Strategy to Identify Areas of Use of Amazon River dolphins
- 1Laboratorio de Ecología Funcional (LEF), Unidad de Ecología y Sistemática (UNESIS), Pontificia Universidad Javeriana, Bogotá, Colombia
- 2Fundación Omacha, Bogotá, Colombia
- 3Departamento de Biología, Grupo de Ecología del Paisaje y Modelación de Ecosistemas (ECOLMOD), Facultad de Ciencias, Universidad Nacional de Colombia, Bogotá, Colombia
- 4Programa de Biología, Centro de Estudios de Alta Montaña (CEAM), Facultad de Ciencias Básicas y Tecnologías, Universidad del Quindío, Armenia, Colombia
- 5Programa de Biología, Grupo de Investigación en Desarrollo y Estudio del Recurso Hídrico y el Ambiente (CIDERA), Facultad de Ciencias Básicas y Tecnologías, Universidad del Quindío, Armenia, Colombia
- 6Laboratório de Ecologia Comportamental e Bioacustica, Programa de PósGraduacão em Ecologia, Universidade Federal de Juiz de Fora, Juiz de Fora, Brazil
- 7Instituto Aqualie, Juiz de Fora, Minas Gerais, Brazil
- 8Grupo de Pesquisa em Mamíferos Aquáticos Amazônicos, Instituto de Desenvolvimento Sustentável Mamirauá, Tefé, Brazil
- 9WWF-Colombia, Cali, Colombia
- 10ProDelphinus, Lima, Peru
- 11School of BioSciences, Penryn, University of Exeter, Exeter, United Kingdom
- 12Carrera de Biología Marina, Universidad Científica del Sur, Lima, Peru
- 13Museo de Historia Natural “Vera Alleman Haeghebaert”, Universidad Ricardo Palma, Lima, Peru
- 14Asociación Solinia, Iquitos, Peru
- 15Laboratorio de Biología de Organismos, Centro de Ecología, Instituto Venezolano de Investigaciones Científicas, San Antonio de los Altos, Venezuela
- 16Fundación Neotropical Cuencas, Arauca, Colombia
- 17Faunagua, Sacaba, Cochabamba, Bolivia
Unsustainable fisheries practices carried out in large parts of the Amazon, Tocantins, and Orinoco basins have contributed to the decline in the populations of the Amazon River dolphins (Inia spp.), considered Endangered by the International Union for Conservation of Nature (IUCN). Amazon River dolphin byproducts are often obtained through unregulated fisheries and from stranded and incidentally caught individuals that are traded for the flesh and blubber used for Calophysus macropterus fisheries, traditional and other medicinal purposes, and more recently for human consumption. To identify localities of use of Amazon River dolphins, we conducted a systematic review of the related literature published since 1980, complemented with structured surveys of researchers that allowed the identification of 57 localities for uses of Inia (33 in the Amazon, two in the Tocantins, and 22 in the Orinoco basins), and two more on the Brazilian Atlantic coast, with recent reports of targeted consumption in the upper Orinoco River. Subsequently, the localities of use or bushmeat markets where Amazon River dolphin byproducts are trafficked were identified. This information was integrated with a kernel density analysis of the distribution of the Inia spp. populations establishing core areas. Our spatial analysis indicated that the use of Inia spp. is geographically widespread in the evaluated basins. It is urgent that decision-makers direct policies towards mitigating the socioeconomic and cultural circumstances associated with illegal practices affecting Amazon River dolphin populations in South America.
Introduction
The use of aquatic mammals for bait in fisheries for traditional and medicinal purposes or as human consumption is geographically widespread and affects at least 42 species (Mintzer et al., 2018). The incidental capture of whales, dolphins, manatees, and pinnipeds with fishing gear, as well as targeted harvesting, is recognized as a major threat for these aquatic mammals and represents a significant cause of mortality that remains poorly quantified (Crespo and Hall, 2002; Heppell et al., 2005; Clapham and Van Waerebeek, 2007; Costello and Baker, 2011; Diniz, 2011; Lewison and Moore, 2012; Iriarte and Marmontel, 2013a; Mintzer et al., 2013). Products from wild aquatic megafauna are obtained through illegal or unregulated hunting, as well as from stranded (dead or alive) and/or incidentally caught animals and are defined with the term “aquatic bushmeat” (CMS, 2016).
The use of body parts of Amazon River dolphins has been reported for traditional and medicinal purposes and as bait in the Amazon, Tocantins, and Orinoco basins (Best and da Silva, 1993; Cravalho, 1999; Aliaga Rossel, 2003; Alves and Rosa, 2008; Gravena et al., 2008; da Silva et al., 2017; da Silva V.M.F. et al., 2018; Siciliano et al., 2018). Individuals are often obtained as products from fishing activities (e.g., such as operational and ecological interactions) or targeted captures (Mintzer et al., 2013; Mintzer et al., 2018). Since the beginning of the 2000s, Inia spp. have been hunted illegally for their meat to use as bait for fishing the scavenger catfish Calophysus macropterus (known as blanquillo in Bolivia, piracatinga or douradinha in Brazil, mota or zamurito in Colombia and Ecuador, simi or mota punteada in Peru, and mapurite in Venezuela; Flores et al., 2008; Trujillo et al., 2010; Alves et al., 2012; Cosentino and Fisher, 2016; Trujillo et al., 2020). This catfish largely replaces either explicitly or implicitly the overfished Pimelodus grosskopfii, or capaz distributed in the Magdalena-Cauca basin in Colombia (Gómez et al., 2008; Salinas et al., 2014; Mosquera-Guerra et al., 2015); and its trading has spread to domestic markets in Brazil (Cunha et al., 2015), and Venezuela (Diniz, 2011).
One of the main reasons for the current re-categorization of I. geoffrensis from Data Deficient to Endangered by the International Union for Conservation of Nature (IUCN) Red List of Threatened Species is the increase in mortality of individuals in the last three decades due to conflicts with fishermen in a significant portion of its distribution (da Silva V. et al., 2018) and in smaller proportion as traditional uses or consumption (da Silva V.M.F. et al., 2018; Trujillo et al., 2020). In addition, the populations of this top predator of aquatic food webs (Gómez-Salazar et al., 2011) and regulator of the structure and composition of fish populations (da Silva, 1983; Best and da Silva, 1989) are being threatened by the degradation of their habitats by tensors like the following: (1) construction and operation of 307 dams in the Amazon basin, 10 in Tocantins basin, and four in the Orinoco basin, (2) mining, (3) high rates of deforestation and fire in flood plains, and (4) the negative effects of climate change on the flood pulse (Mosquera-Guerra et al., 2018; Anderson et al., 2019; Mosquera-Guerra et al., 2019a; Mosquera-Guerra et al., 2019b; Campbell et al., 2020; Armenteras et al., 2021; Barbosa et al., 2021; Brum et al., 2021; Fearnside et al., 2021; Pivari et al., 2021). In this context, Amazon River dolphins are considered among the most threatened aquatic mammals globally (Reeves et al., 2003; Trujillo et al., 2010).
In this paper, we identify the geographic distribution of illegal practices using Amazon River dolphins across their area of occurrence. Additionally, we implemented spatial analyses to determine areas of risk for the Inia spp. populations. Specifically, our objectives were the following: (1) to identify the localities where these types of practices have been reported, and (2) to establish the core areas for Amazon River dolphin populations.
Methods
Systematic Review of Literature and Surveys
We accessed 57 literature references (dated between 1980 and 2021) to obtain information on the use of Amazon River dolphins as bushmeat, medicinal and traditional purposes, and human consumption. The search and selection of publications followed the PRISMA methodological approach (Moher et al., 2009; Nakagawa et al., 2017). A search for information was conducted in the following databases: (1) Scopus, (2) Science Direct, (3) Springer Link, and (4) Google Scholar. Different search terms were used: (1) Amazon River dolphin (TI) AND targeted captures AND bushmeat AND piracathinga fishery*, (2) Amazon River dolphin (TI) AND flesh and blubber OR bushmeat (TI) AND piracathinga fishery*, (3) [TITLE-ABS KEY (Amazon River dolphin * AND piracathinga fishery) * AND (flesh * OR blubber * OR bushmeat * OR traditional medicine * AND piracathinga fishery *) AND TITLE (Amazon River dolphin *)]. In addition, 14 structured surveys were carried out with researchers of Bolivia, Brazil, Colombia, Perú, and Venezuela to identify areas where Amazon River dolphin are captured and opportunistic uses are reported. Subsequently, the information was classified in a database considering the following criteria: (1) country, (2) locality, (3) river, (4) basin, (5) subspecies, (6) category of use: traditional/medicinal purposes, bycatch/bushmeat, and consumption, (7) period(s) of recorded bushmeat use (1980-2000/2001-2021), and (8) references.
