, no dementia, no severe comorbidities, no second line therapies, etc.) which subsequently entails a bias toward early PD. This aspect may explain the lower frequency of admissions in our analysis after 1-year of follow-up. On the other hand, with regards to the causes of hospitalization, our findings are in line with two recent review studies [26, 39], in which infections, worsening motor features, falls/fractures, cardiovascular co-morbidities, neuropsychiatric, and gastrointestinal complications were the main reasons for hospitalization among people with PD. Specifically, in our study, falls was the most frequent cause despite more than 90% of the patients had a stage 2 of the H&Y. By the contrary, admissions related to a direct PD-related event were infrequent since ambulatory PD symptoms management is often preferred.
A worse QoL and a greater disability and NMS burden at baseline was observed in PD patients who presented an acute hospitalization in our study. We observe this finding because, unlike most other studies, the assessment at baseline was exhaustive using different validated scales. Differences were observed between patients who were admitted during the follow-up and those who did not as those who were admitted had a worse motor and non-motor status and worse autonomy for activities of daily living and QoL. In other words, PD patients more affected by their disease may be more vulnerable and have a higher risk of acute hospitalization. Symptoms such as pain, dysphagia, falls, motor fluctuations, dyskinesia, a worse QoL, and a greater motor disability were more frequent in patients who presented an acute hospitalization, but no differences were observed in fatigue and motor phenotype. Falls, fractures, infections, cognitive, and motor decline have been identified as risk factors for acute hospital admissions in patients with PD [11, 14]. More specifically, longer timed up and go test, higher number of comorbidities, number of medications, the presence of motor fluctuations, having deep brain stimulation, and the degree of caregiver burden have been associated with hospitalization and/or rehospitalization in PD [35]. A higher PDQ-39 total score was observed to be associated with a higher risk of rehospitalization as well [35]. In our study, the factors associated with acute hospitalization are not surprising. However, the only independent predictor of hospitalization was falls. In this context, in a pooled data of 7 studies selected [27], the main causes of general ward admission were falls (30%) and PD-related causes (16%), whereas the main causes of neurological ward were motor (42.3%) and psychiatric complications (21.2%). In any case, our finding of falls as a predictor of acute hospitalization in PD should be interpreted with caution because the rates of admission due to fall (N = 9) were very low. Ideally, these findings should be reproduced over a longer period to capture more admission and arguably with patients with a broader range of severity.
A very important point is that some of these symptoms can be treated with the aim of reducing acute PD hospital admission [14]. For example, in our cohort, motor fluctuations were associated with a doubled probability of acute hospitalization, so reducing OFF time could reduce the risk. Many factors are correlated. Falls were associated with motor fluctuations (falls were present in 22.4% of patients with motor fluctuations compared to 8.5% of patients without them; p < 0.0001). One strategy for reducing them could be to increase the ON time in PD patients. However, falls can have different etiology. Optimizing motor symptom control and managing medication side effects may prevent falls and hence hospital admissions [14]. Furthermore, physiotherapy is thought to improve PD motor symptoms, mobility, and balance [39], which also may reduce the risk of falls. However, there is a lack of evidence about interventions or negative results in the few trials performed [14]. In a randomized clinical trial comparing the incidence of pneumonia among 515 patients with dementia and PD who were randomized to either chin-down posture technique or two types of thickened fluids (nectar- and honey-thick consistencies) over a 3 month follow up period, at least one hospital admission was observed in 20% of each intervention arms [40]. Considering the complexity of managing PD, it is likely that a multimodal approach which addresses motor and non-motor complications, as well as palliatives aspects in end-stage disease, may be more effective compared to a single intervention approach [14, 41, 42]. The risk of acute hospitalization seems to be more related with PD symptoms, proper care, medication adherence, and comorbidity than age, gender or disease duration [4, 14, 26, 27, 41, 42]. Both patients who were admitted and those who were not had a similar mean disease duration. However, in the model and after adjustment to covariates related to disease progression, longer disease duration was associated with a lower risk of hospitalization. Lower risk of falls in more advanced patients because they have limited walking or prioritized care towards those with a more recent diagnosis could explain these findings.
The most important limitation of this study is the fact that information about admission in 88 of 693 PD patients was not recorded (12.7%). However, this is a limitation observed in other prospective studies. Of 7,507 PD patients, follow-up data was available only for 4,680 participants (62.3%) [35]. Although hospital admissions or mortality cannot be ruled out in this group from our study (N = 88), there were no significant differences between this group and the rest of patients from the cohort with regards to age, disease duration, LEDD, and all the covariates included in the model (data not shown). A very important second limitation is that PD patients older than 75 years old were excluded from participation by COPPADIS study protocol [16], which is a major weakness because older patients with PD are more likely to have complex disease (cognitive impairment, high comorbidity, etc.) and falls. Other limitations are the bias toward early PD in this cohort, as we previously commented, and the lack of a control group. Moreover, falls diary, calendar, or postcard were not used [43] and a patient was considered to suffer from falls based on the interview conducted. On the contrary, the strengths of our study include the large sample size, the prospective longitudinal follow-up design, the fact that this analysis was “a priori” planned as one objective of the multicenter COPPADIS project [16], and the extensive clinical and demographic information recorded.
