Peripheral Delta Opioid Receptors Mediate Formoterol Anti-allodynic Effect in a Mouse Model of Neuropathic Pain
- 1Institut des Neurosciences Cellulaires et Intégratives, Centre National de la Recherche Scientifique, Université de Strasbourg, Strasbourg, France
- 2Chronobiotron, Centre National de la Recherche Scientifique, Strasbourg, France
- 3Centre d’Evaluation et de Traitement de la Douleur, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
- 4Institut de Génétique et de Biologie Moléculaire et Cellulaire, Centre National de la Recherche Scientifique, Université de Strasbourg, INSERM, Illkirch, France
Neuropathic pain is a challenging condition for which current therapies often remain unsatisfactory. Chronic administration of β2 adrenergic agonists, including formoterol currently used to treat asthma and chronic obstructive pulmonary disease, alleviates mechanical allodynia in the sciatic nerve cuff model of neuropathic pain. The limited clinical data currently available also suggest that formoterol would be a suitable candidate for drug repurposing. The antiallodynic action of β2 adrenergic agonists is known to require activation of the delta-opioid (DOP) receptor but better knowledge of the molecular mechanisms involved is necessary. Using a mouse line in which DOP receptors were selectively ablated in neurons expressing Nav1.8 sodium channels (DOP cKO), we showed that these DOP peripheral receptors were necessary for the antiallodynic action of the β2 adrenergic agonist formoterol in the cuff model. Using a knock-in mouse line expressing a fluorescent version of the DOP receptor fused with the enhanced green fluorescent protein (DOPeGFP), we established in a previous study, that mechanical allodynia is associated with a smaller percentage of DOPeGFP positive small peptidergic sensory neurons in dorsal root ganglia (DRG), with a reduced density of DOPeGFP positive free nerve endings in the skin and with increased DOPeGFP expression at the cell surface. Here, we showed that the density of DOPeGFP positive free nerve endings in the skin is partially restored and no increase in DOPeGFP translocation to the plasma membrane is observed in mice in which mechanical pain is alleviated upon chronic oral administration of formoterol. This study, therefore, extends our previous results by confirming that changes in the mechanical threshold are associated with changes in peripheral DOP profile. It also highlights the common impact on DOP receptors between serotonin noradrenaline reuptake inhibitors such as duloxetine and the β2 mimetic formoterol.
Introduction
Neuropathic pain arises from traumatic nerve injury or from a disease that affects the somatosensory system and is characterized by spontaneous pain, mechanical allodynia and/or thermal hypersensitivity (von Hehn et al., 2012). First-line treatments include antidepressants such as serotonin and noradrenaline reuptake inhibitors (SNRIs), or anticonvulsants such as gabapentinoids (Kremer et al., 2016a). In preclinical studies, activation of the β2-adrenergic receptors has been shown to be mandatory for the antiallodynic action of antidepressants (Yalcin et al., 2009; Kremer et al., 2016a, 2018) and chronic administration of several β2-adrenergic agonists such as formoterol has been successfully used to alleviate mechanical allodynia (Choucair-Jaafar et al., 2009, 2014; Yalcin et al., 2010; Jourdain and Hatakeyama, 2019). Formoterol is already routinely used in clinics to treat chronic obstructive pulmonary disease (Vanfleteren et al., 2018). Also, inhalation of β2-agonists during the perioperative period was associated with a five-fold lower risk of developing post-thoracotomy neuropathic pain whereas chronic antidepressants or chronic gabapentanoids appeared ineffective (Salvat et al., 2015). Drug repurposing could, therefore, be envisaged to treat neuropathic pain.
The mechanisms underlying the relief of mechanical allodynia are the topic of extensive research in various preclinical models but remain unclear. Interestingly, delta-opioid (DOP) receptors are essential for the antiallodynic effect of DOP agonists (Nozaki et al., 2012; Vicario et al., 2016) but also for the antiallodynic effect of chronic administration of both antidepressants (Benbouzid et al., 2008b; Yalcin et al., 2010; Ceredig et al., 2018; Kremer et al., 2018) and β2 mimetics (Yalcin et al., 2010; Choucair-Jaafar et al., 2014). More specifically, our previous work using the cuff model pointed to peripheral DOP receptors expressed in Nav1.8 positive neurons as mandatory for the antiallodynic action of DOP agonists (Gaveriaux-Ruff et al., 2011; Nozaki et al., 2012) as well as the SNRI duloxetine (Ceredig et al., 2018). Our data also revealed that changes in the expression profile of peripheral DOP receptors correlated with mechanical allodynia. Indeed, we observed decreased DOP receptor expression in unmyelinated calcitonin gene-related peptide (CGRP) positive neurons and free nerve endings in the skin and increased surface expression in the neurons still expressing the receptor in neuropathic conditions but not in mice chronically treated with duloxetine after cuff surgery (Ceredig et al., 2018). Here, we sought to determine whether the same mechanisms are triggered by chronic treatment with the β2 adrenergic agonist formoterol by identifying changes in the expression of peripheral DOP receptor using a mouse line in which peripheral DOP receptors are selectively ablated in neurons expressing the Nav1.8 sodium channel (DOP cKO; Gaveriaux-Ruff et al., 2011) and a knock-in mouse line expressing DOP receptors fused to the green fluorescent protein eGFP (DOPeGFP; Scherrer et al., 2006). Our data indicate that peripheral DOP receptors expressed in Nav1.8+ neurons were mandatory for formoterol anti-allodynic activity. DOP surface expression was lower in animals treated with chronic formoterol compared to neuropathic conditions. However, DOP receptor expression was only partially restored in nerve free endings in the skin and remained similar to the neuropathic conditions in the dorsal root ganglia (DRG) after chronic formoterol. Altogether, data suggest that antidepressants and β2 mimetics effects engage similar DOP-dependent mechanisms.
Materials and Methods
Animals
DOPeGFP knock-in mice expressing the DOP receptor infusion with the green fluorescent protein were generated by homologous recombination. In these animals, the eGFP cDNA preceded by a five amino acid linker (G-S-I-A-T) was introduced into the exon 3 of the DOP receptor gene, in the frame and 5’ from the stop codon (Scherrer et al., 2006). The genetic background of DOPeGFP mice was C57BL/6J:129SvPas (50%:50%). DOP-floxed (Oprd1fl/fl) mice were interbred with Nav1.8-Cre mice to generate conditional knockout (cKO) of DOP in primary nociceptive neurons (Nav1.8-Cre × Oprd1fl/fl or DOPcKO) as previously reported (Gaveriaux-Ruff et al., 2011). The genetic background of conditional DOP knock-out mice and their floxed controls was C57BL/6J:129SvPas (62.5%:37.5%). These mice were bred at the ICS animal facility, Illkirch, France, and kindly provided by Pr. Claire Gavériaux-Ruff. Adult male and female mice aged 6–20 weeks, weighing 20–32 g for females and 20–38 g for males were used. Animals from independent cohorts were distributed at best to provide groups of similar size for each gender and treatment (n = 88 DOPeGFP mice, n = 22 DOPcKO mice, n = 20 DOP-floxed mice). Mice were group-housed 2–5 per cage, under standard laboratory conditions (12 h dark/light cycle, lights on at 7 am) in temperature (21 ± 1°C) and humidity (55 ± 10%) controlled rooms with food and water ad libitum. All experiments were approved by the “Comité d’Ethique en Matière d’Expérimentation Animale de Strasbourg” [authorization number 201503041113547 (APAFIS#300).02] and conducted in agreement with the EU Directive 2010/63/EU for animal experiments.