Spatial Analysis
Spatial analyses included the mapped localities of use of Inia spp. in the assessed basins, derived from the literatura review. Additionally, 39,135 georeferenced locations from 23 boat-surveys (n = 11,519 locations) conducted in the Amazon (n = 16), Tocantins (n = 1) and the Orinoco basins (n = 6), and 33 tagged individuals from satellite monitoring (n = 27,616 locations) in Amazon (n = 20 individuals) and Orinoco basins (n = 13 individuals; Figure 1) were integrated into a kernel density (KD) estimation analysis on percentage volume contours from (K10) 10% to (K90) 90% at 10% intervals (Oshima et al., 2010; Sveegaard et al., 2011; Wells et al., 2017; Mosquera-Guerra et al., 2021). This means that the area within the (K10) 10% contour represented the areas with the highest density or core area and the (K90) 90% contour represented almost the entire range of Amazon River dolphins (Sveegaard et al., 2011). Kernel density analyses allowed us to spatially locate the Inia spp. populations at greater risk from use by calculating the following spatial metrics: (1) number of the core areas (K50), (2) distance from the nearest the Amazon River dolphin core area to a locality use, and (3) distance from the nearest core area to a protected area for the assessed basin (Protected Planet Report, 2020). Mapping was performed using the geostatistical analyst and spatial analyst extensions in ESRI ArcGIS version 10.8.1 (ESRI Environmental Systems Research Institute, 2021; Table 1).
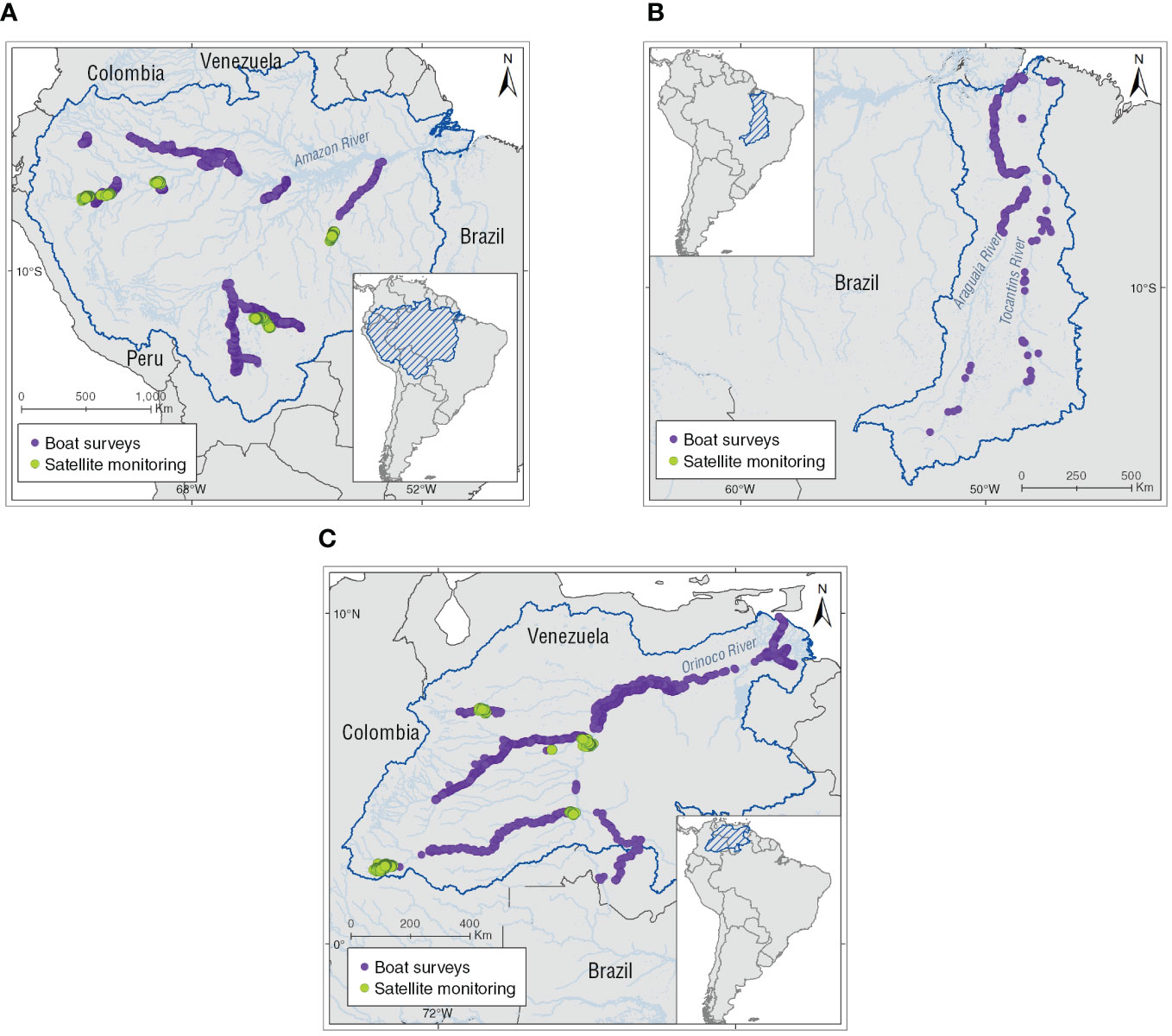
Figure 1 Locations of Amazon River dolphins obtained through boat surveys and satellite monitoring that were used in the spatial analyses. (A) Amazon basin, (B) Tocantins basin, and (C) Orinoco basin.
Statistical Analysis
Shapiro-Wilk normality test was performed to the variables: (1) Inia spp. population size, (2) Inia spp. population density, (3) Number of the Amazon River dolphin use localities, (4) Number of core areas (K50) in the assessed river basin sections, (5) Distance of core area (K50) to the nearest locality of use, and (6) Distance of core area (K50) to the nearest protected areas. These tests were developed using the open-source software R.4.0.3 (R Core Team, 2020). In all cases, a value of p < 0.05 was considered statistically significant.
Results
Localities that Reported the Use of Amazon River Dolphins
We identified 57 localities where Inia spp. individuals were used under the evaluated categories in the study areas, and reported two more on the Brazilian Atlantic coast. The localities were distributed in the basins as follow: Amazon (n = 33, 58%), Tocantins (n = 2, 3%), and Orinoco basins (n = 22, 39%). Based on the number of records, the country with the highest number of localities is Brazil (n = 20, 34%), followed by Venezuela (n = 17, 29%), Peru (n = 13, 22%), Colombia (n = 7, 12%), and Bolivia (n = 2, 3%; see Figure 2 and Supplementary Table 1).
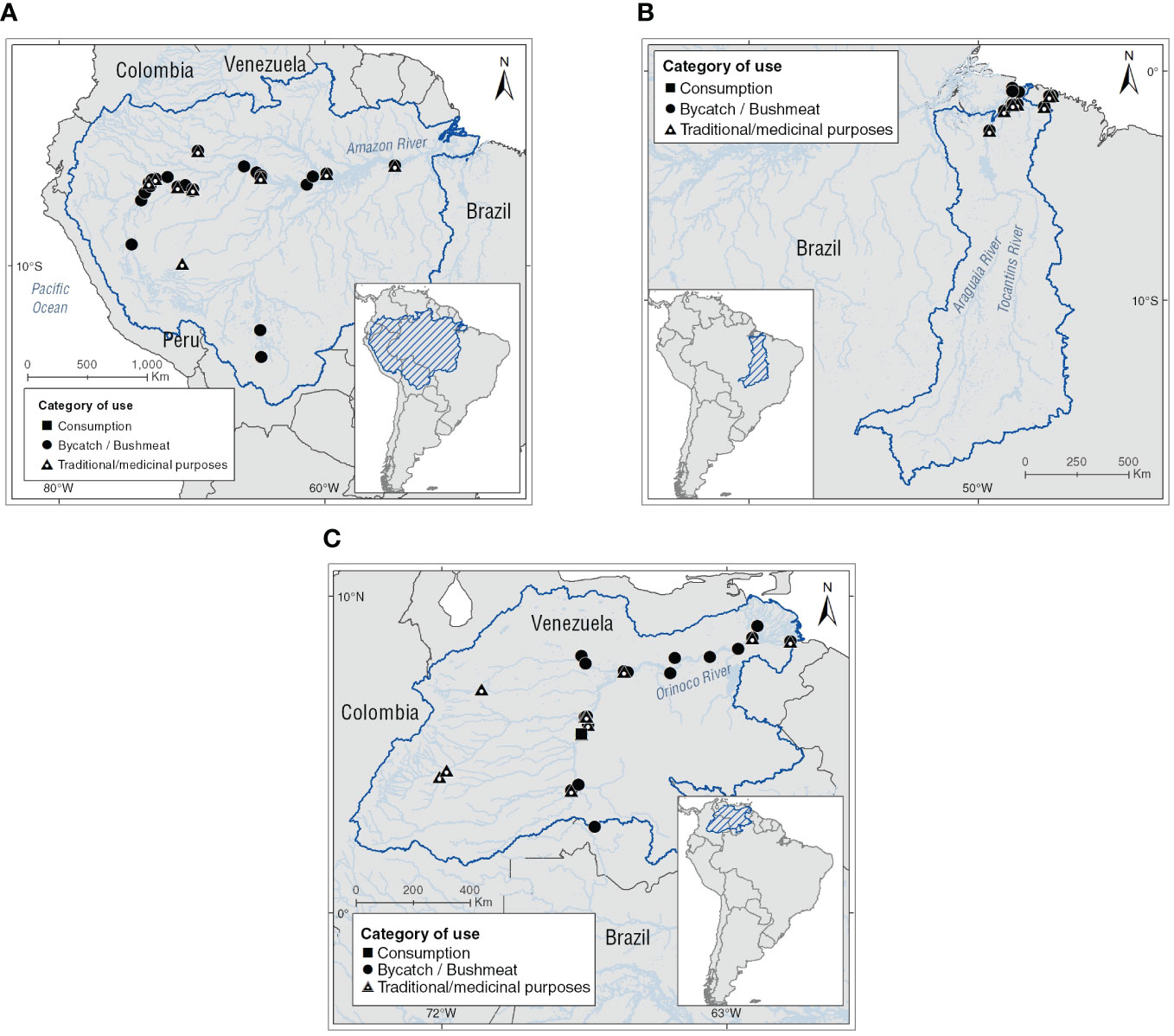
Figure 2 Locations where use categories assessed for Amazon River dolphins are reported. (A) Amazon basin, (B) Tocantins basin, and (C) Orinoco basin.
