Vestibular symptoms are related to the proportion of REM sleep in people with sleep complaints: A preliminary report
Abstract
OBJECTIVE/BACKGROUND:
Though sleep problems (apnea, insomnia) and related daytime symptoms (fatigue, anxiety, depression) have been associated with vestibular problems (falls, dizziness), it is not well known which particular sleep features relate to vestibular problems. We thus assessed symptoms of vestibular problems in patients visiting a sleep clinic and evaluated how they were associated with objective sleep parameters derived from polysomnography and relevant daytime symptoms.
PATIENTS/METHODS:
The polysomnography data of thirty-one patients (61% female, between 20 and 79 years of age) who were referred for clinical sleep assessment was collated with subjective measures of symptoms linked to vestibular problems (rated on the Situational Characteristics Questionnaire), as well as fatigue, anxiety and depression symptoms. Multiple linear regression was used to identify factors associated with vestibular symptoms, including analyses adjusted for age, sex, medication use and total sleep time.
RESULTS:
A higher percentage of REM sleep and more severe anxiety symptoms were independently associated with more severe vestibular symptoms, which survived adjusted analyses. Other sleep stages, as well as as sleep efficiency, apnea-hypopnea index and oxygen saturation were not significantly related to vestibular symptoms.
CONCLUSIONS:
These results point at vestibular symptoms as possible important and overlooked correlates of variations in sleep architecture in individuals with sleep complaints. Though replication is needed to confirm findings from this limited sample, the results highlight the importance of assessing vestibular symptoms in people with sleep complaints. In particular, further investigations will need to address the potential implication of REM sleep for vestibular functions and the directionality of this relation.
1Introduction
Sleep disturbances can affect cognitive and affective functioning, but much less is known about their relation to vestibular functioning. Experimental sleep deprivation can cause vestibular problems, such as postural instability and disruption of visuo-spatial perception [4, 8, 34]. Furthermore, people with obstructive sleep apnea syndrome (OSAS) have an elevated risk of falling and showing postural instability [15, 36] which can decrease after OSAS treatment [8, 36]. Also, insomnia has been related to vestibular symptoms such as nausea and oculomotor symptoms, with increased sensitivity to motion sickness in a driving simulator [2]. However, specific sleep features which may relate to vestibular problems are not well known.
Vestibular disorders can be linked to central (e.g. stroke, demyelinating disease, brain injury etc.) or peripheral involvement (Ménière’s disease, benign paroxysmal positional vertigo, etc.). Yet, in about 40% of cases, no organic cause is found, leading to a diagnosis of psychogenic dizziness [25]. People with vestibular disorders were found to have abnormal sleep durations (e.g. very short or very long sleep duration), and worse sleep problems compared to people without vestibular disorders [7, 8]. In addition, those with psychogenic dizziness reported worse quality of sleep and higher insomnia severity than those with other vestibular diagnoses [1, 21]. The term ‘psychogenic dizziness’ has more recently changed into ‘persistent postural-perceptual dizziness (PPPD)’ and this new terms has been accepted as part of the Classification of Vestibular Disorders [35]. Sleep may thus be one of the factors linked to certain types of seemingly idiopathic vestibularproblems.
Although previous studies suggested a link between sleep problems and vestibular dysfunctions, the specific sleep features which may be involved remain poorly understood. In support of the potential involvement of deep sleep in vestibular function, lower percentage of N3 sleep has been linked to worse postural stability in people with OSAS [41]. In addition, fatigue and mental health symptoms frequently co-occuring with sleep disturbances could possibly also contribute to vestibular problems. For instance, anxiety, depression and fatigue are highly comorbid with both sleep disorders and vestibular disorders [30, 35, 40]. This is of high relevance since anxiety disorders and major depression, the two most prevalent types of mental disorders worldwide [42], are known to share a bidirectional relationship with sleep disturbances [16, 33]. Notably, alterations in both N3 and REM sleep are common in people with anxiety disorders or major depression [3, 18], and REM abnormalities have been related to more prominent depression comorbid with sleep-related breathing disorders [17, 27]. There is thus a need to investigate how vestibular functions relate to sleep disturbances, fatigue, anxiety and depression.