Neuropathic Pain Model
Neuropathic pain was induced by cuffing the main branch of the right sciatic nerve as previously described (Benbouzid et al., 2008c; Yalcin et al., 2014). Before surgeries, mice were anesthetized with ketamine (Vibrac, Carros, France)/xylazine (Rompun, Kiel, Germany; 100/10 mg/kg, i.p.). The common branch of the right sciatic nerve was exposed, and a cuff of PE-20 polyethylene tubing (Harvard Apparatus, Les Ulis, France) of standardized length (2 mm) was unilaterally inserted around it (Cuff group). Sham-operated animals underwent the same surgical procedure without cuff implantation (Sham group). Animals were placed on their left side in a clean home cage immediately after surgery and kept under the heat lamp until they awoke. Water and chow were placed directly in the home cage. The surgical site was checked daily during the next 3 days, and animals were monitored for signs of unusual suffering or infection with endpoints defined in agreement with the recommendations of the ethical committee.
Assessment of Mechanical Allodynia
Mechanical allodynia was tested using von Frey filaments and results were expressed in grams as described in Yalcin et al. (2014). Briefly, calibrated von Frey filaments (Bioseb, Vitrolles, France) were applied to the plantar surface of each hind paw until they just bent, in a series of ascending forces up to the mechanical threshold. Filaments were tested five times per paw and the paw withdrawal threshold (PWT) was defined as the lower of two consecutive filaments for which three or more withdrawals out of the five trials were observed (Yalcin et al., 2014).
Treatment Procedures
Treatment with the β2-adrenergic agonist formoterol began 4 weeks after the surgical procedure and lasted 4 weeks. Formoterol (Cat. Nr BG0369, Biotrend AG, Switzerland) was delivered by ad libitum access and as sole source of fluid dissolved in drinking water at a dose of 0.5 μg/ml (equivalent to 0.05 mg/kg/day) with 0.2% saccharin (Cat. Nr S1002, Sigma Aldrich, St Louis, MO, USA). Experimental groups for DOPeGFP mice included the Sham group (n = 36, 29 females and seven males), Cuff group (n = 29, 16 females and 13 males), and Formoterol group corresponding to Cuff mice treated with formoterol (n = 23, 14 females and nine males). Experimental groups for DOP cKO mice included Sham group (n = 6, two females and four males), Cuff group (n = 5 males), and Formoterol group corresponding to Cuff mice treated with formoterol (n = 11, seven females and four males). Experimental groups for control littermates Oprd1fl/fl (floxed mice) included Sham group (n = 7, five females and two males), Cuff group (n = 5 males), and Formoterol group corresponding to Cuff mice treated with formoterol (n = 8, two females and six males). Sham and Cuff groups both received control saccharin solution 0.2% in drinking water. Sham and Cuff groups were identical to those published previously in Ceredig et al. (2018).
Tissue Preparation and Immunohistochemistry
Mice were anesthetized with ketamine (Vibrac, Carros, France)/xylazine (Rompun, Kiel, Germany; 100/10 mg/kg, i.p.) and perfused intracardially with 100 ml of ice-cold (2–4°C) 4% paraformaldehyde (PFA) in phosphate buffer 0.1 M pH 7.4 (PB). Ipsilateral (right) and contralateral (left) L4 to L6 lumbar DRG were dissected out and post-fixed for 90–120 mins at 4°C in 4% PFA in PB, cryoprotected at 4°C in 30% sucrose in PB for 24 h, embedded in OCT (Optimal Cutting Temperature medium, Thermo Fisher Scientific, Waltham, MA, USA), frozen and kept at −80°C. DRG longitudinal sections (16 μm thick) were cut with a cryostat (Microm Cryo-star HM560) and kept floating in PB.
Immunohistochemistry was performed according to standard protocols as previously described in Ceredig et al. (2018). Briefly, DRG sections were incubated for 1 h at room temperature (RT) in the blocking solution consisting of PB with 0.2% Tween 20 (PBT; Cat. Nr 85114, Thermo Fisher Scientific, Waltham, MA, USA), 3% normal goat serum (Invitrogen, Paisley, UK) and 3% donkey serum when needed (D9663 Sigma-Aldrich, St Quentin Fallavier, France). The sections were then incubated overnight at 4°C in the blocking solution with the appropriate primary antibodies: polyclonal rabbit anti-GFP (Cat. Nr A-11122, Invitrogen, dilution 1:1,000), sheep polyclonal anti-CGRP (Cat. Nr. AB 22560, Abcam, dilution 1:2,000). Three washes were performed with PBT before sections incubated for 2 h at RT in dim light with goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Cat. Nr A-11012, Molecular Probes, dilution 1:2,000) and donkey anti-sheep IgG conjugated with Alexa Fluor 594 (Cat. Nr A-11016, Molecular Probes, dilution 1:2,000). Following three washes with PBT, the sections were mounted with MOWIOL (Calbiochem, Darmstadt, Germany) and 4,6-diamino-phenylindole (DAPI; Roche Diagnostic, Mannheim, Germany; 0.5 μg/ml).
Plantar skin of both hind paws (footpad and glabrous skin, 1 cm long) were fixed at 4°C in 4% PFA solution overnight, cryoprotected overnight with 30% sucrose in PB, embedded in OCT, frozen and kept at −80°C. Longitudinal cross-sections (50 μm thickness) were cut with a cryostat (MicromCryo-star HM560) and kept floating in PB. Paw tissue samples were then processed to visualize primary afferent terminals in the skin of the hind paw as previously described in Ceredig et al. (2018). Briefly, sections were treated with 0.3% H2O2, dehydrated with successive baths in ethanol then rehydrated, washed 3 times with PBS and incubated in blocking solution (PBS, 0.5% Triton X100 (PBST) with 3% normal goat serum or normal donkey serum) for 30 min at RT. After overnight incubation at 4°C in the blocking solution, the sections were incubated with the primary antibodies against anti-GFP (1:1,000) or anti-CGRP (1:2,000) antibody. The sections were then washed three times with PBST, respectively incubated for 2 h at RT with anti-rabbit or anti-goat biotinylated secondary antibody (1:400) in PBST and washed again three times with PBST before staining with Vector SG (Sk-4700, VectorLab). Samples were mounted with MOWIOL.
Image Acquisition and Analysis
Image acquisition and analysis were performed as previously described in Ceredig et al. (2018). Briefly, images were acquired with the Leica TCS SP5 confocal microscope using a 20× dry objective (Numerical Aperture: 0.7), the 40× resolution was achieved with a digital zoom factor. Confocal acquisitions in the sequential mode (single excitation beams: 405, 488 and 568 nm) were used for marker co-localization to avoid potential crosstalk between the different fluorescence emissions. Images were acquired with the LCS (Leica) software using randomly selected sections.
The ImageJ® software cell counter (approximately 15 non-adjacent sections per condition and per animal) was used to count on-screen neurons expressing a given fluorescent marker manually and blindly. Threshold was applied to fluorescence detection. Only neurons from L4-L6 DRGs with a visible nucleus were considered. Cells expressing a given marker and eGFP fluorescence were analyzed separately. During the analysis, we recorded all cross-sectional areas of cell profiles for each marker. No difference was observed in the distribution of the neuronal populations between male and female mice and data were pooled for subsequent analysis.
DOPeGFP subcellular distribution was expressed as a ratio of membrane-associated vs. cytoplasmic fluorescence densities determined as previously described (Erbs et al., 2016). Acquisitions using 63× (NA: 1.4) oil objective were performed to determine DOPeGFP subcellular distribution. Briefly, quantification of internalization was performed using the ImageJ software on 8-bit raw confocal images from neurons randomly sampled. Nuclear fluorescence was used to define the background level (no threshold was applied). Cytosolic fluorescence intensity was subtracted from whole-cell fluorescence intensity to obtain surface fluorescence intensity. Fluorescence intensity values were divided per surface unit (pixel) to obtain densities. Ratio of membrane-associated (Df memb) vs. cytoplasmic (Df cyto) fluorescence densities was calculated to normalize data across neurons examined. A value of 1.0 results from equal densities of DOPeGFP at the cell surface and in the cytoplasm.
Free nerve endings in the glabrous part of the skin were visualized using a 20× dry objective (Nikon Eclipse 80i). Counting on blinded samples was performed manually on screen using the Neurolucida software (V.10 MBF Bioscience) on at least four randomly chosen sections per animal. Density was obtained by dividing the number of afferents crossing the dermal-epidermal junction excluding secondary branching, by the total length of the section (Lauria et al., 2005).