Amazon Basin
The use of I. g. geoffrensis in the upper Amazon basin has been reported in 20 localities. The highest number of these is situated on the Amazon River from the Napo River to the tripartite border of Peru, Colombia, and Brazil. In the middle basin, six localities of use have been identified from the confluence of the Putumayo/Içá and Caquetá/Japurá rivers with the Amazon River to the Negro River in Brazil and in the Tijamuchi and Mamoré rivers in Bolivia in these last two rivers, where use is made of I. g. boliviensis individuals. Finally, in the lower basin, seven localities from the confluence of the Tapajos and Amazon rivers to the island of Marajó in the vicinity of the Belém city and the mouth of the Amazon River at the Atlantic Ocean in Brazil were identified (see Figure 2 and Supplementary Table 1).
Tocantins Basin
The Tocantins basin is currently isolated from the Amazon River basin. This condition makes it a biogeographic area of interest for genetic and ecological studies of Inia spp.; recently populations of this basin were proposed as a new species I. araguaiaensis (Hrbek et al., 2014). Since the 2000s, the use of the Amazon River dolphin individuals in the Mocajuba in the Tocantins River and Ourém in the Guamá River have been documented. In addition, the following are reported Bragança and Tracuateua localities in the Caeté River in the Brazilian Atlantic Coast (see Figure 2 and Supplementary Table 1).
Orinoco Basin
In the Upper Orinoco the Amazon River dolphin use is documented from the San Miguel River to the confluence of the Meta-Orinoco rivers in 12 localities located at the border between Colombia and Venezuela. In this section of the basin, consumption of individuals of I. g. geoffrensis is reported for the locality of Puerto Ayacucho (Venezuela). Smoked meat of the Amazon River dolphin is marketed as the meat of lowland tapir (Tapir terrestris) traditionally consumed by local communities. Furthermore, Inia geoffrensis oil is marketed from the city of Puerto Ayacucho to other localities such as Casuarito, Puerto Carreño and Inírida in Colombia to treat symptoms of respiratory ailments. In the middle basin, the use of Amazon River dolphins has been evidenced in the Camaguán, Caicara del Orinoco, San Fernando de Apure, and Puruey localities. In these, the use of Inia’s oil for the treatment of SARS-CoV-2 derived respiratory symptoms by indigenous communities who live in these localities has been documented. Finally, in the lower basin this use has been reported in the Ciudad Bolivar, Uverito, Puerto Barranca, San Felix, Tucupita, and Curipao localities (see Figure 2 and Supplementary Table 1).
The most represented use category for Amazon River dolphins in the basins was bushmeat (n = 55, 64%), followed by traditional/medical purposes (n = 30, 35%), and finally consumption (n = 1, 1%). The taxa of the genus Inia that report the highest number of use localities in the basin evaluated is I. g. geoffrensis (n = 53, 90%), subsequently of I. araguaiaensis (n = 4, 7%), and finally I. g. boliviensis (n = 2, 3%; see Figure 2 and Supplementary Table 1).
Spatial Analysis
Kernel density analyses show that most of the core areas (K50) of Inia spp. are in heterogeneous habitat types as follow: (1) main rivers, (2) confluences, (4) lagoons, and (5) channels of the river basins. In the Amazon basin there are four core areas: (1) Napo-Amazonas rivers confluence, (2) Loretayacu-Amazonas rivers confluence, including the wetland complex of Tarapoto, (3) Iténez River, and (4) Tapajós River. The Orinoco basin has three core areas: (1) Guayabero River, (2) Guaviare-Inírida rivers confluence, and (3) Meta-Bita-Orinoco rivers confluence, and in the Tocantins basin, the core area was in the lower basin (Figure 3).
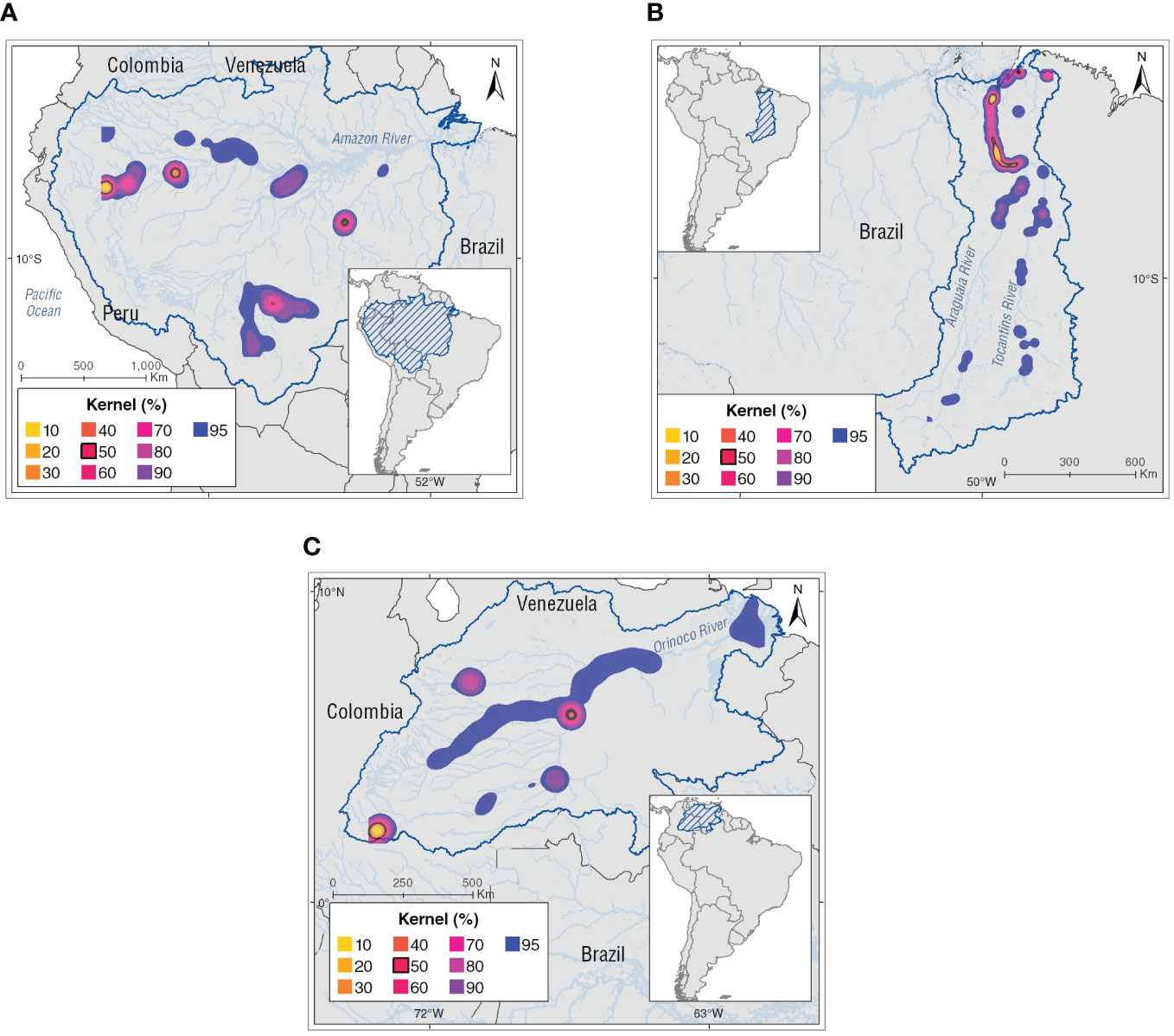
Figure 3 Kernel density (KD) estimation analysis for the Inia spp. in the basins with percentage volume contours from (K10) 10% to (K90) 90% at 10% intervals. The black line indicates high-density areas for the Amazon River dolphins defined as the 50% kernel contour or core areas. (A) Amazon basin, (B) Tocantins basin, and (C) Orinoco basin.