The present study investigated the relation between vestibular symptoms, sleep features, daytime fatigue, and anxiety and depression symptoms in people undergoing clinical sleep assessment. It was expected that: (1) lower proportion of N3 and REM sleep, fragmented sleep, and more frequent respiratory events would be associated with worse vestibular symptoms, and (2) sleep would have a stronger weight in explaining vestibular symptoms than daytime fatigue, anxiety or depression symptoms.
2Methods
2.1Participants
Thirty-one participants were sequentially recruited from the Sleep Disorders Clinic of the Royal Ottawa Mental Health Center (ROMHC) prior to their first consultation. The only entry criteria was to be a new patient referred for clinical sleep assessment. The ROMHC Research Ethics Board approved this study. All experimental procedures were conducted according to the Declaration of Helsinki and all participants provided informed consent.
2.2Questionnaires
For descriptive purposes, participants were asked to report all current mental disorders and medication intake on the day of polysomnography, and the Pittsburgh Sleep Quality Index (PSQI [9]) was used to characterize subjective sleep quality. Vestibular symptoms were evaluated by the Situational Characteristics Questionnaire (SCQ; [20]), which has been used as an index of vestibular symptoms in several studies [20, 31]. Through the 41 items of the SCQ, levels of discomfort related to vestibular challenges (e.g. being in a starting elevator) were rated on a Likert scale ranging from 0 to 3, yielding total scores ranging between 0 and 123. Anxiety and depression symptoms were assessed with the Beck Anxiety Inventory (BAI; [5]) and the Beck Depression Inventory-II (BDI; [6]) respectively. Daytime fatigue was rated on the Fatigue Severity Scale (FSS; [22]). There were missing data for self-reported current mental disorders (n = 1), BAI/BDI (n = 1), FSS (n = 2), and PSQI (n = 1).
2.3Objective sleep assessment
All paticipants underwent level 1 polysomnography at the Sleep Disorders Clinic. A Registered Polysomnographic Technologist placed electrodes according to the 10–20 system with three electroencephalogram channels (F3, C3 and O1), ground and reference channels, right and left electrooculograms, two chin and leg electromyograms (EMG), and two electrocardiogram channels. Respiration was monitored with an airflow cannula (pressure transducer), oronasal thermistor, and chest and abdomen plethysmography. Pulse Oximetry was used to assess the minimum oxygen saturation percentage reached during sleep (minimum SaO2%). Sleep stages, respiratory events, arousals, and periodic limb movements were visually scored by a Registered Polysomnographic Technologist according to guidelines established by the American Academy of Sleep Medicine [7].
2.4Statistical analysis
Multiple linear regression were performed using the enter method with SCQ total scores as the dependent variable and the following independent variables: sleep parameters, BAI, BDI, and FSS total scores. Due to multicollinearity issues and to minimize the number of parameters, two distinct regression models were run with different sets of sleep parameters: one with the percentages of sleep stages (using % N2 as a reference), and one with sleep efficiency, the apnea/hypopnea index (AHI) and minimum SaO2%. All analyses were repeated while adjusting for age, sex, medication use, and total sleep time to assess the effects of potential confounders. Multicollinearity was verified with the variation inflation factors (VIF).
3Results
3.1Sample characteristics
Table 1 presents global demographic characteristics. In brief, this sample included 61% females and ages ranged from 20 to 79 years. All participants were referred for suspected sleep disordered breathing, except for one individual who had suspected restless legs syndrome. Overall, the average total sleep time during polysomnography was 5.8 + 1.1 hours and mean scores on the PSQI (8.0±3.6) crossed the standard threshold for poor subjective sleep quality. Of all participants, 77% (n = 24) had an AHI > 5 and 35% (n = 10) reported having a current diagnosis of a mental disorder. Twenty participants (65%) used a psychotropic, cardiovascular or diuretic medication on the day of polysomnography. Vestibular symptoms rated on the SCQ ranged from 2 to 116, with a mean of 25.8 + 27.5.
