Sunflower Seed Husk as Promising By-Product for Soil Biodisinfestation Treatments and Fertility Improvement in Protected Lettuce Crop
- 1Department of Plant Production and Protection, NEIKER, Basque Institute for Agricultural Research and Development, Basque Research and Technology Alliance (BRTA), Derio, Spain
- 2Centro de Investigación Apícola y Agroambiental, Instituto Regional de Investigación y Desarrollo Agroalimentario y Forestal de Castilla-La Mancha (IRIAF), Marchamalo, Spain
One of the major challenges in biodisinfestation treatments against soilborne pathogens is the selection of the proper organic amendments and mixture features. The use of agro-industrial by-products is a sustainable alternative with proven efficacy, but the availability has to be considered in terms of location and quantity. Sunflower seed is one of the five major oil crops widely cultivated and the husk constitutes a significant part that is discarded. This by-product brings together the features to be considered an interesting organic amendment in agricultural soils because of its lignocellulose content, but no references have been found in this field. In this study, sunflower seed husk was used with fresh cow manure in biodisinfestation treatments, alone or combined with other by-products (rapeseed cake, beer bagasse and wheat bran). The assay was performed in summer in a commercial greenhouse with significant yield losses in lettuce crops caused by the root-knot nematode Meloidogyne incognita. Four different amendment mixtures were applied which included 3kg/m2 cow manure, as common waste, and 1 kg/m2 of by-products (dry weight), considering 6mgC/g soil in all treatments but different C/N ratio (23, 29, 31, 34) and by-products. Data was collected in three moments: (i) before and (ii) after biodisinfestation treatments and (iii) after harvesting the first crop after biodisinfestations. Crop damage was assessed through root galling index and the number of eggs in roots. The effects on the pathogen population and the whole soil nematode community were assessed along with some physicochemical and soil microbiological variables (respiration rate, microbial organic C, water-soluble organic C and physiological profile of heterotrophic bacteria through Biolog Ecoplates™). All treatments reported effectiveness in disease control without significant differences among them, but among times. However, soil temperatures during biodisinfestations were higher at higher C/N ratios and fertility variables also increased in these cases, mainly in the treatment with husk as the only by-product. Sunflower seed husk proved to be an interesting source of organic C to improve both biodisinfestation treatments and soil fertility in humid temperate climate zones.
Introduction
In the last decades, many pesticides have been banned for use worldwide because of human health and environmental concerns. Besides, farmers also deal with the problem of resistance development by the pathogens to the allowed pesticides. In fact, that was the reason why this study could be performed on an agricultural holding, none of the allowed pesticides for lettuce (Lactuca sativa) crop was effective against the root-knot nematode Meloidogyne incognita. Among the less harmful alternatives, soil biodisinfestation is one of the most effective and, at the same time, improves soil quality (Klein et al., 2012; Mocali et al., 2015). The term biodisinfestation includes all the different approaches that consist on the incorporation of organic matter and plastic film cover during, at least, 4 weeks. These approaches were named to emphasize the main desired effect, thus the following terms can be found: biosolarization (thermal effect), biofumigation (release of isothiocyanates) and anaerobic or reductive soil disinfestation (ASD or RSD, anaerobic conditions) (Katan, 2017; Rosskopf et al., 2020). However, we prefer the term biodisinfestation because many effects might converge in any of the approaches and this term is more inclusive.
Soil biodisinfestation is implemented in production systems in warm climate regions with high solar radiation, because of the proven efficacy of high temperatures against many soilborne pathogens (Katan and Gamliel, 2014). Although these treatments are mainly performed during hot seasons, ASD tries to compensate for the lack of lethal temperatures in temperate regions through soil saturation and high microbial activity (Blok et al., 2000; Shinmura, 2000). This last effect is mainly subject to the characteristics of the organic amendment. The concentration of C, N and their ratio in the final mixture seems to play an important role in treatment efficacy (Shrestha et al., 2021a; Testen et al., 2021). Besides, the type of C-source used might also condition the processes given during biodisinfestations. Labile C-sources are rapidly degraded by microorganism, whereas less labile or recalcitrant C takes more time to be decomposed (Liu et al., 2016). Therefore, in areas with limited solar radiation, like in our case, the selection of proper amendments is a challenge, since many organic by-products and vegetal materials can be used. Among them, some agro-industrial by-products have exhibited positive results (Lacasa et al., 2021; Testen et al., 2021) and might be a worthwhile alternative because it is cost-effective and promotes circular economy.