The values of the Shapiro-Wilk normality test concluded that the data for all variables do not come from a normal distribution (Table 2).
Discussion
Geographic Distribution of the Illegal Uses of Amazon River Dolphin
Our results are in line with previous reports on the widespread use of Amazon River dolphin (Inia spp.), such as bait in the C. macropterus fisheries. This practice is an unsustainable practice that is widespread in the Amazonian countries of Bolivia, Brazil, Colombia, and Peru, along the lower Tocantins River in Brazil, and along the Orinoco basin shared between Colombia and Venezuela. It is considered a significant threat to the populations of these species (Brum, 2011; Iriarte and Marmontel, 2013a; Iriarte and Marmontel, 2013b; Mintzer et al., 2013; Botero-Arias et al., 2014; Brum et al., 2015; da Silva V.M.F. et al., 2018; Mintzer et al., 2018; Trujillo et al., 2020).
In the last thirty years, the increase of the human population in the hydrographic areas assessed, as well as the internal and external demand for the fishery resources in these countries, have led to overexploitation and the rapid decline of stocks of fishes of commercial interest to fisheries (e.g., large catfish Brachyplatystoma spp.), and has resulted in a shift of target species of fisheries from increasingly scarce large fish to smaller species (e.g., small catfishes with C. macropterus; Gómez et al., 2008; Barthem, 2013; Barthem et al., 2017). Change in the fisheries in the Amazon, Tocantins and Orinoco basins has involved the use of unsustainable practices (e.g., monofilament nets, trammel nets, and even the use of endangered species such as bait) thus increasing the biological and operational interactions with aquatic vertebrates (e.g., Amazon River dolphins). These events generally result in the incidental capture and retaliatory killing of individuals that in some cases are traded in the bushmeat markets for bait or traditional purposes and in lower proportions for consumption (Hernández and Gonzalves, 2009; Diniz, 2011; da Silva et al., 2011; da Silva V.M.F. et al., 2018; Escobar-WW et al., 2020; Trujillo et al., 2020; Brum et al., 2021).
Governments of Brazil and Colombia have generated instruments such as moratoriums to regulate or prohibit the commercialization of C. macropterus (da Silva V.M.F. et al., 2018; Trujillo et al., 2020). This interaction is considered a serious threat for Inia spp. populations in management plans formulated in Brazil, Bolivia, Colombia, Peru, and Venezuela. However, the implementation of actions proposed in these strategies for the mitigation of this threat has not been effective due to factors such as a: (1) lack of transboundary regulatory instruments for the management of the fishery resource (e.g., moratoriums and ban unified between neighboring countries), (2) reduced institutional capacity to control extensive areas in transboundary zones, and (3) high levels of economic vulnerability and low levels of education of the local communities that facilitate their insertion into extractive models (e.g., illegal trade of wild species), and the use of species of fauna (e.g., river dolphins) for the treatment of diseases without scientific evidence.
Spatial Ecology of Amazon River Dolphins
Our kernel density results (K10 – K95) coincide with those reported by Mosquera-Guerra et al. (2021) on the heterogenous distribution of the core areas in the different habitat types used by Amazon River dolphins (e.g., confluences, channels, tributaries and lagoons) that are influenced by the ecology of the species and environmental aspects of the basin, such as: (1) wide variations in the home range sizes (K95 = 6.2 – 234 km², mean = 59 ± 13.5 km²), and core area sizes (K50 = 0.6 – 54.9 km², mean = 9 ± 2.6 km ²), (2) broad and specific habitat uses, (3) movements influenced by the lateral and longitudinal migration of fish, (4) sexual segregation of Inia individuals, and (5) ecological characteristics of the aquatic systems where they occur (productivity levels; Mosquera-Guerra et al., 2021).
Although Inia spp. is distributed over >1,000,000 km2 of the Amazon, Orinoco, and Tocantins basins, its occurrence is represented by only 15% of their distribution inside protected areas (Mosquera-Guerra et al., 2018). This is evidence that Inia spp. populations throughout much of their range are exposed to different types of human-induced threats such as bycatch (Hernández and Gonzalves, 2009; da Silva et al., 2011; Diniz, 2011; da Silva V.M.F. et al., 2018; Escobar-WW et al., 2020; Trujillo et al., 2020; Brum et al., 2021).
The results obtained through our statistical analysis show that the variables do not come from a normal distribution. This condition may be due to the widespread occurrence of this practice on the Inia spp. and the reduced management and control of the protected areas in the basins evaluated (da Silva V.M.F. et al., 2018; Trujillo et al., 2020). The population aspects considered in our analyses-such as population size of Amazon river dolphins- are influenced by: (1) the abundance and availability of fish prey, (2) the accessibility to foraging locations, determined mainly by the flooding pulse and river geomorphology, and (3) group sizes (Martin and da Silva, 1998; McGuire and Winemiller, 1998; Trujillo, 2000; Martin and da Silva, 2004; McGuire and Henningsen, 2007; Yamamoto et al., 2015; Mintzer et al., 2016; Mosquera-Guerra et al., 2021). Amazon River dolphins make up one of the smallest group sizes among odontocetes as a strategy to increase individual fitness and reduce competition for prey during declines in fish abundance during the high-water period (Gómez-Salazar et al., 2011).
Additionally, the spatial ecology of Amazon River dolphins is influenced by strong sexual segregation of individuals. The documented differential behaviors in the intensity of habitat use between males and females is reported by Trujillo (2000) for the lakes of Tarapoto and the Colombian Amazonas, Martin and da Silva (1998) and Martin and da Silva (2004) using data from 24 individuals monitored with radio telemetry in the Mamirauá Sustainable Development Reserve, and Mosquera-Guerra et al. (2021) from 24 individuals monitored by satellite in Bolivia, Brazil, Colombia, and Peru. This sexual segregation of Inia spp. differentially exposes males and females to targeted or incidental captures as well as other types of threats (Mintzer et al., 2016). The interactions between Amanimals with 20 individuals killed for use as bait inazon River dolphins and fisheries generally occur in highly productive habitats, such as: (1) confluences, (2) channels, and (3) lagoons, where capturing mostly sexually mature individuals and possibly larger numbers of females that have minor movements and are restricted to specific habitats where they care for their calfs. This could explain the rapid population decline in their area of occurrence (Williams et al., 2016; Martin and da Silva, 2021), possibly due to the special reproductive conditions of Inia spp. including (1) extended periods to reach sexual maturity of individuals that on average is considered to be 9.7 years, (2) extensive gestation periods (12.3–13 months), (3) prolonged parental care of calves (1.5–5.8 years), and (4) average intervals between births of 4.6 years (Martin and da Silva, 2018).
Knowledge Gaps
Our study highlights the need to continue with Inia population trend studies in order to monitor in a standardised way the fast population decline of Amazon River dolphins reported in the last three decades in the study areas (Williams et al., 2016; Martin and da Silva, 2021). This information is essential to complement spatial analyses and to focus conservation efforts in priority areas. Population studies conducted in the upper and middle Amazon and Orinoco rivers highlight the negative impact of bycatch on I. g. geoffrensis populations. For example, Williams et al. (2016) assess Amazon River dolphin abundance estimates made in the Colombian Amazon trapezoid in 1993, 2002 and 2007, and report an annual decline probability for I. geoffrensis of > 0.75. Hernández and Gonzalves (2009) report that the population of I. geoffrensis in the Javari River, a tributary of the Amazon River, is 250 animals with 20 individuals killed for use as bait in C. macropterus fisheries annually (8%). da Silva et al. (2011), and Martin and da Silva (2021) report between the 5.5–10% annual decline of populations of I. g. geoffrensis in the Central Amazon in the vicinity of the Mamiruá Reserve and document that 1650 Amazon River dolphins are captured annually near the Brazilian Amazonian city of Tefé. Finally, in the Venezuelan Orinoco basin, Diniz (2011) estimates that 840 individuals are killed for piracatinga fisheries (da Silva V.M.F. et al., 2018). In this context, it is a priority to continue with this type of population dynamic studies and thus contribute to an understanding of the effect of this threat on the health of Inia populations.