In conclusion, this study observes that acute hospital admission is not infrequent in PD patients, that the risk is higher in patients with different complication (such as dysphagia, motor fluctuations, dyskinesia, and greater motor disability and NMS burden), and that falls is an independent predictor of acute hospitalization in PD patients. Future prospective studies are required to identify other predictors of acute hospitalization in PD and, more interestingly, to evaluate the effectiveness of proposed interventions over them.
CONFLICT OF INTEREST
Santos García D. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, and Teva.
de Deus Fonticoba T: None.
Cores C. has received honoraria for educational presentations and advice service by Lundbeck and UCB Pharma.
Suárez Castro E: None.
Hernández Vara J. has received honoraria for advice service from Britannia, travel bursaries and educational grants from Abbvie, and has received honoraria for educational presentations from Abbvie, Teva, Bial, Zambon, Italfarmaco, and Sanofi-Genzyme.
Jesús S. has received honoraria from AbbVie, Bial, Merz, UCB, and Zambon and holds the competitive contract “Juan Rodés” supported by the Instituto de Salud Carlos III. She has received grants from the Spanish Ministry of Economy and Competitiveness (PI18/01898) and the Consejería de Salud de la Junta de Andalucía (PI-0459-2018).
Mir P. has received honoraria from AbbVie, Abbott, Allergan, Bial, Merz, UCB, and Zambon. He has received grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Subdirección General de Evaluación y Fomento de la Investigación) and by Fondo Europeo de Desarrollo Regional (FEDER), the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía [CVI-02526, CTS-7685], the Consejería de Salud y Bienestar Social de la Junta de Andalucía [PI-0437-2012, PI-0471-2013], the Sociedad Andaluza de Neu-rología, the Jacques and Gloria Gossweiler Foundation, the Fundación Alicia Koplowitz, and the Funda-ción Mutua Madrileña.
Cosgaya M: None.
Martí MJ. received honoraria for advice and lecture from Abbvie, Bial, and Merzt Pharma and grants from Michael J. Fox Foundation for Parkinson Disease (MJFF): MJF_PPMI_10_001, PI044024; Fondo de Investigacuiones Sanitarias of Spain (FIS PI17/00096) and from Generalitat de Catalunya (AGAUR Exp 2017 SGR 1502).
Pastor P: None.
Cabo I. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
Seijo M. has received honoraria for educational services from KRKA, UCB, Zambon, and Bial; travel grants from Daiichi and Roche.
Legarda I. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva.
Vives B: None.
Caballol N. has received honoraria from Bial, Italfármaco, Qualigen, Zambon, UCB, Teva, and KRKA and sponsorship from Zambon, TEVA, and Abbvie for attending medical conferences.
Rúiz Martínez J. has received honoraria for educational presentations, attending medical conferences, and advice service by Abbvie, UCB Pharma, Zambon, Italfarmaco, Bial, and Teva.
Croitoru I: None.
Cubo E: Travel grants: Abbvie, Allergan, Boston; Lecturing honoraria: Abbvie, International Parkinson’s disease Movement Disorder Society.
Miranda J: None.
Alonso Losada MG. has received honoraria for educational presentations and advice service by Zambon and Bial.
Labandeira C. has received honoraria for educational presentations and advice service by Abbvie, Italfarmaco, Zambon, and Bial.
López Ariztegui N. has received honoraria for educational presentations and advice service by Abbvie, Italfarmaco, Zambon, and Bial.
Morales-Casado M. has received honoraria for educational presentations and advice service by Bial, Zambon, UCB, Ferrer and Fressenius-kabi.
González Aramburu I: None.
Infante J. has received travel bursaries and honoraria for educational presentations from Abbvie and Zambon.
Escalante S. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
Bernardo N: None.
Blázquez Estrada M. has received honoraria for educational presentations and advice service by Abbvie, Abbott, UCB Pharma, Allergan, Zambon, Bial, and Qualigen.
Menéndez M. has received honoraria for educational presentations by KRKA and Zambon.
Seijo M. has received honoraria for educational services from KRKA, UCB, Zambon, and Bial; travel grants from Daiichi and Roche.
García Caldentey J. has received honoraria for educational presentations and advice service by Qualigen, Nutricia, Abbvie, Italfarmaco, UCB Pharma, Lundbeck, Zambon, Bial, and Teva.