Sunflower seed husk (SSH) contains around 48% cellulose and 17% lignin (Shaukat et al., 2021), which might be interesting C-sources in biodisinfestation treatments. Besides, the sunflower (Helianthus annuus) is one of the most important edible oil crops, widely cultivated because of its adaptation and resistance to different environmental conditions. Currently, sunflower is cultivated in six continents with an estimated production of around 50 million tons per year of which roughly half is produced in European countries (Perea-Moreno et al., 2018; FAO, 2021). Considering that the husk constitutes 30–50% of the seed, millions of waste material is generated every year. This biomass is mainly used in livestock feed but different uses are being assessed because of its high energy content (Perea-Moreno et al., 2018; Shaukat et al., 2021). However, all the references regarding agricultural use are related to livestock feeding (Robertiello et al., 1984; Osman et al., 2018) and no publication was found as organic amendment in agricultural soils. The type of organic amendment can boots certain microbial populations and lignocellulose degrading microorganisms induce antagonistic effects which exert pressure on the pathogen population, including Meloidogyne spp. (Simmons et al., 2016; Fernández-Bayo et al., 2019; Shea et al., 2022).
The root-knot nematode Meloidogyne incognita Chitwood (1949) is one of the most damaging species with wide host ranges and widely distributed (Avato et al., 2013; Jones et al., 2013). Root galling is the most important symptom and it is caused by mature females that lay their eggs in the roots and induce gall formation. According to the galling level, the root development and nutrient uptake capacity might diminish leading to important yield loss (Bridge and Page, 1980; Jones et al., 2013). However, soil nematodes are the most abundant of the Metazoa and only a few species can cause crop damage. Most are free-living species with different feeding habits that occupy key positions in the soil food web (Bongers, 1990; Bongers and Ferris, 1999). Therefore, the study of the soil nematode community structure might be an indicator of changes in the microbial structure (Bongers and Ferris, 1999; Cesarz et al., 2015) and can be implemented in the evaluation of biodisinfestation treatments (Ney et al., 2019; Gandariasbeitia et al., 2021; Shea et al., 2022). Biodisinfestations suppose a transitional disturbance in the whole soil microbial community and change the soil physicochemical properties. However, the type of organic amendment plays an important role in boosting certain microbial mechanisms which can affect the results (Liu et al., 2016; Fernández-Bayo et al., 2019). In fact, some of these mechanisms can supply the lack of thermal effect in areas with low solar radiation. Therefore, the assessment of microbiological and physicochemical properties can also help better understand the biodisinfestation processes and changes in fertility variables, which is worthy of serious consideration in agricultural fields.
This study was performed in a humid region on a family-run farmland with natural infection of M. incognita in protected lettuce crop. After 20 years of intensive monoculture and conventional management, the pathogen developed resistance to chemical pesticides leading to important yield losses. The previous year, some greenhouse tunnels were biodisinfested using fresh cow manure with rapeseed cake and beer bagasse with positive results regarding the non-treated tunnels (Gandariasbeitia et al., 2021). In this trial, we compared this treatment with others that contained sunflower seed husks with fresh cow manure in different proportions, alone or in combination with the other by-products and including wheat bran which is a common amendment in ASD treatments. All treatments had the same quantity of amendment and similar C content but differed in total N concentration, thus C/N ratio. The aim was to assess the sunflower seed husk as organic amendment and differences in C/N ratios in biodisinfestation treatments along with the fertilizing effect. For that, disease development and the pathogen population were assessed and changes in nematode community structure and microbial activity were evaluated together with soil physicochemical properties. The assessment was done in three different moments, before treatments, after treatment and after harvesting the first crop after treatments.
Materials and Methods
Study Site and Soil
The study site was located in the Basque Country, a humid temperate climate zone in northern Spain (43°17′00”N; 2°45′00”W), on a family-run holding with important losses. The assay was carried out in a greenhouse poly-tunnel (four tunnels, 8 × 50 m each) in which an intensive monoculture of lettuce (5 yields/year), varietal type “Batavia”, has been grown for the last 20 years under conventional management.