Table 1
Sample characteristics and statistics from unadusted multiple regression examining the relative contribution of sleep, fatgigue (FSS: Fatigue Severity Scale), anxiety (BAI: Beck Anxiety Inventory) and depression (BDI: Beck Depression Inventory) symptoms for vestibular symptoms as assessed by the SCQ (Situational Characteristics Questionnaire). A) Multiple regression model focused on sleep stages, B) Multiple regression model focused on respiratory and fragmentation indices. Sleep effects remained significant after adusting for total sleep time, age, sex and medication use. % N1/2/3: Percentage of Non-Rapid Eye Movement sleep stages (N2 was used as a reference in multiple regression); ‡Self-reported current diagnoses of mental disorders (some participants reported multiple diagnosis). SD: Standard Deviation. REM: Rapid Eye Movement sleep; B: unstandardized coefficient (calculated per unit); 95% CI: confidence interval of B; β: standardized coefficient; VIF: variation inflation factors
| Multiple Regression | |||||||||
| Mean±SD or % | A) Sleep Stages | B) Respiratory and Fragmentation Indices | |||||||
| (frequency) | B [95% CI] | Beta | p | VIF | B [95% CI] | Beta | p | VIF | |
| General Demographics | |||||||||
| Sex (F/M) | 19/12 | ||||||||
| Age (Years) | 48.5±15.4 | ||||||||
| Medicated | 64.5% (20) | ||||||||
| Any Current Mental Disorders‡ | 34.5% (10) | ||||||||
| Anxiety disorders | 13.8% (4) | ||||||||
| Mood disorders | 27.6% (8) | ||||||||
| Other mental disorders | 13.8% (4) | ||||||||
| Daytime Symptoms | |||||||||
| Fatigue (FSS) | 37.8±13.8 | 0.59 [–0.01; 1.20] | 0.30 | 0.054 | 1.8 | 0.81 [0.06; 1.57] | 0.41 | 0.036 | 2.0 |