Borrué C: None.
Vela L. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva.
Catalán MJ: None.
Gómez-Mayordomo V: None.
Kurtis M. has received honoraria from Bial, the Spanish Neurology Society and the International and Movement Disorders Society.
Prieto C: None.
Ordás C: None.
Nogueira V: None.
López Manzanares L: Compensated advisory services, consulting, research grant support, or speaker honoraria: AbbVie, Acorda, Bial, Intec Pharma, Italfarmaco, Pfizer, Roche, Teva, UCB, and Zambon.
Ávila Rivera MA. has received honoraria from Zambon, UCB Pharma, Qualigen, Bial, and Teva, and sponsorship from Zambon and Teva for attending conferences.
Puente V. has served as consultant for Abbvie and Zambon; has received grant/research from Abbvie.
García Moreno JM. has received honoraria for educational presentations and advice service by Abbvie, Ital-Pharma, Lundbeck, Merz, KRKA, UCB, Pharma, Zambon, Bial and Teva.
Solano Vila B. has received honoraria for educational presentations and advice service by UCB, Zambon, Teva, Abbvie, and Bial.
Álvarez Sauco M. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva.
Carrillo Padilla F. has received honoraria from Zambon (SEN Congress assistance).
Martínez Castrillo JC. has received research support from Lundbeck, Italfarmaco, Allergan, Zambon, Merz, and Abbvie. He has also received speaking honoraria from AbbVie, Bial, Italfarmaco, Lundbeck, Krka, TEVA, UCB, Zambon, Allergan, Ipsen, and Merz.
Sánchez Alonso P. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva.
Gastón I. has received research support from Abbvie and Zambon and has served as a consultant for Abbvie, Exelts, and Zambon.
Kulisevsky J: (1) Consulting fees: Roche, Zambon; (2) Stock / allotment: No; (3) Patent royalties / licensing fees: No; (4) Honoraria (e.g., lecture fees): Zambon, Teva, Bial, UCB; (5) Fees for promotional materials: No; (6) Research funding: Roche, Zambon, Ciberned; Instituto de SaludCarlos III; FundacióLa Maratóde TV3; (7) Scholarship from corporation: No; (8) Corporate laboratory funding: No; (9) Others (e.g., trips, travel, or gifts): No.
Valero C. has received honoraria for educational services from Zambon, Abbvie and UCB.
de Fábregues O. has received honoraria for educational presentations and advice service by Bial, Zambon, Abbvie, KRKA, and Teva.
González Ardura J. has recieved honoraria for speking from italofarma, Krka, Genzyme, UCB, Esteve, Psyma iberica marketing research SL and Ferrer, course grant from Teva and travel grant from Merck.
López Díaz L. has received honoraria from UCB, Lundbeck and KRKA.
Martinez-Martin P. has received honoraria from Editorial Viguera and Takeda Pharmaceuticals for lecturing in courses; from Britannia for writing an article in their Parkinson’s Disease Medical Journal-Kinetic; and from the International Parkinson and Movement Disorder Society (MDS) for management of the Program on Rating Scales. Grants from the MDS for development and validation of the MDS-NMS.
Appendices
APPENDIX 1. COPPADIS STUDY GROUP
Adarmes AD, Almeria M, Alonso Losada MG, Alonso Cánovas A, Alonso Frech F, Alonso Redondo R, Álvarez I, Álvarez Sauco M, Aneiros Díaz A, Arnáiz S, Arribas S, Ascunce Vidondo A, Aguilar M, Ávila Rivera MA, Bernardo Lambrich N, Bejr-Kasem H, Blázquez Estrada M, Botí M, Borrue C, Buongiorno MT, Cabello González C, Cabo López I, Caballol N, Cámara Lorenzo A, Carrillo F, Carrillo Padilla FJ, Casas E, Catalán MJ, Clavero P, Cortina Fernández A, Cosgaya M, Cots Foraster A, Crespo Cuevas A, Cubo E, de Deus Fonticoba T, de Fábregues O, Díez-Fairen M, Erro E, Escalante S, Estelrich Peyret E, Fernández Guillán N, Gámez P, Gallego M, García Caldentey J, García Campos C, García Moreno JM, Gastón I, Guillén Fopiani D, Gómez Garre MP, Gómez Mayordomo V, González Aloy J, González-Aramburu I, González Ardura J, González García B, González Palmás MJ, González Toledo GR, Golpe Díaz A, Grau Solá M, Guardia G, Hernández Vara J, Horta-Barba A, Idoate Calderón D, Infante J, Jesús S, Kulisevsky J, Kurtis M, Labandeira C, Labrador MA, Lacruz F, Lage Castro M, Legarda I, López Ariztegui N, López Díaz LM, López Manzanares L, López Seoane B, Lucas del Pozo S, Macías Y, Mata M, Martí Andres G, Martí MJ, Martínez Castrillo JC, Martinez-Martin P, McAfee D, Meitín MT, Menéndez González M, Méndez del Barrio C, Mir P, Miranda Santiago J, Morales Casado MI, Moreno Diéguez A, Nogueira V, Novo Amado A, Novo Ponte S, Ordás C, Pagonabarraga J, Pareés I, Pascual-Sedano B, Pastor P, Pérez Fuertes A, Pérez Noguera R, Planas-Ballvé A, Planellas LL, Prats MA, Prieto Jurczynska C, Puente V, Pueyo Morlans M, Redondo Rafales N, Rodríguez Méndez L, Rodríguez Pérez AB, Roldán F, Ruíz De Arcos M, Ruíz Martínez J, Sánchez Alonso P, Sánchez-Carpintero M, Sánchez Díez G, Sánchez Rodríguez A, Santacruz P, Santos García D, Segundo Rodríguez JC, Seijo M, Sierra Peña M, Solano Vila B, Suárez Castro E, Tartari JP, Valero C, Vargas L, Vela L, Villanueva C, Vives B, Villar MD
| Name (Last Name, First Name) | Location | Role | Contribution |
| Astrid Adarmes, Daniela | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Almeria, Marta | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Alonso Losada, Maria Gema | Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo (CHUVI), Vigo, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Alonso Cánovas, Araceli | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Alonso Frech, Fernando | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Alonso Redondo, Ruben | Hospital Universitario Lucus Augusti (HULA), Lugo, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Aneiros Díaz, Ángel | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Álvarez, Ignacio | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Álvarez Sauco, María | Hospital General Universitario de Elche, Elche, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Arnáiz, Sandra | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Arribas, Sonia | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Ascunce Vidondo, Arancha | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Aguilar, Miquel | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Ávila Rivera, Maria Asunción | Consorci Sanitari Integral, Hospital General de L’Hospitalet, L’Hospitalet de Llobregat, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Bernardo Lambrich, Noemí | Hospital de Tortosa Verge de la Cinta (HTVC), Tortosa, Tarragona, Spain | Site investigator | Evaluation of participants and/or data management |