The soil was naturally infected by the root-knot nematode M. incognita but different population densities were observed among the tunnels, thus among plots. The soil texture was loamy (21 ± 2.5% sand, 61 ± 1.5% silt, 18 ± 0.9% clay), determined using the Bouyoucos hydrometer, and the pH was measured with a pH meter (GLP 22, Crison InstrumentsTM, Barcelona, Spain) (1:5 dilution) resulting slightly alkaline (pH=7.4 ± 0.1). The soil had 5.5–6% of organic matter measured by calcination method (M.A.P.A., 1994). Traditional lettuce crop management in this region includes the incorporation of fresh cow manure every year and tilling before planting over black plastic film with a planting pattern of 25 × 30 cm.
Organic Amendments
In this assay, fresh cow manure (FCM), beer bagasse (BB), rapeseed cake (RC), wheat bran (WB) and sunflower seed husk (SSH) were used as organic amendments in the biodisinfestation treatments. The fresh cow manure, mixed on about 20% with straw, was supplied by a nearby farm and it was used in all treatments in combination with the by-products.
Beer bagasse and rapeseed (Brassica napus) cake were used in the amendment mixture in a previous study (Gandariasbeitia et al., 2021) with positive results against M. incognita and yield improvement, thus, this treatment served as reference in this trial. Beer bagasse is the residue obtained in beer production before fermentation and contains high amounts of sugars that promote microbial activity. It was offered by BOGA Basque Craft Beer (75% moisture), a local brewery that has to manage this residue weekly. Pellets of defatted rapeseed cake were provided by an experimental sheep farm in Alava (Basque Country). It has shown positive results, despite the low content of glucosinolates but the effect is mainly attributed to the high N concentration that might cause the release of NH3 during decomposition processes (Mazzola et al., 2001; Serrano-Pérez et al., 2017).
Wheat (Triticum aestivum) bran and sunflower (Helianthus annuus) seed husk were selected as main C-sources and are mainly used in livestock feed. Wheat bran has been used in other studies as a labile C-source to enhance microbial activity and promote ASD (Rosskopf et al., 2020). Sunflower seed husk was selected as a rich C-source to ensure microbial degradation and promote lignocellulolytic microorganisms. These husks are mainly composed of cellulose and lignin (48.4 and 17.0% respectively) (Shaukat et al., 2021), which might enhance biodisinfestation processes and favor antagonist microorganisms.
All the by-products were characterized before the assay to design the different treatments. Besides, these analyses helped in better understanding the results. The characterization is presented in the results section and Table 1.
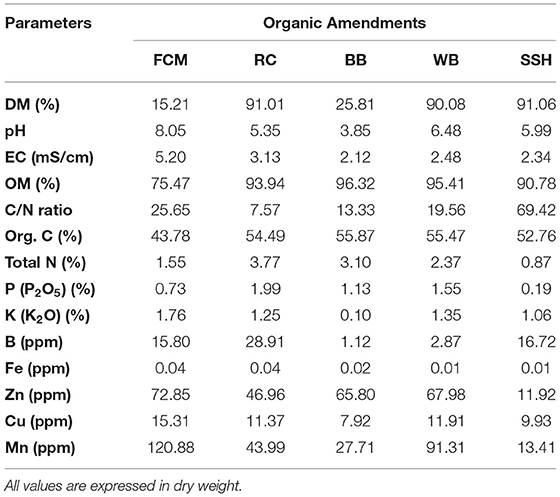
Table 1. Physicochemical values (DM, dry matter; OM, organic matter) of the organic amendments used for the biodisinfestation treatments: FCM (fresh cow manure), RC (rapeseed cake), BB (beer bagasse), WB (wheat bran), SSH (sunflower seed husk).
Treatments and Experimental Design
Four different mixtures of organic amendments were designed as biodisinfestation treatments in this assay: M1, M2, M3, and M4. All contained the same amount of FCM (3 kg/m2, dry weight) and the same amount of by-products (1 kg/m2, dry weight). M1 was the only treatment without SSH, the other three contained SSH in different proportions. In M2 and M3, SSH was incorporated together with BB and WB, respectively, while M4 was only composed of FCM and SSH, thus M4 contained the highest dose of SSH followed by M2. All treatments had 6mgC/kg soil, slightly higher than the minimum proposed in ASD treatments (4mgC/kg soil) (Butler et al., 2014; Shrestha et al., 2021a). The designed treatments had different C/N ratios, the lowest values were 23 and 29 (M1 and M3, respectively) and the highest 31 and 34 (M2 and M4, respectively). The doses and main characteristics of each treatment are shown in Table 2.