Statistical Analysis
Behavioral analysis of von Frey testing was performed using Statistica v12 (StatSoft, France) and Graph-Pad Prism v7 (GraphPad, San Diego, CA, USA). Changes in the PWT, as a function of post-operative time (within factor) and experimental treatment (between factor), were analyzed using two-way ANOVA with repeated measures (two-way rANOVA) analysis followed by Tukey HSD post hoc test. Baseline PWT in males and females were compared using a two-sample student’s t-test. Exact p-values below 0.0001 were not provided for behavioral data (Graph-Pad Prism v7, GraphPad, San Diego, CA, USA). For cross-sectional area measurements, data were pooled per treatment group for each marker (CGRP or eGFP). In all groups, cross-sectional areas were found not normally distributed (p-value always < 10−8, Shapiro–Wilk test) using R (R Core Team, 2017). Sums of Gaussian functions were therefore adapted to the relative cumulative distribution curve of cell size, using non-linear least-square curve fitting (with nls2, nlstools and pracma R packages). Data were expressed as cumulative distributions to allow direct determination of the mean and standard error by adjusting the function of repartition on the experimental points. We compared the cumulative distributions for the various groups using the non-parametric Kolmogorov–Smirnov test (R). Treatment impact in DOPeGFP+ bins was analyzed using multiple t-tests. Statistical analysis of DOPeGFP subcellular distribution and skin fiber was performed with one-way ANOVA followed by Tuckey HSD post hoc test (Graph-Pad Prism v7, GraphPad, San Diego, CA, USA). Co-localization of DOPeGFP with neuronal markers in small size neurons (<300 μm2) was analyzed using the non-parametric Kruskal–Wallis test followed by Dunn’s post hoc test.
Results
Chronic Formoterol Requires DOP Receptors Expressed in Nav1.8+ Neurons for Anti-allodynic Action
We previously established that mechanical allodynia is induced by cuff-implantation, developed directly after surgery and was maintained until up to 12–14 weeks (Yalcin et al., 2011). Also, DOP receptors expressed in Nav1.8+ neurons were shown to be mandatory for the anti-allodynic action of the antidepressant duloxetine in the cuff model (Ceredig et al., 2018). Since DOP receptors are also required for the anti-allodynic effect of a chronic treatment with the β2 agonist clenbuterol (Yalcin et al., 2010), we investigated whether the specific deletion of DOP receptors in primary afferents (peripheral DRG neurons) expressing Nav1.8 voltage-gated sodium channels (DOPcKO; Gaveriaux-Ruff et al., 2011) was also abolishing the antiallodynic action of a β2-adrenergic agonist.
Von Frey testing revealed that sciatic nerve cuffing resulted in unilateral mechanical allodynia in DOPcKO and control floxed (DOPfl/fl) animals (Figures 1A,B) as previously reported (Ceredig et al., 2018). Cuff DOPcKO mice did not recover after formoterol treatment in drinking water (50 μg/ml; two-way rANOVA, interaction treatment × time F(12,114) = 11.31, p < 0.0001, effect of time F(6,114) = 33.37, p < 0.0001, effect of treatment F(2,19) = 112.8, p < 0.0001, Tukey HSD post hoc test: formoterol treatment from day 28 to 39 vs. Sham, p < 0.0001 at each time-point; Figure 1A), whereas control DOPfl/fl Cuff animals treated with formoterol returned to baseline values at day 39, after 11 days of formoterol administration (two-way rANOVA interaction treatment × time F(12,119) = 8.386, p < 0.0001, effect of time F(6,119) = 18.56, p < 0.0001, effect of treatment F(2,119) = 84.47, p < 0.0001, Tukey HSD post hoc test: formoterol treatment vs. Sham: p < 0.0001 from day 28 to 32, p < 0.01 from day 33 to day 38, p = 0.33 on day 39; Figure 1B). Our result therefore established that DOP receptors in Nav1.8 positive neurons were mandatory to alleviate mechanical allodynia upon chronic treatment with the β2 mimetic formoterol. We thus assessed in more detail the impact of chronic formoterol treatment on neuronal populations expressing the DOP receptor using the DOPeGFP fluorescent knock-in mouse line.
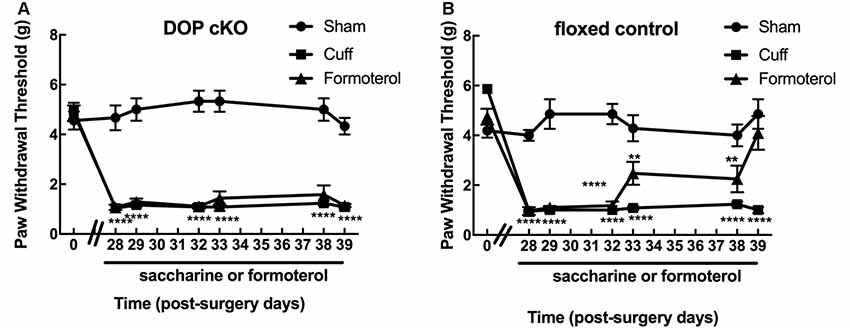
Figure 1. Expression of delta-opioid (DOP) receptors in Nav1.8+ neurons was mandatory for oral formoterol anti-allodynic action. The right (ipsilateral) hind paw mechanical threshold was tested using von Frey calibrated filaments. Following cuff implantation surgery, animals had lowered paw withdrawal thresholds (PWT), displaying sustained mechanical allodynia. Formoterol (0.5 μg/ml) or saccharin 0.2% control per os treatments started 4 weeks after nerve injury and were maintained for 3 weeks. (A) Mechanical threshold of the right (ipsilateral) hind paw in DOP cKO mice. Data from three separate experiments (each including the three conditions) were pooled and are expressed as means ± SEM. Sham (n = 6), Cuff (n = 5), Cuff treated with formoterol (n = 11). Two-way rANOVA and Tukey HSD post hoc test: ****p < 0.001 formoterol treatment vs. baseline. (B) Mechanical threshold of the right (ipsilateral) hind paw in control floxed mice. Data from three separate experiments (each including the three conditions) were pooled and are expressed as means ± SEM. Sham (n = 7), Cuff (n = 5), Cuff treated with formoterol (n = 8). Two-way rANOVA and Tukey HSD post hoc test: ****p < 0.0001, **p < 0.01 formoterol treatment day 39 vs. baseline: p = 0.3284.
Chronic Formoterol Alleviates Cuff-Induced Mechanical Allodynia in DOPeGFP Mice
We first verified that oral administration of the β2 adrenergic agonist-induced the expected anti-allodynic effect in the DOPeGFP knock-in mouse line. As previously reported (Ceredig et al., 2018), males had significantly higher baseline mechanical thresholds compared to females (5.4 ± 0.4 g for males vs. 3.5 ± 0.2 g for females, student’s t-test for baseline values: t = 7.18 p < 0.0001) and cuff implantation induced ipsilateral mechanical allodynia which lasted for at least 8 weeks (time of perfusion) in either sex (two-way rANOVA; males interaction treatment × time F(16,208) = 15.15, p < 0.0001; effect of treatment, F(2,26) = 318.5, p < 0.0001 from day 21 to 57 Cuff vs. Sham; females interaction treatment × time F(16,448) = 11.02, p < 0.0001, effect of treatment F(2,56) = 236, p < 0.0001 from day 21 to 57 Cuff vs. Sham; Figures 2A,B). We did not detect any change in the nociceptive threshold after sham surgery or in the contralateral hind paw of Cuff animals (data not shown).