| Bejr-Kasem, Helena | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Blázquez Estrada, Marta | Hospital Universitario Central de Asturias, Oviedo, Spain | Site investigator | Evaluation of participants and/or data management |
| Botí González, Maria Ángeles | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Borrué, Carmen | Hospital Infanta Sofía, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Buongiorno, Maria Teresa | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Nurse study coordinator |
| Cabello González, Carolina | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Scheduling of evaluations |
| Cabo López, Iria | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Caballol, Nuria | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain. | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Cámara Lorenzo, Ana | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Nurse study coordinator |
| Carrillo, Fátima | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Carrillo Padilla, Francisco José | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Casas, Elena | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Catalán, Maria José | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Clavero, Pedro | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cortina Fernández, A | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Coordination of blood extractions |
| Cosgaya, Marina | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cots Foraster, Anna | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Crespo Cuevas, Ane | Hospital del Mar, Barcelona, Spain. | Site investigator | Evaluation of participants and/or data management |
| Cubo, Esther | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| De Deus Fonticoba, Teresa | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Nurse study coordinator Evaluation of participants and/or data management |
| De Fábregues, Oriol | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Díez Fairen, M | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Erro, Elena | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Escalante, Sonia | Hospital de Tortosa Verge de la Cinta (HTVC), Tortosa, Tarragona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Estelrich Peyret, Elena | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Fernández Guillán, Noelia | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Gámez, Pedro | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Gallego, Mercedes | Hospital La Princesa, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| García Caldentey, Juan | Centro Neurológico Oms 42, Palma de Mallorca, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| García Campos, Cristina | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| García Moreno, Jose Manuel | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Gastón, Itziar | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Guillén Fopiani, Desiré | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Gómez Garre, María del Pilar | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Genetic studies coordination |
| Gómez Mayordomo, Víctor | Hospital Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| González Aloy, Javier | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| González Aramburu, Isabel | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| González Ardura, Jessica | Hospital Universitario Lucus Augusti (HULA), Lugo, Spain | Site investigator | Evaluation of participants and/or data management |
| González García, Beatriz | Hospital La Princesa, Madrid, Spain | Site investigator | Nurse study coordinator |
| González Palmás, Maria Josefa | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Evaluation of participants and/or data management |
| González Toledo, Gabriel Ricardo | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Golpe Díaz, Ana | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Laboratory analysis coordination |
| Grau Solá, Mireia | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Guardia, Gemma | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Hernández Vara, Jorge | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Horta Barba, Andrea | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Idoate Calderón, Daniel | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigaor | neuropsychologist; evaluation of participants |
| Infante, Jon | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Jesús, Silvia | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Kulisevsky, Jaime | Hospital de Sant Pau, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Kurtis, Mónica | Hospital Ruber Internacional, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Labandeira, Carmen | Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo (CHUVI), Vigo, Spain | Site investigator | Evaluation of participants and/or data management |
| Labrador Espinosa, Miguel Ángel | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Neuroimaging data analysis |
| Lacruz, Francisco | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Lage Castro, Melva | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Evaluation of participants and/or data management |
| Legarda, Inés | Hospital Universitario Son Espases, Palma de Mallorca, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| López Ariztegui, Nuria | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator / PI | Evaluation of participants and/or data management |
| López Díaz, Luis Manuel | Hospital Da Costa de Burela, Lugo, Spain | Site investigator | Evaluation of participants and/or data management |