| Anxiety (BAI) | 10.9±9.7 | 2.34 [1.41; 3.27] | 0.83 | < 0.001 | 2.1 | 2.47 [1.32; 3.61] | 0.87 | < 0.001 | 2.3 |
| Depression (BDI) | 10.1±8.8 | –0.87 [-2.04; 0.29] | –0.28 | 0.135 | 2.7 | –1.48 [–2.92; –0.04] | –0.48 | 0.044 | 3.0 |
| Polysomnography | |||||||||
| % N1 | 13.6±6.7 | 0.31 [–0.69; 1.31] | 0.08 | 0.528 | 1.2 | – | – | – | – |
| % N2 | 59.6±9.8 | – | – | – | – | – | – | – | – |
| % N3 | 8.8±6.9 | 0.23 [–0.72; 1.17] | 0.06 | 0.622 | 1.1 | – | – | – | – |
| % REM | 18.0±5.8 | 1.78 [0.63; 2.94] | 0.38 | 0.004 | 1.2 | – | – | – | – |
| Sleep Efficiency (%) | 82.4±11.8 | – | – | – | – | –0.10 [–0.85; 0.66] | –0.04 | 0.796 | 1.4 |
| AHI (event/hour) | 13.4±15.5 | – | – | – | – | –0.33 [–1.05; 0.38] | –0.19 | 0.341 | 2.2 |
| Mininum spO2% | 89.0±3.7 | – | – | – | – | –1.78 [–4.66; 1.10] | –0.24 | 0.212 | 2.1 |
3.2Associations between vestibular symptoms, sleep, fatigue, and anxiety and depression symptoms
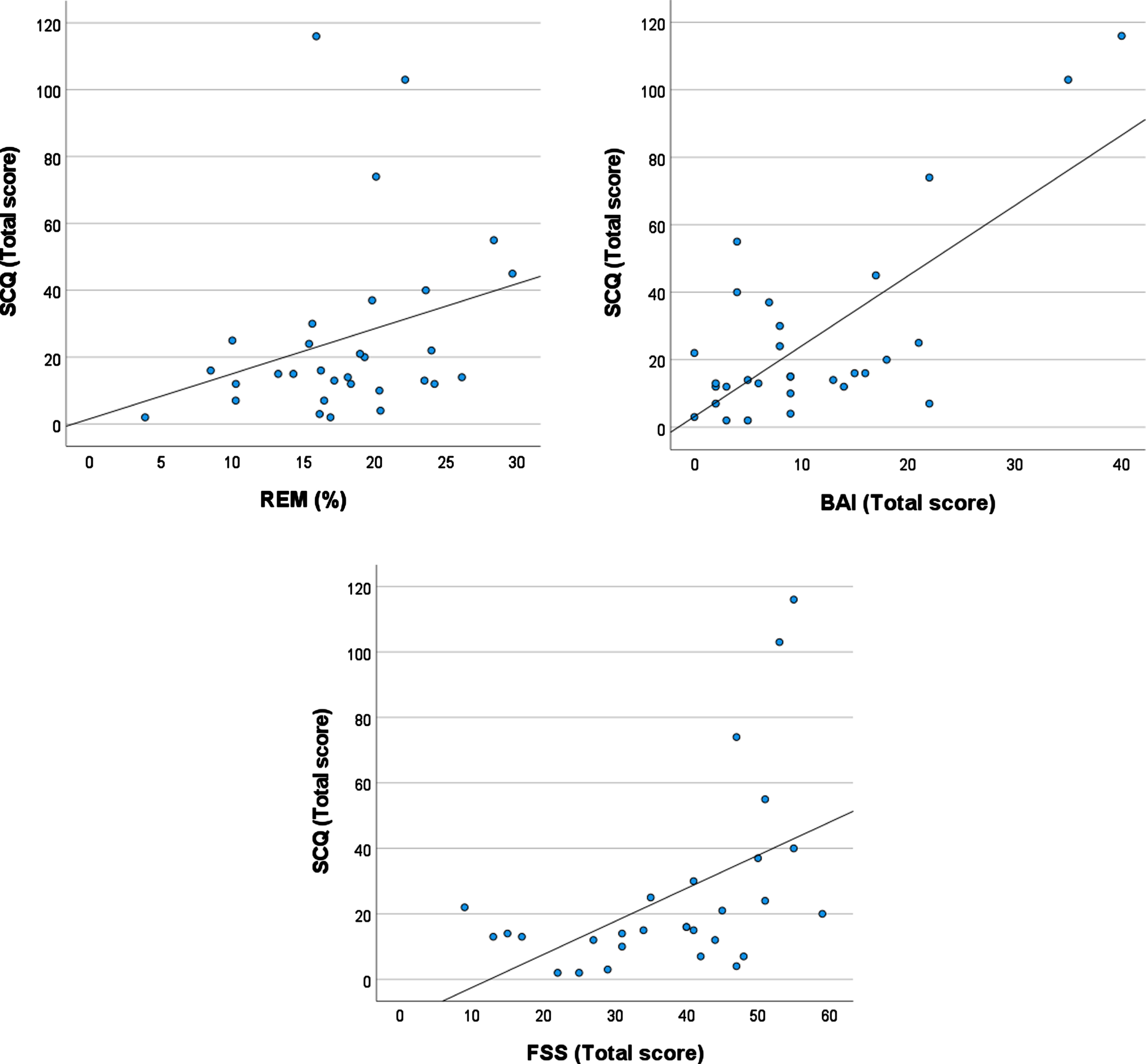
The regression model that focused on sleep stages significantly explained variability in vestibular symptoms on the SCQ (F(6, 27) = 10.5, p < .001, R2 = .75 (Table 1). A higher percentage of REM sleep (β= 0.38; p = .004) and more severe anxiety symptoms (BAI: β= 0.83; p < .001) were independently associated with more severe vestibular symptoms. Higher daytime fatigue (FSS: β= 0.30; p = .054) tended to be associated with more severe vestibular symptoms. These effects, except for the trend for fatigue, persisted after adjusting for age, sex, medication use, and total sleep time (β> 0.41; p < 0.004). Figure 1 shows vestibular symptoms severity on the SCQ plotted against the percentage of REM sleep, anxiety symptoms and daytime fatigue levels.