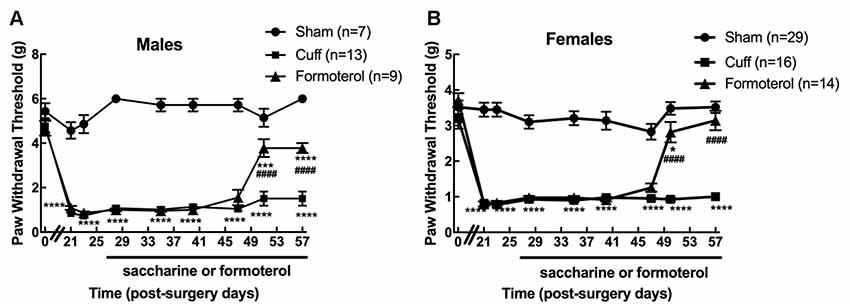
Figure 2. Chronic formoterol treatment per os relieved mechanical allodynia in DOPeGFP knock-in mice. The right (ipsilateral) hind paw mechanical threshold was tested using von Frey calibrated filaments in male and female DOPeGFP KI mice. Males (A) and females (B) had lowered PWT following cuff implantation surgery, i.e., sustained mechanical allodynia. Four weeks after nerve injury, formoterol (0.5 μg/ml) or saccharin 0.2% control per os treatments started and were maintained for 4 weeks. Data from three separate experiments (each including the three conditions) were pooled and are expressed as means ± SEM. Sham (n = 7 males and 29 females), Cuff (n = 13 males and 16 females), Cuff treated with formoterol (n = 9 males and 14 females). Two-way rANOVA and Tukey HSD post hoc test: *p < 0.05, ***p < 0.001, ****p < 0.0001 vs. Sham. #### p < 0.001 vs. Cuff.
Formoterol treatment in drinking water (50 μg/ml) relieved mechanical allodynia at day 51 (following 23 days administration) in DOPeGFP males although not fully (two-way rANOVA Tukey HSD post hoc test: day 51 vs. Sham: p(Males) = 0.0009 and day 57 vs. Sham: p(Males) < 0.0001, day 51 and day 57 vs. Cuff: p(Males) < 0.0001; Figure 2A). In DOPeGFP females, formoterol treatment-induced almost complete relief at day 51 (two-way rANOVA Tukey HSD post hoc test: day 51 vs. Sham: p(Females) = 0.0382) and values were similar to sham mice at day 57 (two-way rANOVA Tukey HSD post hoc test: day 57 vs. Sham: p(Females) = 0.3547, day 51 and day 57 vs. Cuff: p(Females) < 0.0001; Figure 2B). This time course was similar to what we previously observed, in the same model, in male C57BL6/J mice repeatedly injected i.p. twice daily (Yalcin et al., 2010).
DOPeGFP Expression in Formoterol-Treated Mice
We then examined changes in the distribution of DOPeGFP+ neurons in the DRG (Figure 3A). As previously established (Ceredig et al., 2018), the cumulative distribution of DOPeGFP+ cells in the Cuff experimental groups was shifted towards larger cell size values compared to the Sham group (Figure 3B). The loss of DOPeGFP+ neurons with small cross-sectional areas (Multiple t-tests: Cuff vs. Sham: p = 0.03 for the 100–200 μm2 category) indicated a loss of DOPeGFP expression in small and/or medium neurons following 8 weeks of neuropathy (Figure 3C).
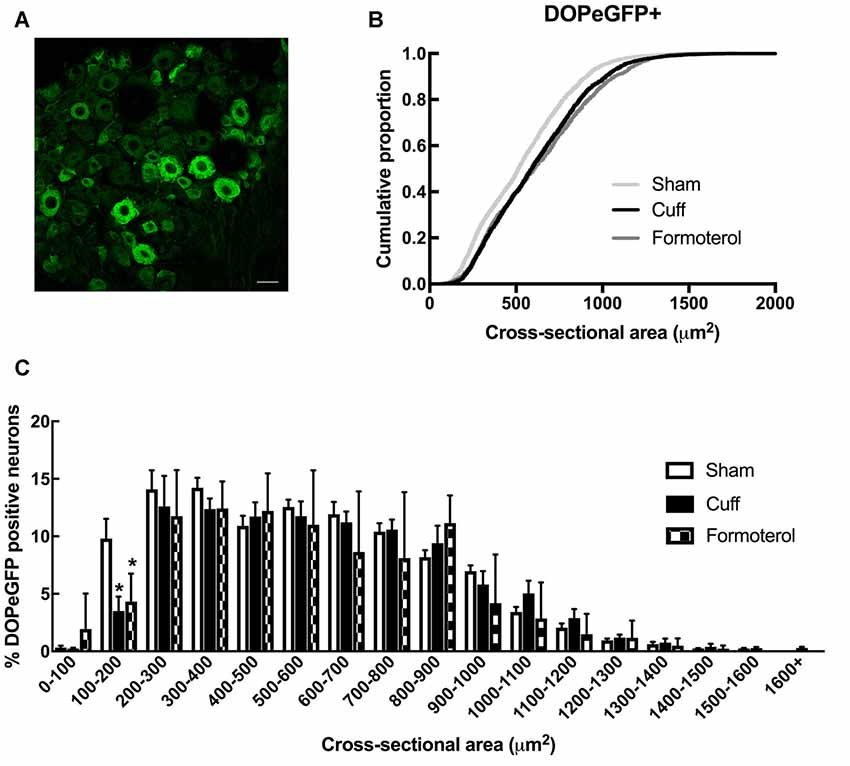
Figure 3. DOPeGFP distribution in Sham, Cuff and Formoterol-treated animals. (A) Representative confocal image of fluorescent DOPeGFP expressing neurons in lumbar dorsal root ganglia (DRG) of sham animals. Scale bar 10 μm. (B) Distribution of DOPeGFP+ neuronal populations in Sham (3,080 neurons, n = 7 animals; light gray), Cuff (3,123 neurons, n = 6 animals; black) and Formoterol (1,917 neurons, n = 5 animals; dark gray) groups. (C) Categorical data plot of the size distribution for DOPeGFP positive neuron cross-sectional areas in Sham (white bars), Cuff (black bars) and Formoterol (checked bars) groups. Multiple t-tests: *p = 0.03 Cuff vs. Sham, *p = 0.01 Formoterol vs. Sham.
In Cuff mice chronically treated with formoterol, the distribution of the DOPeGFP+ neuronal populations remained very similar to the Cuff condition and was significantly different from the Sham group (KS test: D = 0.14212, p < 2.2 10−16; Figure 3B). Indeed, the percentage of small size neurons remained lower compared to the Sham condition (Multiple t-tests: Formoterol vs. Sham: p = 0.0137 for the 100–200 μm2 category; Figure 3C).
Since DOPeGFP expression was decreased in unmyelinated peptidergic populations in Cuff mice (Figure 3) and (Ceredig et al., 2018), we assessed the consequence of chronic formoterol treatment on the global CGRP+ population and on the proportion of the CGRP+ population also expressing DOPeGFP (Figure 4). The cumulative distribution corresponding to CGRP+ neurons showed that the shift towards larger cell size values in the Cuff group is no longer present for the smallest cross-sectional areas after treatment with formoterol although the overall distribution remained significantly distinct from the Sham group (KS test: D = 0.090945, p = 3.398 10−7; Figure 4B). However, the cumulative distribution of the CGRP+DOPeGFP+ neurons remained similar to the cuff condition and was significantly different from the Sham condition (KS test: D = 0.15672, p < 2.2 10−16; Figure 4C) with the proportion of small size neurons (<300 μm2; 6.7 ± 0.8%) remaining similar to the Cuff condition (4.3 ± 0.5%) and lower compared to the Sham condition (16.5 ± 1.3%; KW test: p < 0.0001, Dunn’s post hoc test Sham vs. Cuff p = 0.0007, Sham vs. Formoterol p = 0.0352, Cuff vs. Formoterol p > 0.9999; Figure 4D). Therefore, chronic treatment with formoterol did not appear to restore the loss of DOPeGFP+ neurons induced by the neuropathic condition in small peptidergic neurons.