| López Manzanares, Lydia | Hospital La Princesa, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| López Seoane, Balbino | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Lucas del Pozo, Sara | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Macías, Yolanda | Fundación Hospital de Alcorcón, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Mata, Marina | Hospital Infanta Sofía, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Martí Andres, Gloria | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Martí, Maria José | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Martínez Castrillo, Juan Carlos | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator /PI | Coordination at the center Evaluation of participants and/or data management |
| Martinez-Martin, Pablo | Centro Nacional de Epidemiología y CIBERNED, Instituto de Salud Carlos III. Madrid | Collaborator in statistical and methods analysis | Methods and statistical reviewer |
| McAfee, Darrian | University of Pennsylvania, Philadelphia | Collaborator in english style | English style reviewer |
| Meitín, Maria Teresa | Hospital Da Costa de Burela, Lugo, Spain | Site investigator | Evaluation of participants and/or data management |
| Menéndez González, Manuel | Hospital Universitario Central de Asturias, Oviedo, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Méndez del Barrio, Carlota | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Mir, Pablo | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Miranda Santiago, Javier | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Morales Casado, Maria Isabel | Complejo Hospitalario de Toledo, Toledo, Spain. | Site investigator | Evaluation of participants and/or data management |
| Moreno Diéguez, Antonio | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Nogueira, Víctor | Hospital Da Costa de Burela, Lugo, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Novo Amado, Alba | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Novo Ponte, Sabela | Hospital Universitario Puerta de Hierro, Madrid, Spain. | Site investigator | Evaluation of participants and/or data management |
| Ordás, Carlos | Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Spain. | Site Investigator | Evaluation of participants and/or data management |
| Pagonabarraga, Javier | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Isabel Pareés | Hospital Ruber Internacional, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Pascual-Sedano, Berta | Hospital de Sant Pau, Barcelona, Spain | Site Investigator | Evaluation of participants and/or data management |
| Pastor, Pau | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Pérez Fuertes, Aída | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Blood analysis |
| Pérez Noguera, Rafael | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Planas-Ballvé, Ana | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Planellas, Lluís | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Prats, Marian Ángeles | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Prieto Jurczynska, Cristina | Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Puente, Víctor | Hospital del Mar, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Pueyo Morlans, Mercedes | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Redondo, Nuria | Hospital La Princesa, Madrid, Spain | Site Investigator | Evaluation of participants and/or data management |
| Rodríguez Méndez, Luisa | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Blood analysis |
| Rodríguez Pérez, Amparo Belén | Hospital General Universitario de Elche, Elche, Spain | Site investigator | Evaluation of participants and/or data management |
| Roldán, Florinda | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Neuroimaging studies |
| Ruíz de Arcos, María | Hospital Universitario Virgen Macarena, Sevilla, Spain. | Site investigator | Evaluation of participants and/or data management |
| Ruíz Martínez, Javier | Hospital Universitario Donostia, San Sebastián, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez Alonso, Pilar | Hospital Universitario Puerta de Hierro, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez-Carpintero, Macarena | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Sánchez Díez, Gema | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez Rodríguez, Antonio | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| Santacruz, Pilar | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Santos García, Diego | CHUAC, Complejo Hospitalario Universitario de A Coruña | Coordinator of the Project | Coordination of the COPPADIS-2015 |
| Segundo Rodríguez, José Clemente | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator | Evaluation of participants and/or data management |
| Seijo, Manuel | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Sierra, María | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| Solano, Berta | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Suárez Castro, Ester | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Evaluation of participants and/or data management |
| Tartari, Juan Pablo | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Valero, Caridad | Hospital Arnau de Vilanova, Valencia, Spain | Site investigator | Evaluation of participants and/or data management |
| Vargas, Laura | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Vela, Lydia | Fundación Hospital de Alcorcón, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Villanueva, Clara | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Vives, Bárbara | Hospital Universitario Son Espases, Palma de Mallorca, Spain | Site investigator | Evaluation of participants and/or data management |
| Villar, Maria Dolores | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
ACKNOWLEDGMENTS
We would like to thank all patients and their caregivers who collaborated in this study. Many thanks also to Fundación Española de Ayuda a la Investigación en Parkinson y otras Enfermedades Neurodegenerativas (Curemos el Parkinson; www.curemoselparkinson.org), Alpha Bioresearch (www.alphabioresearch.com), and other institutions helping us.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-212539.