In this context, it is necessary to clarify the taxonomy of the genus Inia using integrative taxonomy studies since currently only two subspecies are recognized (Committee on Taxonomy, 2021); I. g. geoffrensis distributed across the Amazon, and Orinoco basins and I. g. boliviensis, found along the Mamoré, Iténez, and Madeira rivers (Aliaga-Rossel, 2002; Aliaga-Rossel et al., 2006; Gravena et al., 2014; da Silva and Martin, 2014; da Silva V. et al., 2018; Aliaga-Rossel and Guizada-Durán, 2020; Pivari et al., 2021). This condition does not allow for evidence of possible effects on the loss of genetic diversity for the genus caused by the reduction of populations. This is the case of the Inia spp. that are pressured by targeted and incidental catches in the middle Amazon (Bolivia and Brazil), and Tocantins Basins (Brazil). It has been proposed that I. boliviensis corresponds to a valid species (Banguera-Hinestroza et al., 2002; Ruíz-García, 2010, and Gravena et al., 2014); in the same way, Hrbek et al. (2014) have suggested I. araguaiaensis (Tocantins basin) as a new species in the genus, with a population size >3,000 individuals, seriously threatened by infrastructure projects like dams (Hrbek et al., 2014; Paschoalini et al., 2020; Brum et al., 2021). Although these taxa have not yet been recognized as valid species by a section of the scientific community (Committee on Taxonomy, 2021), a precautionary principle should be considered and efforts should be made to preserve the taxonomic diversity of the genus Inia (Trujillo et al., 2010; da Silva V.M.F. et al., 2018).
Additionally, it is a priority to promote the implementation of public health programs in the countries of the region that monitor concentrations of heavy metals in aquatic ecosystems as well as the zoonotic risks generated by the illegal bushmeat market. The best biological models for evidence of mercury concentrations in aquatic food webs are top predators (e.g., Amazon River dolphins; Mosquera-Guerra et al., 2019a; Barbosa et al., 2021), and benthic fish with omnivorous habits (C. macropterus; Mosquera-Guerra et al., 2015). These aquatic vertebrates are extensively used in the region, and this situation could become a public health problem for local communities and external consumers who make multiple uses of these species.
Finally, the bushmeat markets are widely distributed in the Neotropical region, illegally trading massive numbers of wildlife rodents, primates, xenarthrans, and ungulates (Olival et al., 2017), with a significant increment in the trading of Inia since the 2000s for bait for C. macropterus fisheries, and more recently for human consumption. The use of other products of Inia such as oil, eyes, and genitals organs for traditional purposes and non-evaluated treatments of respiratory ailments since the 1980s (Cravalho, 1999; Gravena et al., 2008; Loch et al., 2009; Trujillo et al., 2010; Martins, 2015; Cosentino and Fisher, 2016; Santos, 2017; da Silva V.M.F. et al., 2018; Mintzer et al., 2018; Siciliano et al., 2018), constitutes a risk for the emergence and transmition of zoonotic diseases and future pandemics. Bushmeat markets are centers for the interaction of viral loads of various vertebrate species (Olival et al., 2017), that could, at any time, cause a zoonotic jump in densely populated places with the Amazon basin where recent censuses have reported more than 40 million inhabitants.
Conclusion
Spatial analyses are powerful tools that at different scales contribute to an understanding of the distribution of areas of the ecological importance of the species with wide ranges of occurrence such as Inia spp.; as well they contribute to the identification of their threats, and focus conservation efforts. Species such as the Amazon River dolphin erroneously have been considered relatively safe from human-induced threats due to its wide area of distribution. However, this consideration ignores the broad and specific ecological requirements of river dolphins, as well as the cumulative effect of the multiple threats facing their populations and habitats throughout their range. An example of this condition was the recent ecological extinction in 2006 of the baiji (Lipotes vexillifer) that was widely distributed along 1,700 km in the middle of the Yangtzé River in China.
Over the last three decades in South America, researchers have endeavored to identify threats to the conservation of taxa of the genus Inia, including quantifying the number of individuals that have been captured and killed for illegal use. This scientific knowledge has been essential in the construction of different strategies for the conservation of Amazon River dolphin populations. However, despite the efforts made by civil society and governments, the implementation of these actions lacks effectiveness due to aspects such as the absence of transnational instruments to sustainably manage the habitats and the conservation of healthy populations of this endangered cetacean on a basin scale. The current state of decline Inia spp. populations mainly are caused by of the directed and incidental catch as well as the ecosystemic degradation of the natural environments suitable for Amazon River dolphins. One of the identified problems compromising the effectiveness of management is the reduced management capacity at the transnational level. Finally, in the context of the global health crisis caused by SARS-CoV-2, it is urgent to prevent future pandemics through public health surveillance strategies and the social management of the bushmeat markets, while considering the cultural and economic needs of local populations of these basins.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Pontificia Universidad Javeriana.
Author Contributions
FM-G led and executed the systematic review and spatial analyses, synthesis, and preparation of the first draft. FT, JP-T, HM-M, NF-L, MP, MV, JU, EC, JA-S, JLM, JCM, CG, MZ, YB, KV, PT-F, LS, AF, SB, PD, and DA-P contributed the initial idea and from the first draft on, edited and organized the development of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Whitley Fund For Nature (WFN), World Wildlife Fund (WWF), CORMACARENA, CORPORINOQUIA, CORPOAMAZONIA, Projects Design and Development (PDD), and Pontificia Universidad Javeriana (PUJ). The views expressed are those of the authors and do not necessarily reflect the views of these organizations.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This program is part of the strategic plan of the South American River Dolphin Initiative (SARDI) supported by WWF in Brazil, Colombia, Peru, Ecuador, and Bolivia. Partial support for two tag transmitters installed in individuals from Peru was obtained from the Rufford Foundation through RSG. Special gratitude goes to M. Oliveira da Costa, D. Willems, K. Berg, L. Sainz, J. Rivas, J. Surkin, R. Maldonado, D. Embert, V. Tellez, F. La Rosa, and M. Wulms from WWF. FMG received a Postdoctoral Fellowship from PUJ (ID project: 20389 to 2021). EC received a doctoral scholarship from WWF-EFN. A. Echeverría, L. Cordova, and A. Salinas are acknowledged for their support during tagging campaigns in Bolivia and M. Marmontel in Brazil. We acknowledge the WFN, CORMACARENA, CORPORINOQUIA, CORPOAMAZONIA, PDD, the fishing communities, and the local and national authorities for participating in the Amazon River dolphin capture process.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.838988/full#supplementary-material
References
Aliaga-Rossel E. (2002). Distribution and Abundance of the River Dolphin (Inia Geoffrensis) in the Tijamuchi River, Beni, Bolivia. Aq. Mamm. 28, 312–323.
Aliaga Rossel E. (2003). Situación Actual Del Delfín De Río (Inia Geoffrensis) En Bolivia. Ecol. Bolivia. 38, 167–178.
Aliaga-Rossel E., Guizada-Durán L. A. (2020). Bolivian River Dolphin Site Preference in the Middle-Section of Mamoré River, Upper Madeira River Basin, Bolivia. Theyra 11, 459–465. doi: 10.12933/therya-20-977
Aliaga-Rossel E., McGuire T. L., Hamilton H. (2006). Distribution and Encounter Rates of the River Dolphin (Inia Geoffrensis Boliviensis) in the Central Bolivian Amazon. Cetacean. Res. Manage. 8, 87–92.