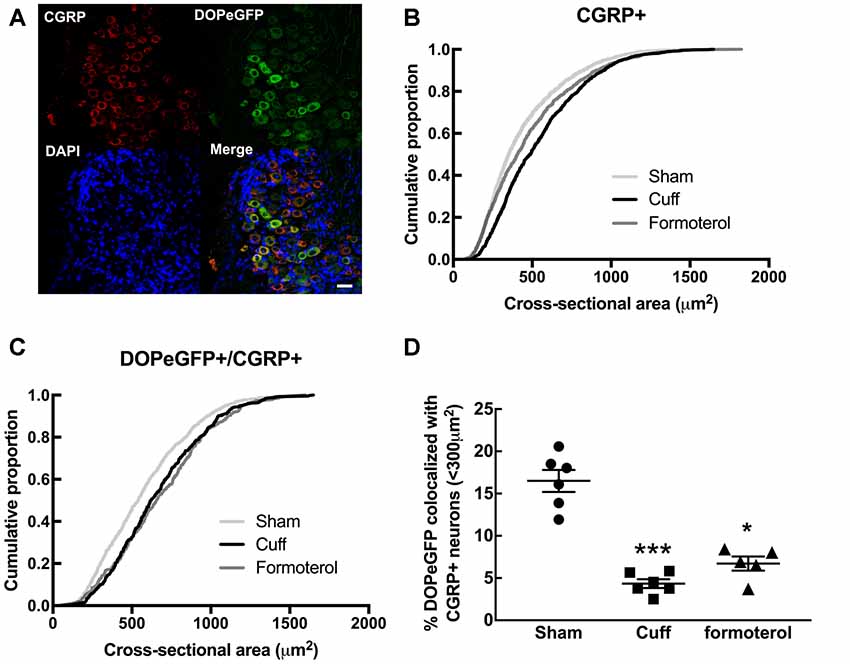
Figure 4. Impact of formoterol treatment on DOPeGFP colocalization with CGRP+ populations. (A) Representative fluorescence micrographs showing DOPeGFP co-localization with the neuronal marker calcitonin gene-related peptide (CGRP; red) as indicated by arrows in the overlay figure. Nuclei are stained with DAPI (blue). Scale bar 10 μm. (B) Distribution of CGRP+ neuronal populations in Sham (3,351 neurons, n = 7 animals; light gray), Cuff (1,331 neurons, n = 6 animals; black), and Formoterol (1,311 neurons, n = 5 animals; dark gray). (C) Distribution of neuronal populations co-expressing DOPeGFP and CGRP in Sham (803 neurons, n = 7 animals; light gray), Cuff (438 neurons, n = 6 animals; black) and Formoterol (294 neurons, n = 5 animals; dark gray) groups. (D) Percentage of cells co-expressing DOPeGFP and CGRP in neurons with areas <300 μm2 in Sham (n = 6; ●), Cuff (n = 6; ▪) or Formoterol (n = 5; ▴) animals. Values expressed as mean ± SEM. Kruskal–Wallis test and Dunn’s post-test: *p < 0.05, ***p < 0.001 vs. Sham.
DOPeGFP Expression at the Plasma Membrane in Formoterol-Treated Mice
Enhanced DOP expression at the plasma membrane was previously described in neuropathic conditions (Gendron et al., 2015). This observation was confirmed in the cuff model by showing that the ratio of fluorescence associated with the cell surface was significantly increased compared to the fluorescence associated with the intracellular compartments in the DOPeGFP+ neurons (Figure 5) as also previously reported (Ceredig et al., 2018). Formoterol treatment decreased membrane-associated fluorescence to values which were even lower than those of neurons in the Sham condition (Sham 1.16 ± 0.03, Cuff: 1.35 ± 0.04, formoterol 0.94 ± 0.05, one-way ANOVA F(2,89 = 28.8 p < 0.0001 Tukey HSD post hoc test Cuff vs. Sham p = 0.002, Cuff vs. Formoterol p < 0.0001, Sham vs. Formoterol p = 0.0002; Figure 5B). This indicates that treatment with formoterol suppressed the increase in DOP receptor surface expression observed in neuropathic mice.
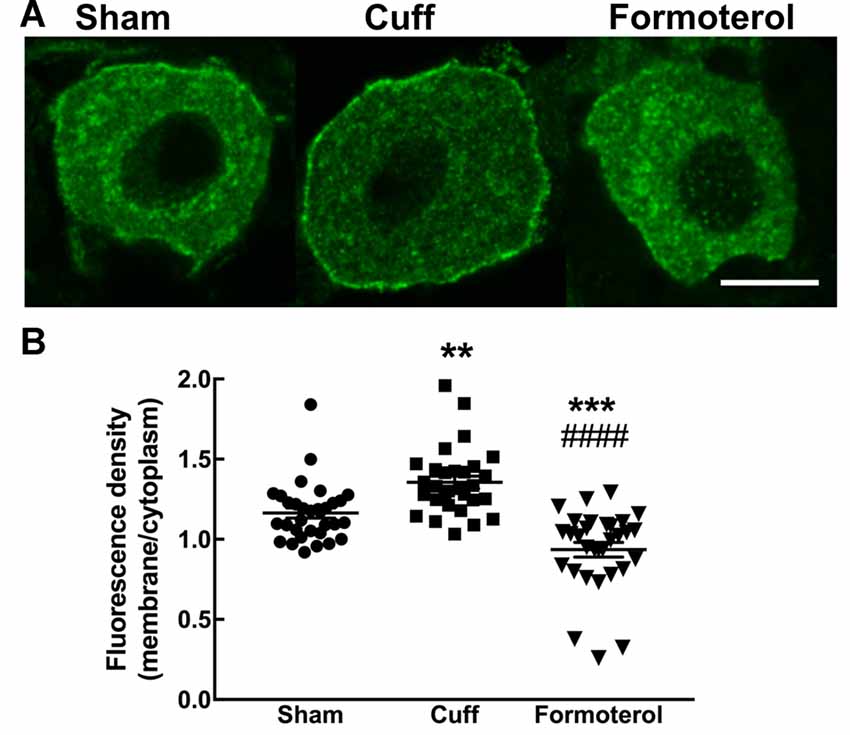
Figure 5. DOPeGFP expression at the cell surface in Formoterol-treated mice. (A) Representative fluorescence micrographs of DOPeGFP-positive neuron in Sham, Cuff and cuff animals treated with formoterol. Scale bar 10 μm. (B) DOPeGFP subcellular distribution was increased in neuropathic conditions and reduced after formoterol treatment compared to Sham. Data are expressed as means ± SEM (Sham: n = 32 cells from four animals, Cuff: n = 30 cells from three animals, Formoterol: n = 30 cells from three animals). One-way ANOVA and Tukey HSD post hoc test: **p < 0.01, ***p < 0.001 vs. Sham and #### p < 0.0001 vs. Cuff, p > 0.5 Formoterol vs. Cuff.
CGRP and DOPeGFP Expression in the Skin of Formoterol Treated Mice
A decrease in CGRP+ intra-epidermal nerve fiber (IENF) fiber length and density, as well as decrease in DOPeGFP+ IENF density, was reported 8 weeks post cuff surgery (Nascimento et al., 2015; Ceredig et al., 2018). We thus assessed whether treatment with formoterol impacted CGRP+ and DOPeGFP+ IENFs (Figure 6). The density of CGRP+ IENFs in the glabrous skin of the hind paw of cuffed mice treated with formoterol remained at a level comparable to the Cuff group (one-way ANOVA F(2,8) = 49.15, p < 0.0001; Tukey HSD post hoc test Sham vs. Cuff p < 0.0001, Sham vs. Formoterol p < 0.0001, Cuff vs. Formoterol p = 0.771; Figure 6B). The density of DOPeGFP+ IENFs was higher in formoterol treated animals compared to the Cuff group but remained significantly lower compared to the Sham condition [one-way ANOVA F(2,9) = 371, p < 0.0001; Tukey HSD post hoc test Sham vs. Cuff p < 0.0001, Sham vs. Formoterol p < 0.0001, Cuff vs. Formoterol p = 0.0008 (Figure 6C)]. As a whole, chronic formoterol treatment did not restore CGRP+ expression and induced partial recovery of DOPeGFP expression in the IENFs.