The regression model focussed on respiratory and fragmentation indices revealed that vestibular symptoms on the SCQ were not significantly related to sleep efficiency (β= –0.04; p = .796), AHI (β= –0.19; p = .341), or the minimum SaO2% (β= –0.24; p = .212).
Fig. 1
Plots showing the association between symptoms for vestibular symptoms as assessed by the SCQ (Situational Characteristics Questionnaire) and: A) the percentage of REM sleep (the association between the SCQ and REM% does persist if the three datum with the highest SCQ scores are excluded), B) Anxiety symptoms as assessed by the Beck Anxiety Inventory (BAI), C) Daytime fatigue as assessed by the Fatigue Severity Scale (FSS).
4Discussion
The current preliminary findings indicate that, independently of sleep duration, a higher proportion of REM sleep is associated with more severe vestibular symptoms in people with sleep complaints. In line with earlier studies, daytime fatigue and anxiety symptoms were also found to contribute to vestibular symptoms, athough to a lower level. The current results thus emphasize that, beyond sleep quantity, sleep architecture may be linked to vestibular symptoms.
The finding that longer REM sleep duration is linked to vestibular symptoms was unexpected. Previous literature in people with OSAS suggested that vestibular symptoms are linked to sleep fragmentation and less stage N3 sleep [41]. However, it has previously been observed that astronauts have increased REM sleep percentage and more rapid eye movements during REM sleep in their first nights in space with zero gravity [32]. Those astronauts showing the highest increase of REM sleep percentage in space also suffered from more nausea and vomiting during the wake state, suggesting worse vestibular functions. While Hobson et al. have suggested that REM sleep may in fact play a role in vestibular adaptation in space [18], the current findings suggest that this REM change may also relate to vestibular functions under normal gravity.
Healthy sleepers typically spend 20 to 25% of their sleep episode in REM [10]. Several of our participants fell at the upper edge or above this range. The current study focused on people with untreated sleep problems, which may induce partial sleep deprivation. These sleep disruptions could possibly have triggered REM rebounds and concomitant alterations in vestibular functions [8]. Previous studies suggested that people with sleep disordered breathing may have decreased REM sleep percentages [12, 13]), but that individuals with depression show the opposite pattern, with increased REM percentage [26]. The current sample mostly contained people with disordered breathing, but many participants also had a mood disorder, which may have pulled the amounts of REM sleep in different directions. Future work should investigate whether depressed states and/or medications such as selective serotonin reuptake inhibitors, which are known to suppress REM sleep [38], may modulate the relationship between increased REM sleep and vestibular symptoms. Of note, experimental studies demonstrated that the amounts of REM sleep can be enhanced by challenges faced during wake such as adapting to living with an inverted visual field (e.g. [14]). If REM sleep does play a role in vestibular adaptation, it may be postulated that REM sleep could increase in response to vestibular challenges.
Although the current study does not enable to assess causality, there are some indications that experimental sleep manipulations can induce vestibular dysfunctions [24]. Similarly, sleep loss is also known to be associated with higher anxiety levels. As such, addressing sleep problems may be a valuable therapeutic target to support the management of vestibular problems, both through direct impacts on the vestibular system and indirect impacts via the attenuation of anxiety levels. Furthermore, vestibular nuclei project to the suprachiasmatic nucleus [11, 19], a brain structure notably involved in the regulation of REM sleep [23, 39]. From this perspective, it may also be possible that dysfunctions of the vestibular system could impact REM sleep dynamics.