Alves R. R. N., Rosa I. L. (2008). Use of Tucuxi Dolphin Sotalia Fluviatilis for Medicinal and Magic/Religious Purposes in North of Brazil. Hum. Ecol. 36, 443–447. doi: 10.1007/s10745-008-9174-5
Alves L. C. P. S., Zappes C. A., Andriolo A. (2012). Conflicts Between River Dolphins (Cetacea: Odontoceti) and Fisheries in the Central Amazon: A Path Toward Tragedy? Zoologia 29, 420–429. doi: 10.1590/S1984-46702012000500005
Anderson E. P., Osborne T., Maldonado-Ocampo J. A., Mills-Novoa M., Castello L., Montoya M., et al. (2019). Energy Development Reveals Blind Spots for Ecosystem Conservation in the Amazon Basin. Front. Ecol. Environ. 17, 521–529. doi: 10.1002/fee.2114
Armenteras D., Dávalos L. M., Barreto J. S., Miranda A., Hernández-Moreno A., Zamorano-Elgueta C., et al. (2021). Fire-Induced Loss of the World’s Most Biodiverse Forests in Latin America. Sci. Adv. 7, eabd3357. doi: 10.1126/sciadv.abd3357
Banguera-Hinestroza E., Cárdenas H., Ruíz-García M., Marmontel M., Gaitán E., Vázquez R., et al. (2002). Molecular Identification of Evolutionarily Significant Units in the Amazon River Dolphin Inia Sp. (Cetacea: Iniidae). J. Hered. 93, 312–322. doi: 10.1093/jhered/93.5.312
Barbosa M. S., Carvalho D. P., Gravena W., de Almeida R., Mussy M. H., Sousa E. A., et al. (2021). Total Mercury and Methylmercury in River Dolphins (Cetacea: Iniidae: Inia Spp.) in the Madeira River Basin, Western Amazon. Environ. Sci. Pollut. Res., (33), 45121–133. doi: 10.1007/s11356-021-13953-z
Barthem R. (2013). “Estado Natural, Amenazas Y Tendencias De Los Recursos Pesqueros En La Amazonia Brasileña,” in Hacia El Manejo De Las Pesquerías En La Cuenca Amazónica, Perspectivas Transfronterizas. Eds. Collado L., Castro E., Hidalgo M. (Miraflores: Instituto del Bien Común), 13–17.
Barthem R. B., Goulding M., Leite R. G., Cañas C., Forsberg B., Venticinque E., et al. (2017). Goliath Catfish Spawning in the Far Western Amazon Confirmed by the Distribution of Mature Adults, Drifting Larvae and Migrating Juveniles. Sci. Adv. 7, 41784. doi: 10.1038/srep41784
Best R. C., da Silva V. M. F. (1989). “Amazon River Dolphin. Boto Inia Geoffrensis (De Blainville 1817),” in Handbook of Marine Mammals, River Dolphins and the Larger Toothed Whales. Eds. Ridgway S. H., Harrison R. (London: Academic Press), 1–24.
Botero-Arias R., Franco D. L., Marmontel M. (2014) Caiman and Dolphin Mortality Associated to the Piracatinga Fshery in the Mid Solimões River Region Amazonas, Brazil. Instituto De Desenvolvimento Sustentável Mamirauá Final Report. Available at: https://mamiraua.org.br/documentos/b30938dff080b2b7f7cfafac696b29a4.pdf (Accessed Aug 21, 2021).
Brum S. M. (2011). Interacão Dos Golfinhos Da Amazônia Com a Pesca No Médio Solimões (Manaus, Brasil: Universidade Federal do Amazonas/Instituto Nacional de Pesquisas da Amazônia).
Brum S., Rosas-Ribeiro P., de Souza Amaral R., de Souza D. A., Castello L., Ferreira da Silva, et al. (2021). Conservation of Amazonian Aquatic Mammals. Aquat Conserv.: Mar. Freshw. Ecosyst. 1, 1–19. doi: 10.1002/aqc.3590
Brum S., Silva V., Rossoni F., Castello L. (2015). Use of Dolphins and Caimans as Bait for Calophysus Macropterus (Lichtenstein 1819) (Siluriforme: Pimelodidae) in the Amazon. J. Appl. Ichthyol. 31, 675–680. doi: 10.1111/jai.12772
Campbell E., Mangel J. C., Alfaro-Shigueto J., Mena J. L., Thurstan R. H., Godley B. J. (2020). Coexisting in the Peruvian Amazon: Interactions Between Fisheries and River Dolphins. J. Nat. Conserv. 56, 125859. doi: 10.1016/j.jnc.2020.125859
Clapham P., Van Waerebeek K. (2007). Bushmeat and Bycatch: The Sum of the Parts. Mol. Ecol. 16, 2607–2609. doi: 10.1111/j.1365-294X.2007.03378.x
Committee on Taxonomy (2021). List of Marine Mammal Species and Subspecies. Society for Marine Mammalogy. Available at: www.marinemammalscience.org/species-information/list-marine-mammal-species-subspecies/
Cosentino A. M., Fisher S. (2016). The Utilization of Aquatic Bushmeat From Small Cetaceans and Manatees in South America and West Africa. Front. Mar. Sci. 3. doi: 10.3389/fmars.2016.00163
Costello M. J., Baker C. S. (2011). Who Eats Sea Meat? Expanding Human Consumption of Marine Mammals. Biol. Conserv. 144, 2745–2746. doi: 10.1016/j.biocon.2011.10.015
Cravalho M. A. (1999). Shameless Creatures: An Ethnozoology of the Amazon River Dolphin. Ethnology 38, 47–58. doi: 10.2307/3774086
Crespo E., Hall M. (2002). “Interactions Between Aquatic Mammals and Humans in the Context of Ecosystem Man-Agement,” in Marine Mammals: Biology and Conservation. Eds. Evans G. H., Raga J. A. (New York: Kluw. Acad. Plen. Publ), 463–490.
Cunha H. A., da Silva V. M., Santos T. E., Moreira S. M., do Carmo N. A., Solé-Cava A. M. (2015). When You Get What You Haven’t Paid for: Molecular Identification of “Douradinha” Fish Fillets can Help End the Illegal Use of River Dolphins as Bait in Brazil. J. Hered. 106, 565–572. doi: 10.1093/jhered/esv040
da Silva V. M. F. (1983). Ecologia Alimentar Dos Golfinhos Da Amazônia (Brazil: Universidade do Amazonas).
da Silva V. M. F., Martin A. R. (2014). “Family Iniidae (Amazon River Dolphis),” in Handbook of the Mammals of the Word. Eds. Wilson D. E., Mittemeier R. A. (Barcelona: Lynx Edicions), 364–379.
da Silva V. M. F., Martin A. R., Carmo N. A. (2011a). Amazonian Fisheries Pose Threat to Elusive Dolphin Species. Species 53, 10–11.
da Silva V. M. F., Nunes A. C. G., de Araújo L. F. B., Batista J. S., Cunha H., et al. (2018). The Use of Amazonian Dolphins (Inia and Sotalia) as Bait the Piracatinga Fishery (Santos: Document SC/68B/SM01 Presented to the International Whaling Commission).
da Silva V. M. F., Shepard G., do Carmo N. A. S. (2017). “Os Mamíferos Aquáticos: Lendas Usos E Interaes Com as PopulaçÕes Humanas Na Amazônia Brasileira,” in Olhares Cruzados Sobre as Relações Silvestres Na AmazôNia. Eds. Marchand G., Vander Velden F. (Brasil, Guiana Francesa: Manaus: EDUA), 193–226.
da Silva V., Trujillo F., Martin A., Zerbini A. N., Crespo E., Aliaga-Rossel E., et al. (2018). “Inia geoffrensis. The IUCN Red List of Threatened Species. e.T10831A50358152. doi: 10.2305/IUCN.UK.2018-2.RLTS.T10831A50358152.en
Diniz K. S. (2011). La Pesca Del Bagre Zamurito (Calophysus Macropterus, Siluriformes: Pimelodidae) Y Su Efecto Potencial Sobre La Extracción De Toninas (Inia Geoffrensis, Cetacea: Iniidae) Y Babas (Caiman Crocodilus, Crocodilia: Aligatoridae) En Venezuela (Miranda, Venezuela: Instituto Venezolano de Investigaciones Científicas).
Escobar-WW M., Rey-Ortiz G., Coca-Méndez C., Córdova-Clavijo L., Sainz L., Moreno-Aulo F., et al. (2020). La Pesquería De Una Espécie Carroñera (Calophysus Macropterus) (Teleostei, Pimelodidae) Y Percepciones Sobre Su Impacto En Las Poblaciones Del Bufeo (Inia Boliviensis) (Cetacea, Iniidae) En La Amazonía Boliviana. Biol. Acuatic. Bolivia. 420, 27–56.
ESRI Environmental Systems Research Institute (2021). ArcGIS Desktop: Release 10.8.1 (Redlands: Environmental Systems Research Institute).
Estupiñán G. M. B., Marmontel M., Queiroz H. L., Souza P. R., Valsecchi J. A., Batista G. S., et al. (2003)A Pesca Da Piracatinga (Calophysus Macropterus) Na Reserva De Desenvolvimento Sustentável Mamirauá. In: Instituto De Desenvolvimento Sustentável Mamirauá Final Report. Available at: https://www.gov.br/agricultura/pt-br/assuntos/aquicultura-e-pesca/pesca/piracatinga/gt-mapa-piracatinga/relatorio/Relatorio_Final_GT_MAPA_Piracatinga:_Aprovado:compactado.pdf (Accessed Aug 27, 2021).