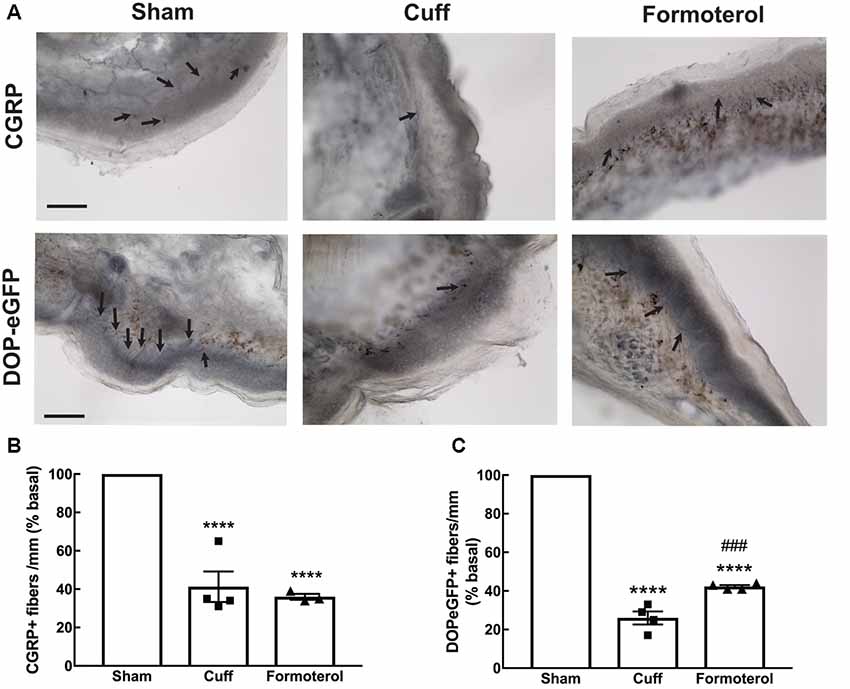
Figure 6. Density of CGRP+ and DOPeGFP+ free nerve endings in the skin. Representative micrograph of (A) CGRP or DOPeGFP intra-epidermal nerve fiber (IENF) labeling in the skin of Sham, Cuff and cuff animals treated with formoterol (black arrows). Scale bar 10 μm. (B) The density of CGRP+ free nerve endings in the glabrous skin of the right hind paw was decreased in Cuff animals and Cuff animals treated with formoterol. Data are expressed as means ± SEM, n = 4 mice for Sham and Cuff and n = 3 for Formoterol. One-way ANOVA and Tukey HSD post hoc test: ****p < 0.0001 vs. Sham. (C) The density of DOPeGFP+ free nerve endings in the glabrous skin of the right hind paw was decreased in Cuff animals and only partially restored in Cuff animals treated with formoterol compared to Sham. Data are expressed as means ± SEM, n = 4 mice per condition. One-way ANOVA and Tukey HSD post hoc test: ****p < 0.0001 vs. Sham, ### p < 0.001 vs. Cuff.
Discussion
We first showed that oral administration of the β2 adrenergic agonist formoterol was as effective as its intraperitoneal injection (Yalcin et al., 2010) to alleviate mechanical allodynia in the cuff model of neuropathy. We then identified peripheral DOP receptors as mandatory for the antiallodynic effect and determined the impact of chronic formoterol on the expression of DOP receptors in the lumbar DRGs and skin IENFs.
Several studies pointed to the role of DOP receptors, and more specifically peripheral DOP receptors present in Nav1.8+ neurons, to counteract mechanical allodynia in neuropathic conditions induced by sciatic nerve ligation (Nadal et al., 2006; Gaveriaux-Ruff et al., 2011; Nozaki et al., 2012). Previous work by the group established that the antiallodynic action of chronic tricyclic antidepressant and SNRI treatments require DOP and β2 adrenergic receptors (Yalcin et al., 2010; Choucair-Jaafar et al., 2014; Kremer et al., 2018). Systemic administration of the DOP antagonist naltrindole also blocked the antiallodynic action of chronic administration of β2 adrenergic agonists (Yalcin et al., 2010; Choucair-Jaafar et al., 2014). In the case of the SNRI duloxetine, peripheral DOP receptors expressed in Nav1.8+ neurons were mandatory for the antiallodynic effect as no recovery was observed in the DOP cKO mouse line in which peripheral DOP receptors were selectively ablated in neurons expressing the Nav1.8 sodium channel (Ceredig et al., 2018). Here, we showed that peripheral DOP receptors expressed in Nav1.8+ neurons were also mandatory for the β2-adrenergic agonist formoterol to alleviate mechanical allodynia. Our results, therefore, establish that peripheral DOP receptors, likely located on C nociceptors, are necessary for the effective antiallodynic effect of the two chronic treatments. They also suggest that DOP receptor expression is modulated by the noradrenergic component of SNRIs.
In our previous work, we have characterized changes in DRG neuronal populations in the neuropathic condition resulting from sciatic nerve cuffing (Ceredig et al., 2018). Our main findings highlighted a decrease in the proportion of small size peptidergic neurons (≤300 μm2) and IENFs expressing DOPeGFP 8 weeks after cuff surgery as well as increased DOPeGFP expression at the plasma membrane (Ceredig et al., 2018), suggesting that these changes may contribute to control mechanical nociception. Here, we showed that chronic formoterol administration promoted partial recovery of DOPeGFP expression in free nerve endings in the skin but not in small CGRP+ neurons in the DRGs. This contrasts with the higher DOPeGFP expression found in small unmyelinated peptidergic neurons (≤300 μm2) following chronic duloxetine administration. The reason for this difference is unknown but it could correspond to the use of a suboptimal dose of formoterol as also suggested by the dose-response curve performed using intraperitoneal injections (Yalcin et al., 2010). It is indeed unlikely to depend on the serotoninergic component of the SNRI action since selective serotonin reuptake inhibitors did not alleviate mechanical allodynia (Benbouzid et al., 2008a). Our results, however, confirmed that DOP expression in the nerve terminals, likely unmyelinated peripheral axons of C mechanonociceptors (Brederson and Honda, 2015) appeared a primary determinant of mechanical sensitivity and might constitute a valuable marker of the neuropathic state.
Chronic pain was shown to increase DOP receptor translocation to the plasma membrane in the DRGs (reviewed in Gendron et al., 2015) and our data showed a similar increase in DOPeGFP surface expression in the cuff model. Chronic formoterol was associated with low DOP receptor expression at the plasma membrane that was even below values observed in the sham condition. The low DOPeGFP expression at the surface was also observed in mice chronically treated with duloxetine (Ceredig et al., 2018) and, therefore, seems a common mechanism by which the two antiallodynic treatments counteract mechanical hypersensitivity. The underlying mechanisms, however, remain elusive. DOP and β2 adrenergic receptors have been proposed to form heteromers based on studies performed in co-transfected cells (Jordan et al., 2001). However, β2 adrenergic receptors are expressed in satellite cells in the DRGs (Bohren et al., 2013) whereas DOP dependent analgesia is mediated at the neuronal level (Gaveriaux-Ruff et al., 2011; Ceredig et al., 2018; this work) and is independent of microglial activation (Mika et al., 2014) which precludes direct molecular interactions. The delayed antiallodynic action of both formoterol and duloxetine suggests that it requires cellular adaptations to take place. Lesion of peripheral noradrenergic fibers with guanethidine, a toxin that does not cross the blood brain barrier abolished the antiallodynic action of duloxetine (Kremer et al., 2018) supporting common involvement of peripheral β2 adrenergic receptors. Formoterol (Bohren et al., 2013) as well as antidepressants (Kremer et al., 2018) counteracted the increase in TNFα associated with neuropathic pain and downregulated the activity of the glial NFκB-TNFα pathway, a key regulator of proinflammatory cytokine production (Leung and Cahill, 2010). However, this anti-neuroinflammatory action is also shared by gabapentinoids that do not need opioid receptors for their antiallodynic action (Kremer et al., 2016b). This rather supports a view in which the anti-neuroinflammatory effect and the modulation of DOP expression and activity are not directly related.