Our preliminary report has some limitations. This is an exploratory correlational study based on a single timepoint, which precludes causal inferences. Since vestibular functions were assessed with a questionnaire, these findings should be replicated with objective vestibular tests. The SCQ measures the frequency of symptoms provoked by environmental challenges known to mobilize vestibular functions and, as such, is an indirect proxy of vestibular functions. Although elevated SCQ scores may be indicative of vestibular problems, they are not specific to particular vestibular disorders, and there is currently no validated clinical cut-off. Nevertheless, scores above 0 are considered to suggest the presence of vestibular problems [20, 28, 29, 31, 37]. Subsequent work is needed to link objective measurements of vestibular functions to sleep in people with sleep disorders. Although we controlled for this factor statistically, many participants were taking various medications which may influence both sleep and vestibular functions. Further, since there was no control group, conclusions are restricted to individuals with sleep complaints referred to a sleep clinic. This was a small heterogenous sample (e.g. large age range, unequal sex distribution, and diverse clinical sleep profiles), but this heterogeneity could possibly more closely resemble clinical populations with sleep complaints.
In conclusion, these preliminary observations suggest that vestibular symptoms may be related to elevated REM sleep, higher levels of anxiety and, to a lower extent, increased fatigue. Further work is required to confirm these findings with objective vestibular measures in larger samples that would enable to tease appart the potential influence of age, sex, mental health status, medication, and different subtypes of sleep problems. If replicated, these findings would highlight the importance of assessing vestibular symptoms in people with sleep complaints and stress the need to for interventional studies assessing whether sleep treatments could attenuate vestibular problems. There is also a need to further investigate the neural networks involved in both vestibular and sleep functions, as well as the potential role of REM sleep to process and integrate vestibular information.
Acknowledgments
The authors wish to thank the participants for their time on this study.
Funding
This work was supported by the University of Ottawa Medical Research Fund.
References
[1] | Albathi M. , Agrawal Y. Vestibular vertigo is associated with abnormal sleep duration, VES 27: ((2017) ), 127–135. |
[2] | Altena E. , Daviaux Y. , Sanz-Arigita E. , Bonhomme E. , Sevin É. Micoulaud-Franchi J. , Bioulac S. , Philip P. How sleep problemscontribute to simulator sickness: Preliminary results from arealistic driving scenario, J Sleep Res 28: ((2019) ). |
[3] | Baglioni C. , Nanovska S. , Regen W. , Spiegelhalder K. , Feige B. Nissen C. Reynolds C.F. Riemann D. Sleep and mental disorders: Ameta-analysis of polysomnographic research, PsychologicalBulletin 142: ((2016) ), 969–990. |
[4] | Batuk I.T. , Batuk M.O. , Aksoy S. Evaluation of the posturalbalance and visual perception in young adults with acute sleepdeprivation, VES 30: ((2020) ), 383–391. |
[5] | Beck A.T. , Brown G. , Epstein N. , Steer R.A. An Inventory for Measuring Clinical Anxiety: Psychometric Properties, 56: , ((1988) ), 893–897. |
[6] | Beck A.T. , Epstein N. , Brown G. , Steer R.A. Manual for Beck Depression Inventory II (BDI-II)., Psychology Corporation, San Antonio, Texas, 1996. |