REFERENCES
[1] | Guttman M , Slaughter PM , Theriault ME , DeBoer DP , Naylor CD ((2003) ) Burden of parkinsonism: A population-based study, Mov Disord 18: , 313–319. |
[2] | Aminoff MJ , Christine CW , Friedman JH , Chou KL , Lyons KE , Pahwa R , Bloem BR , Parashos SA , Price CC , Malaty IA , Iansek R , Bodis-Wollner I , Suchowersky O , Oertel WH , Zamudio J , Oberdorf J , Schmidt P , Okun MS ; National Parkinson Foundation Working Group on Hospitalization in Parkinson’s Disease ((2011) ) National Parkinson Foundation Working Group on Hospitalization in Parkinson’s Disease. Management of the hospitalized patient with Parkinson’s disease: Current state of the field and need for guidelines, Parkinsonism Relat Disord 17: , 139–145. |
[3] | Woodford H , Walker R ((2005) ) Emergency hospital admissions in idiopathic Parkinson’s disease, Mov Disord 20: , 1104–1108. |
[4] | Low V , Ben-Shlomo Y , Coward E , Fletcher S , Walker R , Clarke CE ((2015) ) Measuring the burden and mortality of hospitalisation in Parkinson’s disease: A cross-sectional analysis of the English Hospital Episodes Statistics database 2009-2013, Parkinsonism Relat Disord 21: , 449–454. |
[5] | Ahlskog JE ((2014) ) Parkinson disease treatment in hospitals and nursing facilities: Avoiding pitfalls, Mayo Clin Proc 89: , 997–1003. |
[6] | Martignoni E , Godi L , Citterio A , Zangaglia R , Riboldazzi G , Calandrella D , Pacchetti C , Nappi G ; Parkinson’s Disease Comorbidity Study Group ((2004) ) Comorbid disorders and hospitalisation in Parkinson’s disease: A prospective study, Neurol Sci 25: , 66–71. |
[7] | Dorton DM ((1995) ) The care of patients with Parkinson’s disease, J Post Anesth Nurs 10: , 102–106. |
[8] | Vergenz S ((2007) ) Caring for the Parkinson’s patient: A nurse’s perspective, Dis Mon 53: , 243–251. |
[9] | Stotz M , Thummler D , Schurch M , Renggli JC , Urwyler A , Pargger H ((2004) ) Fulminant neuroleptic malignant syndrome after perioperative withdrawal of antiParkinsonian medication, Br J Anaesth 93: , 868–871. |
[10] | Antonini A , Miro L , Castiglioni C , Pezzoli G ((2008) ) The rationale for improved integration between home care and neurology hospital services in patients with advanced Parkinson’s disease, Neurol Sci 29: (Suppl 5), S392–S396. |
[11] | Hassan A , Wu SS , Schmidt P , Dai Y , Simuni T , Giladi N , Bloem BR , Malaty IA , Okun MS ; NPF-QII Investigators ((2013) ) High rates and the risk factors for emergency room visits and hospitalization in Parkinson’s disease, Parkinsonism Relat Disord 19: , 949–954. |
[12] | Huse DM , Schulman K , Orsini L , Castelli-Haley J , Kennedy S , Lenhart G ((2005) ) Burden of illness in Parkinson’s disease, Mov Disord 20: , 1449–1454. |
[13] | Davis KL , Edin HM , Allen JK ((2010) ) Prevalence and cost of medication nonadherence in Parkinson’s disease: Evidence from administrative claims data., Mov Disord 25: , 474–480. |
[14] | Muzerengi S , Herd C , Rick C , Clarke CE ((2016) ) A systematic review of interventions to reduce hospitalisation in Parkinson’s disease, Parkinsonism Relat Disord 24: , 3–7. |
[15] | Santos García D , Jesús S , Aguilar M , Planellas LL , García Caldentey J , Caballol N , Legarda I , Hernández Vara J , Cabo I , López Manzanares L , González Aramburu I , Ávila Rivera MA , Catalán MJ , López Díaz L , Puente V , García Moreno JM , Borrué C , Solano Vila B , Álvarez Sauco M , Vela L , Escalante S , Cubo E , Carrillo Padilla F , Martínez Castrillo JC , Sánchez Alonso P , Alonso Losada MG , López Ariztegui N , Gastón I , Kulisevsky J , Menéndez González M , Seijo M , Rúiz Martínez J , Valero C , Kurtis M , de Fábregues-Boixar O , González Ardura J , Prieto Jurczynska C , Martinez-Martin P , Mir P ; COPPADIS Study Group ((2019) ) COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015): An ongoing global Parkinson’s disease project about disease progression with more than 1000 subjects included. Results from the baseline evaluation, Eur J Neurol 26: , 1399–1407. |