There are few clues as to which DOP-dependent mechanisms mediate the anti-allodynic action following formoterol or SNRI chronic treatment. Current data point to an indirect effect through increased endogenous opioid peptide release by the native and adaptative immune systems, which can activate neuronal opioid receptors to alleviate mechanical allodynia (Binder et al., 2004; Celik et al., 2016). Indeed, noradrenergic sprouting consequent to nerve injury would activate the β2-adrenoceptors expressed by immune cells and promote the release of enkephalin, dynorphin and β-endorphin by these cells (Binder et al., 2004; Celik et al., 2016; Pannell et al., 2016). Similarly, enkephalins are also present in the skin (Slominski et al., 2011). Moreover, sympathetic fiber sprouting is known to take place in the skin in the cuff model (Nascimento et al., 2015) and is located close to cells expressing β2 adrenergic receptors and β-endorphin in inflamed paw tissue providing a way to activate not only MOP but also DOP receptors (Binder et al., 2004). Activation of DOP receptors could, in turn, induce pain relief by reducing Nav1.8 channel activity via inhibition of p38 mitogen-activated protein kinase as described for Nav1.7 channels in a rat model of diabetic neuropathy (Chattopadhyay et al., 2008).
In summary, our work established that DOP receptors expressed in Nav1.8+ neurons were mandatory for the anti-allodynic action of chronic treatment with the β2 adrenergic agonist formoterol. It also revealed that chronic formoterol partially reversed the loss of peripheral DOP receptors in the skin and counteracted enhanced DOP expression at the plasma membrane. Our study thus adds to current literature pointing to potential interest in repositioning β2 adrenergic agonists for the treatment of neuropathic pain.
Data Availability Statement
Images and raw data will be available upon request to the corresponding author.
Ethics Statement
All experiments were approved by the “Comité d’Ethique en Matière d’Expérimentation Animale de Strasbourg” [authorization number 201503041113547 (APAFIS#300).02] and conducted in agreement with the EU Directive 2010/63/EU for animal experiments.
Author Contributions
RC, IY, CG-R, MB, ES, and DM designed experiments. RC, FP, SD, UA, PH, IY, and DM performed experiments. RC, UA, FP, and DM analyzed the data. RC, IY, CG-R, MB, and DM wrote the manuscript.
Funding
This work was supported by Centre National de la Recherche Scientifique (CNRS), University of Strasbourg (Université de Strasbourg), and Institut National de la Santé et de la Recherche Médicale (INSERM; contracts UPR3212, UMS3415, UMR7104 and U964) and by the European Union Seventh Framework Programme FP7-Health-2013-Innovation (grant 1602919). RC was funded by the Ministère de l’Enseignement Supérieur et de la Recherche et de l’Innovation and the Fondation pour la Recherche Médicale (DPA20140629804).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Chronobiotron animal facility (UMS 3415 CNRS), and the imaging Platform of the Institut des Neurosciences Cellulaires et Intégratives (UPS 3156 CNRS) for their assistance. We are also thankful to the Institut de la Clinique de la Souris (Illkirch, France) animal facility, Anne Robé and David Reiss at IGBMC for breeding and genotyping of Nav1.8 + DOP cKO mouse line. They would like to acknowledge Dr. Jean-Luc Rodeau for his instrumental help with the statistical analysis, Prof. Nelly Boehm, Prof. Remy Schlichter and Dr. Sylvain Hugel for valuable scientific discussions, and Elisabeth Waltisperger for excellent technical contribution.
Abbreviations
ANOVA, analysis of variance; CGRP, calcitonin gene-related peptide; cKO, conditional knockout; DOP, delta-opioid; DRG, dorsal root ganglion; eGFP, enhanced green fluorescent protein; IENF, intraepidermal nerve fiber; KS, Kolmogorov–Smirnov; KW, Kruskal–Wallis; PWT, paw withdrawal threshold; SNRI, serotonin noradrenaline reuptake inhibitor.
References
Benbouzid, M., Choucair-Jaafar, N., Yalcin, I., Waltisperger, E., Muller, A., Freund-Mercier, M. J., et al. (2008a). Chronic, but not acute, tricyclic antidepressant treatment alleviates neuropathic allodynia after sciatic nerve cuffing in mice. Eur. J. Pain 12, 1008–1017. doi: 10.1016/j.ejpain.2008.01.010
Benbouzid, M., Gaveriaux-Ruff, C., Yalcin, I., Waltisperger, E., Tessier, L. H., Muller, A., et al. (2008b). Delta-opioid receptors are critical for tricyclic antidepressant treatment of neuropathic allodynia. Biol. Psychiatry 63, 633–636. doi: 10.1016/j.biopsych.2007.06.016
Benbouzid, M., Pallage, V., Rajalu, M., Waltisperger, E., Doridot, S., Poisbeau, P., et al. (2008c). Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur. J. Pain 12, 591–599. doi: 10.1016/j.ejpain.2007.10.002
Binder, W., Mousa, S. A., Sitte, N., Kaiser, M., Stein, C., and Schafer, M. (2004). Sympathetic activation triggers endogenous opioid release and analgesia within peripheral inflamed tissue. Eur. J. Neurosci. 20, 92–100. doi: 10.1111/j.1460-9568.2004.03459.x
Bohren, Y., Tessier, L. H., Megat, S., Petitjean, H., Hugel, S., Daniel, D., et al. (2013). Antidepressants suppress neuropathic pain by a peripheral β2-adrenoceptor mediated anti-TNFα mechanism. Neurobiol. Dis. 60, 39–50. doi: 10.1016/j.nbd.2013.08.012
Brederson, J. D., and Honda, C. N. (2015). Primary afferent neurons express functional delta opioid receptors in inflamed skin. Brain Res. 1614, 105–111. doi: 10.1016/j.brainres.2015.04.023
Celik, M. Ö., Labuz, D., Henning, K., Busch-Dienstfertig, M., Gaveriaux-Ruff, C., Kieffer, B. L., et al. (2016). Leukocyte opioid receptors mediate analgesia via Ca2+-regulated release of opioid peptides. Brain Behav. Immun. 57, 227–242. doi: 10.1016/j.bbi.2016.04.018
Ceredig, R. A., Pierre, F., Doridot, S., Alduntzin, U., Salvat, E., Yalcin, I., et al. (2018). Peripheral delta opioid receptors mediate duloxetine anti-allodynic effect in a mouse model of neuropathic pain. Eur. J. Neurosci. 48, 2231–2246. doi: 10.1111/ejn.14093
Chattopadhyay, M., Mata, M., and Fink, D. J. (2008). Continuous δ-opioid receptor activation reduces neuronal voltage-gated sodium channel (NaV1.7) levels through activation of protein kinase C in painful diabetic neuropathy. J. Neurosci. 28, 6652–6658. doi: 10.1523/JNEUROSCI.5530-07.2008
Choucair-Jaafar, N., Salvat, E., Freund-Mercier, M. J., and Barrot, M. (2014). The antiallodynic action of nortriptyline and terbutaline is mediated by β2 adrenoceptors and δ opioid receptors in the ob/ob model of diabetic polyneuropathy. Brain Res. 1546, 18–26. doi: 10.1016/j.brainres.2013.12.016
Choucair-Jaafar, N., Yalcin, I., Rodeau, J. L., Waltisperger, E., Freund-Mercier, M. J., and Barrot, M. (2009). β2-adrenoceptor agonists alleviate neuropathic allodynia in mice after chronic treatment. Br. J. Pharmacol. 158, 1683–1694. doi: 10.1111/j.1476-5381.2009.00510.x