[7] | Berry R.B. , Budhiraja R. , Gottlieb D.J. , Gozal D. , Iber C. , Kapur V.K. , Marcus C.L. , Mehra R. , Parthasarathy S. , Quan S.F. , Redline S. , Strohl K.P. , Ward S.L.D. , Tangredi M.M. Rules for Scoring Respiratory Events in Sleep: Update of the AASM Manual for the Scoring of Sleep and Associated Events: Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine, Journal of Clinical Sleep Medicine 08: , ((2012) ) 597–619. |
[8] | Besnard S. , Tighilet B. , Chabbert C. , Hitier M. , Toulouse J. , Le Gall A. Machado M.-L. Smith P.F. The balance of sleep: Role of the vestibular sensory system, Sleep Medicine Reviews 42: ((2018) ), 220–228. |
[9] | Buysse D.J. , Reynolds C.F. , Monk T.H. , Berman S.R. , Kupfer D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research, Psychiatry Research 28: , ((1989) ), 193–213. |
[10] | Carskadon M.A. , Dement W.C. Chapter 2 –Normal Human Sleep: An Overview, (n.d.). |
[11] | Çavdar S. Onat F. Aker R. ŞehiRli Ü Şan T. Raci Yananli H. The afferent connections of the posterior hypothalamic nucleus in the rat using horseradish peroxidase, Journal of Anatomy 198: ((2001) ), 463–472. |
[12] | Chervin R.D. , Aldrich M.S. The Relation Between Multiple Sleep Latency Test Findings and the Frequency of Apneic Events in REM and Non-REM Sleep, Chest 113: ,((1998) ) 980–984. |
[13] | Colt H.G. , Haas H. , Rich G.B. Hypoxemia vs Sleep Fragmentation as Cause of Excessive Daytime Sleepiness in Obstructive Sleep Apnea, Chest 100: ((1991) ), 1542–1548. |
[14] | De Koninck J. , Prévost F. ,Paradoxical sleep and informationprocessing: exploration by inversion of the visual field, 45: ((1991) ), 125–139. |
[15] | Degache F. , Goy Y. , Vat S. , Rubio J.H. , Contal O. , Heinzer R. Sleep-disordered breathing and daytime postural stability, Thorax 71: ((2016) ), 543–548. |
[16] | Fang H. , Tu S. , Sheng J. , Shao A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment, J Cellular Molecular Medi 23: ((2019) ), 2324–2332. |
[17] | Geckil A.A. , Ermis H. The relationship between anxiety, depression, daytime sleepiness in the REM-related mild OSAS and the NREM-related mild OSAS, Sleep Breath 24: ((2020) ), 71–75. |
[18] | Hobson J.A. , Stickgold R. , Pace-Schott E.F. , Leslie K.R. Sleep and Vestibular Adaptation: Implications for Function in Microgravity, VES 8: ((1998) ), 81–94. |
[19] | Horowitz S.S. , Blanchard J. , Morin L.P. Medial vestibular connections with the hypocretin (orexin) system, J Comp Neurol 487: ((2005) ), 127–146. |
[20] | Jacob R.G. , Woody S.R. , Clark D.B. , Lilienfeld S.O. , Hirsch B.E. , Kucera G.D. , Furman J.M. , Durrant J.D. Discomfort with space and motion: A possible marker of vestibular dysfunction assessed by the situational characteristics questionnaire, J Psychopathol Behav Assess 15: ((1993) ), 299–324. |
[21] | Kim S.K. , Kim J.H. , Jeon S.S. , Hong S.M. Relationship between sleep quality and dizziness,e, PLoS ONE 13: ((2018) ), 0192705. |
[22] | Krupp L.B. The Fatigue Severity Scale: Application to Patients With Multiple Sclerosis and Systemic Lupus Erythematosus, Arch Neurol 46: ((1989) ), 1121. |
[23] | Lee M.L. , Swanson B.E. , de la Iglesia H.O. Circadian Timing of REM Sleep Is Coupled to an Oscillator within the Dorsomedial Suprachiasmatic Nucleus, Current Biology 19: ((2009) ), 848–852. |
[24] | Lin B.-Y. , Young Y.-H. Effect of short-duration sleep deprivation on the vestibulo-ocular reflex system evaluated by ocular vestibular-evoked myogenic potential test, Acta Oto-Laryngologica 134: ,((2014) ) 698–703. |