[16] | Santos-García D , Mir P , Cubo E , Vela L , Rodríguez-Oroz MC , Martí MJ , Arbelo JM , Infante J , Kulisevsky J , Martínez-Martín P ; COPPADIS Study Group ((2016) ) COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015), a global–clinical evaluations, serum biomarkers, genetic studies and neuroimaging–prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression., BMC Neurol 16: , 26. |
[17] | Gibson M , Andres R , Isaacs B , Radebaugh T , Worm-Peterson J ((1987) ) The prevention of falls in later life. A report of the Kellogg International Work Group on the Prevention of Falls by the Elderly, Danish Med Bull 34: (Suppl 4), 1–24. |
[18] | Gerlach OH , Winogrodzka A , Weber WE ((2011) ) Clinical problems in the hospitalized Parkinson’s disease patient: Systematic review, Mov Disord 26: , 197–208. |
[19] | Martinez-Martin P , Ray Chaudhuri K ((2018) ) Comprehensive grading of Parkinson’s disease using motor and non-motor assessments: Addressing a key unmet need, Expert Rev Neurother 18: , 41–50. |
[20] | Pagonabarraga J , Kulisevsky J , Llebaria G , García-Sánchez C , Pascual-Sedano B , Gironell A ((2008) ) Parkinson’s disease-cognitive rating scale: A new cognitive scale specific for Parkinson’s disease, Mov Disord 23: , 998–1005. |
[21] | Santos-García D , de Deus-Fonticoba T , Suárez Castro E , M Aneiros Díaz Á , Feal-Painceiras MJ , Paz-González JM , García-Sancho C , Jesús S , Mir P , Planellas L , García-Caldentey J , Caballol N , Legarda I , Hernández-Vara J , González-Aramburu I , Ávila-Rivera MA , Catalán MJ , Nogueira V , Álvarez-Sauco M , Vela L , Escalante S , Cubo E , Sánchez-Alonso P , Alonso-Losada MG , López-Ariztegui N , Martinez-Martin P ; COPPADIS Study Group ((2020) ) The impact of freezing of gait on functional dependency in Parkinson’s disease with regard to motor phenotype, Neurol Sci 41: , 2883–2292. |
[22] | Schade S , Mollenhauer B , Trenkwalder C ((2020) ) Levodopa equivalent dose conversion factors: An updated proposal including opicapone and safinamide, Mov Disord Clin Pract 7: , 343–345. |
[23] | Santos García D , de Deus Fonticoba T , Suárez Castro E , Borrué C , Mata M , Solano Vila B , Cots Foraster A , Álvarez Sauco M , Rodríguez Pérez AB , Vela L , Macías Y , Escalante S , Esteve P , Reverté Villarroya S , Cubo E , Casas E , Arnaiz S , Carrillo Padilla F , Pueyo Morlans M , Mir P , Martinez-Martin P ; Coppadis Study Group ((2019) ) Non-motor symptoms burden, mood, and gait problems are the most significant factors contributing to a poor quality of life in non-demented Parkinson’s disease patients: Results from the COPPADIS Study Cohort, Parkinsonism Relat Disord 66: , 151–157. |
[24] | Vargas AP , Carod-Artal FJ , Nunes SV , Melo M ((2008) ) Disability and use of healthcare resources in Brazilian patients with Parkinson’s disease, Disabil Rehabil 30: , 1055–1062. |
[25] | Cosentino M , Martignoni E , Michielotto D , Calandrella D , Riboldazzi G , Pacchetti C , Frigo G , Nappi G , Lecchini S ((2005) ) Medical healthcare use in Parkinson’s disease: Survey in a cohort of ambulatory patients in Italy, BMC Health Serv Res 5: , 26. |
[26] | Vossius C , Nilsen OB , Larsen JP ((2010) ) Parkinson’s disease and hospital admissions: Frequencies, diagnoses and costs, Acta Neurol Scand 121: , 38–43. |
[27] | Koay L , Rose J , Abdelhafiz AH ((2018) ) Factors that lead to hospitalisation in patients with Parkinson disease-A systematic review, Int J Clin Pract 72: , doi: 10.1111/ijcp.13039 |