Fearnside P. M., Berenguer E., Armenteras D., Duponchelle F., Mosquera-Guerra F., Jenkins C. N., et al. (2021). “Drivers and Impacts of Changes in Aquatic Ecosystems,” in Science Panel for the Amazon (SPA). Eds. Nobre C., Encalada A. (New York: United Nations Sustainable Development Solutions Network), 305–343.
Flores P. A. C., Trujillo F., Rocha-Campos C. C., Marini-Filho O. J., da Silva V. M. F., Martin A. R., et al. (2008). The Status of “Piracatinga” Fishery Using Amazon Botos as Bait in South America (Portoroz: Document SC/60/SM17 Presented to the International Whaling Commission).
Gómez C., Trujillo F., Diazgranados M. C., Alonso J. (2008). “Capturas Dirigidas De Delfines De Río En La Amazonia Para La Pesca De Mota (Calophysus Macropterus): Una Problemática Regional De Gran Impacto,” in Fauna Acuática Amenazada En La Amazonia Colombiana – Análisis Y Propuestas Para Su Conservación. Eds. Trujillo F., Alonso J. C., Diazgranados M. C., Gómez C. (Bogotá D.C: Fundación Omacha, Fundación Natura, Instituto Sinchi, Corpoamazonia), 39–57.
Gómez-Salazar C., Trujillo F., Whitehead H. (2011). Ecological Factors Influencing Group Sizes of River Dolphins (Inia Geoffrensis and Sotalia Fluviatilis). Mar. Mamm. Scien. 28, 24–42. doi: 10.1111/j.1748-7692.2011.00496.x
Gravena W., Farias I. P., da Silva M. N. F., da Silva V. M. F., Hrbek T. (2014). Looking to the Past and the Future: Were the Madeira River Rapids a Geographical Barrier to the Boto (Cetacea: Iniidae). Conserv. Genet. 15, 619–629. doi: 10.1007/s10592-014-0565-4
Gravena W., Hrbek T., da Silva V. M. F., Farias I. P. (2008). Amazon River Dolphin Love Fetishes: From Folklore to Molecular Forensics. Mar. Mamm. Sci. 24, 969–978. doi: 10.1111/j.1748-7692.2008.00237.x
Heppell S. S., Heppell S. A., Read A. J., Crowder L. B. (2005). “Effects of Fishing on Long-Lived Marine Organisms,” in Marine Conservation Biology: The Science of Maintaining the Sea’ s Biodiversity. Eds. Norse E. A., Crowder L. B. (Washington, DC: Island Press), 211–231.
Hernández S., Gonzalves J. (2009) Evaluation of Deliberate Killing of Amazon River Dolphins Used as Bait for Mota Fishery in the Javari River, Brazil. Instituto De Desenvolvimento Socioambiental Vale do Javari Final Report. Available at: http://idsavj.org/pages/mota_project.html (Accessed September 02, 2021).
Hrbek T., da Silva V. M. F., Dutra N., Gravena W., Martin A. R., Farias I. P. (2014). A New Species of River Dolphin From Brazil or: How Little do We Know Our Biodiversity. PloS One 9, e83623. doi: 10.1371/journal.pone.0083623
Iriarte V., Marmontel M. (2013a). River Dolphin (Inia Geoffrensis, Sotalia Fluviatilis) Mortality Events Attributed to Artisanal Fisheries in the Western Brazilian Amazon. Aquat. Mamm. 39, 116–124. doi: 10.1578/AM.39.2.2013.116
Iriarte V., Marmontel M. (2013b). Insights on the Use of Dolphins (Boto, Inia Geoffrensis and Tucuxi, Sotalia Fluviatilis) for Bait in the Piracatinga (Calophysus Macropterus) Fishery in the Western Brazilian Amazon. J. Cetacean Res. Manage. 13, 163–173.
Lewison R., Moore J. (2012). Improving Interview-Based Assessments of Sea Turtle and Marine Mammal Bycatch in West Africa: Putting Fishing Activity Into A Socio-Economic Context (San Diego: San Diego State University and Southwest Fisheries Science Center, NOAA), 81. Project Report.
Loch C., Marmontel M., Simões-Lopes P. C. (2009). Conflicts With Fisheries and Intentional Killing Offreshwater Dolphins (Cetacea: Odontoceti) in the Western Brazilian Amazon. Biodivers. Conserv. 18, 3979–3988. doi: 10.1007/s10531-009-9693-4
Martin A. R., da Silva V. M. F. (1998). Tracking Aquatic Vertebrates in Dense Tropical Forest Using VHF Telemetry. MTS J. 32, 82–88.
Martin A. R., da Silva V. M. F. (2004). Number, Seasonal Movements, and Residency Characteristics of River Dolphins in an Amazonian Floodplain Lake System. Canad. J. Zool. 82, 1307–1315. doi: 10.1139/z04-109
Martin A. R., da Silva V. M. F. (2018). Reproductive Parameters of the Amazon River Dolphin or Boto, Inia Geoffrensis (Cetacea: Iniidae); an Evolutionary Outlier Bucks No Trends. Biol. J. Linn. Soc 123, 666–676. doi: 10.1093/biolinnean/bly005
Martin A. R., da Silva V. M. F. (2021). Amazon River Dolphins Inia Geoffrensis are on the Path to Extinction in the Heart of Their Range. Oryx 33, 1–5. doi: 10.1017/S0030605320001350
Martins B. M. L. (2015). A Pesca E Os Botos: PercepçÃo Dos Pescadores E Análise Das Capturas Acidentais De Pequenos Cetáceos No Estuário AmazôNico (Ilhéus, Brasil: Universidade Estadual de Santa Cruz).
McGuire T. L., Henningsen T. (2007). Movement Patterns and Site Fidelity of River Dolphins (Inia Geoffrensis and Sotalia Fluviatilis) in the Peruvian Amazon as Determined by Photo-Identification. Aqua. Mamm. 33, 359–367. doi: 10.1578/AM.33.3.2007.359
McGuire T. L., Winemiller K. (1998). Occurrence Patterns, Habitat Associations, and Potential Prey of the River Dolphin, lnia geoffrensis, in the Cinaruco River, Venezuela. Biotrop. 30, 625–638.
Mintzer V. J., da Silva V. M. F., Martin A. R., Barbour A. B., Frazer T. K., Lorenzen K. (2013). Effect of Illegal Harvest on Apparent Survival of Amazon River Dolphins (Inia Geoffrensis). Biol. Conserv. 158, 280–286. doi: 10.1016/j.biocon.2012.10.006
Mintzer V. J., Diniz K., Frazer T. K. (2018). The Use of Aquatic Mammals for Bait in Global Fisheries. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00191
Mintzer V. J., Lorenzen K., Frazer T. K., da Silva V. M. F., Martin A. R. (2016). Seasonal Movements of River Dolphins (Inia Geoffrensis) in a Protected Amazonian Floodplain. Mar. Mamm. Sci. 32, 664–681. doi: 10.1111/mms.12298
Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med. 6, e1000097. doi: 10.1371/journal.pmed.1000097
Mosquera-Guerra F., Aya-Cuero C., Acosta-Lugo E., Trujillo F., Parra-Sandova C. A., Franco-León N., et al. (2019c). Aspectos Poblacionales De La Tonina, Inia Geoffrensis Humboldtiana (Cetartiodactyla, Iniidae) En El Río Guayabero, Colombia. Rev. Biodivers. Neotrop. 9, 1–11. doi: 10.18636/bioneotropical.v9i1.819
Mosquera-Guerra F., Trujillo F., Aya-Cuero C., Franco-León N., Valencia K., Vásquez A., et al. (2019b). Population Estimate and Identification of Major Conservation Threats for the River Dolphin (Inia Geoffrensis Humboldtiana) at the Colombian Orinoquia. Therya 11, 9–21. doi: 10.12933/therya-20-854
Mosquera-Guerra F., Trujillo F., Caicedo-Hererra D., Zoque-Cancelado J., Mantilla-Meluk H. (2015). Impactos De Las Pesquerías De Calophysus Macropterus Un Riesgo Para Salud Pública Y La Conservación De Los Delfines De Río En Colombia. Mom. Cien. 12, 76–87.
Mosquera-Guerra F., Trujillo F., Danni P., Oliveira-da-Costa M., Marmontel M., Armenteras-Pascual D., et al. (2018). Analysis of Distribution of River Dolphins (Inia and Sotalia) in Protected and Transformed Areas in the Amazon and Orinoco Basins (Bled: Document SC/67B/SM16 Presented to the International Whaling Commission).