[25] | Neuhauser H.K. , Radtke A. , von Brevern M. Lezius F. , Feldmannand M. , Lempert T. Burden of Dizziness and Vertigo in the Community, Arch Intern Med 168: ((2008) ), 2118. |
[26] | Palagini L. , Baglioni C. , Ciapparelli A. , Gemignani A. and D.Riemann, REM sleep dysregulation in depression: State of the art, Sleep Medicine Reviews 17: ((2013) ), 377–390. |
[27] | Pamidi S. , Knutson K.L. , Ghods F. , Mokhlesi B. Depressive symptoms and obesity as predictors of sleepiness and quality of life in patients with REM-related obstructive sleep apnea: Cross-sectional analysis of a large clinical population, Sleep Medicine 12: ((2011) ), 827–831. |
[28] | Pavlou M. , Bronstein A.M. , Davies R.A. Randomized Trial of Supervised Versus Unsupervised Optokinetic Exercise in Persons With Peripheral Vestibular Disorders, Neurorehabil Neural Repair 27: ((2013) ), 208–218. |
[29] | Pavlou M. , Davies R.A. , Bronstein A.M. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness, VES 16: ((2007) ), 223–231. |
[30] | Post R.E. Dizziness: A Diagnostic Approach, 82: ((2010) ), 8. |
[31] | Powell G. , Derry-Sumner H. , Rajenderkumar D. , Rushton S.K. , Sumner P. Persistent postural perceptual dizziness is on a spectrum in the general population, Neurology ((1929) ) 94: , e1929–e1938.. |
[32] | Quadens O. , Green H. Eye movements during sleep in weightlessness, Science 225: , ((1984) ), 221–222. |
[33] | Richards A. , Kanady J.C. , Neylan T.C. Sleep disturbance in PTSD and other anxiety-related disorders: an updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms, Neuropsychopharmacol 45: ((2020) ), 55–73. |
[34] | Robillard R. , Prince F. , Boissonneault M. , Filipini D. , Carrier J. Effects of increased homeostatic sleep pressure on postural control and their modulation by attentional resources, Clinical Neurophysiology 122: ((2011) ), 1771–1778. |
[35] | Staab J.P. , Eckhardt-Henn A. , Horii A. , Jacob R. , Strupp M. T.Brandt and A. Bronstein, Diagnostic criteria for persistentpostural-perceptual dizziness (PPPD): Consensus document of thecommittee for the Classification of Vestibular Disorders of theBárány Society, VES 27: ((2017) ), 191–208. |
[36] | Stevens D. , Jackson B. , Carberry J. , McLoughlin J. , Barr C. , Mukherjee S. , Oh A. , McEvoy R.D. , Crotty M. , Vakulin A. The Impact of Obstructive Sleep Apnea on Balance, Gait, and Falls Risk: A Narrative Review of the Literature, The Journals of Gerontology: Series A 75: ((2020) ), 2450–2460. |
[37] | Vaillancourt L. , Bélanger C. , Léger-Bélanger M.-P. , Jacob R.G. Validation de la version française du questionnairedes caractéristiques situationnelles dans la mesure del’inconfort spatio-moteur, L’Enc&phale 38: ((2012) ), 248–256. |
[38] | Wichniak A. , Wierzbicka A. , Walęcka M. Jernajczyk W. Effects of Antidepressants on Sleep, Curr Psychiatry Rep 19: ((2017) ), 63. |
[39] | Wurts S.W. , Edgar D.M. Circadian and Homeostatic Control of Rapid Eye Movement (REM) Sleep: Promotion of REM Tendency by the Suprachiasmatic Nucleus, J Neurosci 20: ((2000) ), 4300–4310. |
[40] | Yardley L. OVERVIEW OF PSYCHOLOGIC EFFECTS OF CHRONIC DIZZINESS AND BALANCE DISORDERS, Otolaryngologic Clinics of North America 33: ((2000) ), 603–616. |
[41] | Yilmaz Gokmen G. Gurses H.N. , Zeren M. , Ozyilmaz S. Kansu A. , Akkoyunlu M.E. , Postural stability and fall risk in patients with obstructive sleep apnea: a cross-sectional study, Sleep Breath 25: ((2021) ), 1961–1967. |
[42] | Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx), https://vizhub.healthdata.org/gbd-results/, accessed 14 May 2022)., (n.d.). |