[28] | Guneysel O , Onultan O , Onur O ((2008) ) Parkinson’s disease and the frequent reasons for emergency admission, Neuropsychiatr Dis Treat 4: , 711–714. |
[29] | Kessler II ((1972) ) Epidemiologic studies of Parkinson’s disease. II. A hospital-based survey, Am J Epidemiol 95: , 308–318. |
[30] | Tan LC , Tan AK , Tjia HT ((1998) ) The profile of hospitalised patients with Parkinson’s disease, Ann Acad Med Singapore 27: , 808–812. |
[31] | Temlett JA , Thompson PD ((2006) ) Reasons for admission to hospital for Parkinson’s disease, Intern Med J 36: , 524–526. |
[32] | Louis ED , Henchcliffe C , Bateman BT , Schumacher C ((2007) ) Young onset Parkinson’s disease: Hospital utilization and medical comorbidity in a nationwide survey, Neuroepidemiology 29: , 39–43. |
[33] | Klein C , Prokhorov T , Miniovitz A , Dobronevsky E , Rabey JM ((2009) ) Admission of Parkinsonian patients to a neurological ward in a community hospital, J Neural Transm 116: , 1509–1512. |
[34] | Lubomski M , Rushworth RL , Tisch S ((2015) ) Hospitalisation and comorbidities in Parkinson’s disease: A large Australian retrospective study, J Neurol Neurosurg Psychiatry 86: , 324–330. |
[35] | Shahgholi L , De Jesus S , Wu SS , Pei Q , Hassan A , Armstrong MJ , Martinez-Ramirez D , Schmidt P , Okun MS ((2017) ) Hospitalization and rehospitalization in Parkinson disease patients: Data from the National Parkinson Foundation Centers of Excellence, PLoS One 12: , e0180425. |
[36] | Paul BS , Paul G , Singh G , Kaushal S , Verma U ((2017) ) Pattern of hospital admission and outcome in Parkinson’s disease: A study from Punjab, India, Neurol Asia 22: , 33–39. |
[37] | Braga M , Pederzoli M , Antonini A , Beretta F , Crespi V ((2014) ) Reasons for hospitalization in Parkinson’s disease: A case-control study, Parkinsonism Relat Disord 20: , 488-492; discussion 488. |
[38] | Santos-García D , Castro ES , de Deus Fonticoba T , Panceiras MJF , Enriquez JGM , González JMP , Bartolomé CC , Planellas LL , Caldentey JG , Caballol N , Legarda I , López IC , Manzanares LL , Rivera MAÁ , Catalán MJ , Nogueira V , Borrué C , Sauco MÁ , Vela L , Cubo E , Castrillo JCM , Alonso PS , Losada MGA , Ariztegui NL , Gastón MI , Kulisevsky J , Pagonabarraga J , Seijo M , Martínez JR , Valero C , Kurtis M , Ardura JG , Prieto C , Mir P , Martinez-Martin P ((2020) ) Sleep problems are related to a worse quality of life and a greater non-motor symptoms burden in Parkinson’s disease. J Geriatr Psychiatry Neurol, doi: 10.1177/0891988720964250 |
[39] | Tomlinson CL , Patel S , Meek C , Clarke CE , Stowe R , Shah L , Sackley CM , Deane KH , Herd CP , Wheatley K , Ives N ((2012) ) Physiotherapy versus placebo or no intervention in Parkinson’s disease, Cochrane Database Syst Rev 7: , CD002817. |
[40] | Robbins J , Gensler G , Hind J , Logemann JA , Lindblad AS , Brandt D , Baum H , Lilienfeld D , Kosek S , Lundy D , Dikeman K , Kazandjian M , Gramigna GD , McGarvey-Toler S , Miller Gardner PJ ((2008) ) Comparison of 2 interventions for liquid aspiration on pneumonia incidence: A randomized trial, Ann Intern Med 148: , 509–518. |
[41] | Kulkarni AS , Balkrishnan R , Anderson RT , Edin HM , Kirsch J , Stacy MA ((2008) ) Medication adherence and associated outcomes in medicare health maintenance organization-enrolled older adults with Parkinson’s disease, Mov Disord 23: , 359–365. |
[42] | Bloem BR , Henderson EJ , Dorsey ER , Okun MS , Okubadejo N , Chan P , Andrejack J , Darweesh SKL , Munneke M ((2020) ) Integrated and patient-centred management of Parkinson’s disease: A network model for reshaping chronic neurological care, Lancet Neurol 19: , 623–634. |
[43] | Allen NE , Schwarzel AK , Canning CG ((2013) ) Recurrent falls in Parkinson’s disease: A systematic review, Parkinsons Dis 2013: , 906274. |