Mosquera-Guerra F., Trujillo F., Oliveira-da-Costa M., Marmontel M., Van Damme P. A., Franco N., et al. (2021). Home Range and Movements of Amazon River Dolphins (Inia Geoffrensis) in the Amazon and Orinoco River Basins. Endang. Species Res. 45, 269–282. doi: 10.3354/esr01133
Mosquera-Guerra F., Trujillo F., Parks D., Oliveira da Costa M., Van Damme P. A., Echeverría A., et al. (2019a). Mercury in Populations of River Dolphins of the Amazon and Orinoco Basins. EcoHealth 16, 743–758. doi: 10.1007/s10393-019-01451-1
Nakagawa S., Noble D. W. A., Senior A. M., Lagisz M. (2017). Meta-Evaluation of Meta-Analysis: Ten Appraisal Questions for Biologists. BMC Biol. 15, 3–18. doi: 10.1186/s12915-017-0357-7
Olival K., Hosseini P., Zambrana-Torrelio C., Ross N., Bogich T. L., Daszak P. (2017). Host and Viral Traits Predict Zoonotic Spillover From Mammals. Nature 546, 646–650. doi: 10.1038/nature22975
Oshima J. E. D. F., Oliveira Santos M. C. D., Bazzalo M., Carvalho Flores P. A. D., Nascimento Pupim F. D. (2010). Home Ranges of Guiana Dolphins (Sotalia Guianensis) (Cetacea: Delphinidae) in the Cananeia Estuary, Brazil. J. Mar. Biol. Ass. UK. 90, 1641–1647. doi: 10.1017/S0025315410001311
Paschoalini M., Almeida R. M., Trujillo F., Melo-Santos G., Marmontel M., Pavanato H. J., et al. (2020). On the Brink of Isolation: Population Estimates of the Araguaian River Dolphin in a Human-Impacted Region in Brazil. PloS One 15, e0231224. doi: 10.1371/journal.pone.0231224
Paschoalini M., Trujillo F., Marmontel M., Mosquera-Guerra F., Paitach R. L., Pavanato H. J., et al. (2021). Density and Abundance Estimation of Amazonian River Dolphins: Understanding Popula-Tion Size Variability. J. Mar. Sci. Eng. 9, 1184. doi: 10.3390/jmse9111184
Pavanato H., Melo-Santos G., Lima D., Portocarrero-Aya M., Paschoalini M., Mosquera-Guerra F., et al. (2016). Risk of Dam Construction for South American River Dolphins: A Case of Study of the Tapajós River. Endang. Species Res. 31, 47–60. doi: 10.3354/ESR00751
Pivari D., Pagliani B., Lemos L., Lima D., Gravena W. (2021). Monitoring a Critical Population of the Bolivian River Dolphin, Inia Boliviensis, Before and After Closing the Floodgates of a Hydroelectric Dam in the Amazon Basin, Brazil: A Quantitative Analysis. J. Nat. Conser. 64, 126082. doi: 10.1016/j.jnc.2021.126082
Protected Planet Report (2020) Protected Planet. Available at: www.protectedplanet.net/en.
R Core Team (2020). R: A Language and Environment for Statistical Computing (Vienna: R Foundation for Statistical Computing).
Reeves R. R., Smith B., Crespo E. A., Notarbartolo Di Sciara G. (2003). Dolphins, Whales and Porpoises. 2002–2010 Conservation Action Plan for the World’s Cetaceans (Gland: IUCN/SSC).
Ruíz-García M. (2010). “Changes in the Demographic Trends of Pink River Dolphins (Inia) at the Microgeographical Level in Peruvian and Bolivian Rivers and Within the Upper Amazon: Microsatellites and mtDNA Analyses and Insights Into Inia’s Origin,” in Biology, Evolution and Conservation of River Dolphins. Eds. Ruíz-García M., Shostell J. M. (New York: Nov. Sci. Publ), 161–193.
Salinas C., Cubillos J. C., Gómez R., Trujillo F., Caballero S. (2014). Pig in a Poke (Gato Por Liebre): The “Mota” (Calophysus Macropterus) Fishery, Molecular Evidence of Commercialization in Colombia and Toxicological Analyses. EcoHealth 11, 197–206. doi: 10.1007/s10393-013-0893-8
Santos I. R. (2017). O Boto É Pescador? As Dimensões Humanas Das InteraçÕes Entre a Pesca E Os Pequenos Cetáceos Na Amazônia Oriental (Belém, Brazil: Universidade Federal do Pará).
Siciliano S., Viana M. C., Emin-Lima R., Bonvicino C. R. (2018). Dolphins, Love and Enchantment: Tracing the Use of Cetacean Products in Brazil. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00107
Sveegaard S., Teilmann J., Tougaard J., Dietz R., Mouritsen K. N., Desportes G., et al. (2011). High-Density Areas for Harbor Porpoises (Phocoena Phocoena) Identified by Satellite Tracking. Mar. Mamm. Sci. 27, 230–246. doi: 10.1111/j.1748-7692.2010.00379.x
Trujillo F. (2000). Habitat Use and Social Behavior of the Freshwater Dolphin Inia Geoffrensis (De Blainville 1817) in the Amazon and Orinoco Basins. Phylosophal Doctoral thesis (Scotland: University of Aberdeen).
Trujillo F., Crespo E., Van Damme P. A., Usma J. S. (2010). The Action Plan for South American River Dolphins 2010 – 2020 (Bogotá, D.C: WWF, Fundación Omacha, WDS, WDCS, Solamac).
Trujillo-González F., Mosquera-Guerra F., Franco N. (2019). Delfines De Río: Especies Indicadoras Del Estado De Salud De Los Ecosistemas Acuáticos De La Amazonia Y La Orinoquia. RACCEFYN 43, 199–211. doi: 10.18257/raccefyn.765
Trujillo F., Marmontel M., van Damme P. A., Mosquera-Guerra F., Campbell E., Gilleman C., et al. (2020). The Piracatinga (Calophysus Macropterus) Fishery and its Impact on River Dolphin Conservation: An Update (Bled: Document SC/68B/SM01 Presented to the International Whaling Commission).
Wells R. S., Schwacke L. H., Rowles T. K., Balmer B. C., Zolman E., Speakman T., et al. (2017). Ranging Patterns of Common Bottlenose Dolphins Tursiops Truncatus in Barataria Bay, Louisiana, Following the Deepwater Horizon Oil Spill. Endang. Species Res. 33, 159–180. doi: 10.3354/esr00732
Williams R., Moore J. E., Gómez-Salazar C., Trujillo F., Burt L. (2016). Searching for Trends in River Dolphin Abundance: Designing Surveys for Looming Threats, and Evidence for Opposing Trends of Two Species in the Colombian Amazon. Biol. Conserv. 195, 136–145. doi: 10.1016/j.biocon.2015.12.037
Keywords: amazon basin, Inia spp, artisanal fisheries, conservation, fishery-dolphin interactions, intentional catches, orinoco basin, tocantins basin
Citation: Mosquera-Guerra F, Trujillo F, Pérez-Torres J, Mantilla-Meluk H, Franco-León N, Paschoalini M, Valderrama MJ, Usma Oviedo JS, Campbell E, Alfaro-Shigueto J, Mena JL, Mangel JC, Gilleman C, Zumba M, Briceño Y, Valencia KY, Torres-Forero PA, Sánchez L, Ferrer A, Barreto S, van Damme PA and Armenteras-Pascual D (2022) Strategy to Identify Areas of Use of Amazon River dolphins. Front. Mar. Sci. 9:838988. doi: 10.3389/fmars.2022.838988
Received: 19 December 2021; Accepted: 24 March 2022;
Published: 20 April 2022.
Edited by:
Carolyn J. Lundquist, National Institute of Water and Atmospheric Research (NIWA), New ZealandReviewed by:
Mario Barletta, Federal University of Pernambuco, BrazilEmigdio Marín-Enríquez, National Council of Science and Technology (CONACYT), Mexico
Copyright © 2022 Mosquera-Guerra, Trujillo, Pérez-Torres, Mantilla-Meluk, Franco-León, Paschoalini, Valderrama, Usma Oviedo, Campbell, Alfaro-Shigueto, Mena, Mangel, Gilleman, Zumba, Briceño, Valencia, Torres-Forero, Sánchez, Ferrer, Barreto, van Damme and Armenteras-Pascual. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federico Mosquera-Guerra, federico.mosqueraguerra@gmail.com
 Federico Mosquera-Guerra
Federico Mosquera-Guerra Fernando Trujillo
Fernando Trujillo Jairo Pérez-Torres
Jairo Pérez-Torres Hugo Mantilla-Meluk4,5
Hugo Mantilla-Meluk4,5  Mariana Paschoalini
Mariana Paschoalini María J. Valderrama
María J. Valderrama Elizabeth Campbell
Elizabeth Campbell Joanna Alfaro-Shigueto
Joanna Alfaro-Shigueto Yurasi Briceño
Yurasi Briceño Leonardo Sánchez
Leonardo Sánchez Sebastian Barreto
Sebastian Barreto Dolors Armenteras-Pascual
Dolors Armenteras-Pascual