Erbs, E., Faget, L., Ceredig, R. A., Matifas, A., Vonesch, J. L., Kieffer, B. L., et al. (2016). Impact of chronic morphine on delta opioid receptor-expressing neurons in the mouse hippocampus. Neuroscience 313, 46–56. doi: 10.1016/j.neuroscience.2015.10.022
Gaveriaux-Ruff, C., Nozaki, C., Nadal, X., Hever, X. C., Weibel, R., Matifas, A., et al. (2011). Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain 152, 1238–1248. doi: 10.1016/j.pain.2010.12.031
Gendron, L., Mittal, N., Beaudry, H., and Walwyn, W. (2015). Recent advances on the δ opioid receptor: from trafficking to function. Br. J. Pharmacol. 172, 403–419. doi: 10.1111/bph.12706
Jordan, B. A., Trapaidze, N., Gomes, I., Nivarthi, R., and Devi, L. A. (2001). Oligomerization of opioid receptors with b2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc. Natl. Acad. Sci. U S A 98, 343–348. doi: 10.1073/pnas.011384898
Jourdain, M., and Hatakeyama, S. (2019). A novel tissue-selective β2-adrenoceptor agonist with minimized cardiovascular effects, 5-HOB, attenuates neuropathic pain in mice. BMC Res. Notes 12:413. doi: 10.1186/s13104-019-4466-y
Kremer, M., Salvat, E., Muller, A., Yalcin, I., and Barrot, M. (2016a). Antidepressants and gabapentinoids in neuropathic pain: mechanistic insights. Neuroscience 338, 183–206. doi: 10.1016/j.neuroscience.2016.06.057
Kremer, M., Yalcin, I., Nexon, L., Wurtz, X., Ceredig, R. A., Daniel, D., et al. (2016b). The antiallodynic action of pregabalin in neuropathic pain is independent from the opioid system. Mol. Pain 12:1744806916633477. doi: 10.1177/1744806916633477
Kremer, M., Yalcin, I., Goumon, Y., Wurtz, X., Nexon, L., Daniel, D., et al. (2018). A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J. Neurosci. 38, 9934–9954. doi: 10.1523/JNEUROSCI.1004-18.2018
Lauria, G., Cornblath, D. R., Johansson, O., McArthur, J. C., Mellgren, S. I., Nolano, M., et al. (2005). EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur. J. Neurol. 12, 747–758. doi: 10.1111/j.1468-1331.2005.01260.x
Leung, L., and Cahill, C. M. (2010). TNF-α and neuropathic pain—a review. J. Neuroinflammation 7:27. doi: 10.1186/1742-2094-7-27
Mika, J., Popiolek-Barczyk, K., Rojewska, E., Makuch, W., Starowicz, K., and Przewlocka, B. (2014). Delta-opioid receptor analgesia is independent of microglial activation in a rat model of neuropathic pain. PLoS One 9:e104420. doi: 10.1371/journal.pone.0104420
Nadal, X., Banos, J. E., Kieffer, B. L., and Maldonado, R. (2006). Neuropathic pain is enhanced in δ-opioid receptor knockout mice. Eur. J. Neurosci. 23, 830–834. doi: 10.1111/j.1460-9568.2006.04569.x
Nascimento, F. P., Magnussen, C., Yousefpour, N., and Ribeiro-da-Silva, A. (2015). Sympathetic fibre sprouting in the skin contributes to pain-related behaviour in spared nerve injury and cuff models of neuropathic pain. Mol. Pain 11:59. doi: 10.1186/s12990-015-0062-x
Nozaki, C., Le Bourdonnec, B., Reiss, D., Windh, R. T., Little, P. J., Dolle, R. E., et al. (2012). δ-Opioid mechanisms for ADL5747 and ADL5859 effects in mice: analgesia, locomotion, and receptor internalization. J. Pharmacol. Exp. Ther. 342, 799–807. doi: 10.1124/jpet.111.188987
Pannell, M., Labuz, D., Celik, M. Ö., Keye, J., Batra, A., Siegmund, B., et al. (2016). Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J. Neuroinflammation 13:262. doi: 10.1186/s12974-016-0735-z
R Core Team. (2017). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Salvat, E., Schweitzer, B., Massard, G., Meyer, N., de Blay, F., Muller, A., et al. (2015). Effects of β2 agonists on post-thoracotomy pain incidence. Eur. J. Pain 19, 1428–1436. doi: 10.1002/ejp.673
Scherrer, G., Tryoen-Tóth, P., Filliol, D., Matifas, A., Laustriat, D., Cao, Y. Q., et al. (2006). Knockin mice expressing fluorescent δ-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc. Natl. Acad. Sci. U S A 103, 9691–9696. doi: 10.1073/pnas.0603359103
Slominski, A. T., Zmijewski, M. A., Zbytek, B., Brozyna, A. A., Granese, J., Pisarchik, A., et al. (2011). Regulated proenkephalin expression in human skin and cultured skin cells. J. Invest. Dermatol. 131, 613–622. doi: 10.1038/jid.2010.376
Vanfleteren, L., Fabbri, L. M., Papi, A., Petruzzelli, S., and Celli, B. (2018). Triple therapy (ICS/LABA/LAMA) in COPD: time for a reappraisal. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 3971–3981. doi: 10.2147/copd.s185975
Vicario, N., Parenti, R., Arico, G., Turnaturi, R., Scoto, G. M., Chiechio, S., et al. (2016). Repeated activation of delta opiod receptors counteracts nerve injury-induced TNF-α up-regulation in the sciatic nerve of rats with neuropathic pain: a possible correlation with delta opiod receptors-mediated antiallodinic effect. Mol. Pain 12:1744806916667949. doi: 10.1177/1744806916667949
von Hehn, C. A., Baron, R., and Woolf, C. J. (2012). Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73, 638–652. doi: 10.1016/j.neuron.2012.02.008
Yalcin, I., Bohren, Y., Waltisperger, E., Sage-Ciocca, D., Yin, J. C., Freund-Mercier, M. J., et al. (2011). A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol. Psychiatry 70, 946–953. doi: 10.1016/j.biopsych.2011.07.017
Yalcin, I., Choucair-Jaafar, N., Benbouzid, M., Tessier, L. H., Muller, A., Hein, L., et al. (2009). β2-adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann. Neurol. 65, 218–225. doi: 10.1002/ana.21542
Yalcin, I., Megat, S., Barthas, F., Waltisperger, E., Kremer, M., Salvat, E., et al. (2014). The sciatic nerve cuffing model of neuropathic pain in mice. J. Vis. Exp. 89:e51608. doi: 10.3791/51608
Keywords: mechanical allodynia, beta-mimetics, peripheral nerve injury, cuff model, delta opioid receptor, beta adrenergic receptor
Citation: Ceredig RA, Pierre F, Doridot S, Alduntzin U, Hener P, Salvat E, Yalcin I, Gaveriaux-Ruff C, Barrot M and Massotte D (2020) Peripheral Delta Opioid Receptors Mediate Formoterol Anti-allodynic Effect in a Mouse Model of Neuropathic Pain. Front. Mol. Neurosci. 12:324. doi: 10.3389/fnmol.2019.00324
Received: 29 August 2019; Accepted: 17 December 2019;
Published: 14 February 2020.
Edited by:
Meritxell Canals, University of Nottingham, United KingdomReviewed by:
Jerome Busserolles, Clermont Université, FranceSangsu Bang, Duke University, United States
Copyright © 2020 Ceredig, Pierre, Doridot, Alduntzin, Hener, Salvat, Yalcin, Gaveriaux-Ruff, Barrot and Massotte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominique Massotte, d.massotte@unistra.fr
† Present address: Florian Pierre Institut Européen de Chimie et Biologie, Centre National de la Recherche Scientifique, INSERM, Université de Bordeaux, Pessac, France Unai Alduntzin Department of Biochemistry and Molecular Biology, Faculty of Science and Technology, University of the Basque Country, Leioa, Spain
 Rhian Alice Ceredig
Rhian Alice Ceredig Florian Pierre1†
Florian Pierre1†  Stéphane Doridot
Stéphane Doridot Unai Alduntzin
Unai Alduntzin Eric Salvat
Eric Salvat Ipek Yalcin
Ipek Yalcin Claire Gaveriaux-Ruff
Claire Gaveriaux-Ruff Dominique Massotte
Dominique Massotte