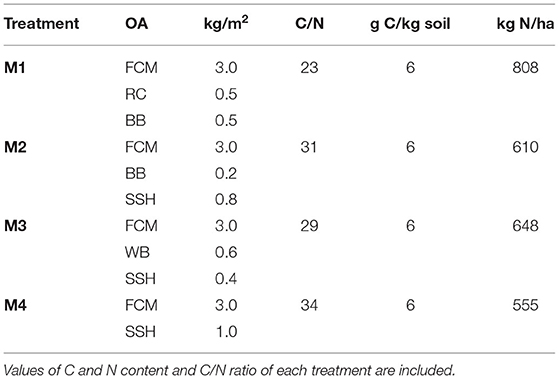
Table 2. Doses in dry weight of the organic amendments used (FCM, fresh cow manure; RC, rapeseed cake; BB, beer bagasse; WB, wheat bran; SSH, sunflower seed husk) in each biodisinfestation treatments (M1, M2, M3, M4).
The four treatments were arranged in a randomized complete block design (RCBD) with three plots per treatment distributed in four greenhouse tunnels. For that, each of the tunnels (50 × 6 m each) of a poly-tunnel affected by M. incognita was virtually divided crosswise into three parts obtaining a plot size of 100 m2 each and a total of 12 plots (3 replicates/treatment). The assessment was done at three different moments: (i) before treatment (T1), (ii) after treatment (T2) and (iii) after the first crop post-treatment (T3). The effects of time and treatment were analyzed individually but also the combination of both factors. This trial was performed without a control due to farmers' management. However, in a previous assay at the same farm, treatment M1 was assessed together with a non-treated control and positive long-term results were proved regarding the control (Gandariasbeitia et al., 2021). Thus, in this case, the aim was to assess possible differences between mixtures with different organic by-products and, for this reason, M1 was used as reference given the lack of control plots. Besides, the results at the different moments were compared to study the effects of the biodisinfestations and after growing the first crop after the treatments.
The trial was carried out for 6 weeks in summer, from July 18th 2019 to September 3rd. First, each amendment was applied in the corresponding plots and mixed with a rotary tiller. Then, the soil was spray irrigated for 20 min (40 l/h/m2) and manually covered with a transparent total impermeable film (TIF plastic provided by Riviera Blumen). The edges of the plastic were buried 10–15cm deep to avoid air diffusion. After 6 weeks, the plastic cover was removed and soil was aerated 1 week before tilling to transplanting on September 9th.
Sampling Procedure and Data Collection
Soil samples (0–25 cm topsoil) were randomly collected at ten different points in each plot using a core soil sampler (2.5 cm diameter). A single composed sample (1.8–2 kg fresh weight) was collected in each plot. This procedure was followed at the three sampling times. First, on July 15th, after harvesting the last yield before biodisinfestation treatments (T1); second, on September 7th, after biodisinfestations once the soil was aerated, i.e., before transplanting, (T2); and third, on November 6th, after harvesting the first lettuce crop after the treatments (T3). The samples were stored at 4°C and used to perform soil physicochemical analysis and study the community structure of nematodes and the pathogen M. incognita along with some soil microbiological properties. Besides, root samples (10 units/plot) were randomly collected, avoiding border effects, from the crop harvested prior (T1) and follow (T3) biodisinfestation to assess root galling index and, at T3 the number of eggs in the roots was also quantified.
During biodisinfestation treatments, the temperature inside the greenhouse was recorded together with soil temperature at 15 and 30 cm depth every 15 min.
Analyzed Variables
Organic Amendments and Soil Physicochemical Characterization
The by-products were characterized to design the different mixtures considering the values in dry weight. For that, dry matter (DM), organic matter (OM), total organic C and total N (Kjeldahl) were measured together with P2O5, K2O, B, Fe, Zn, Cu and Mn (Table 2). For soil characterization, part of the soil samples (1 kg fresh weight) were air-dried at room temperature and ground to analyze the following physicochemical parameters: pH (1:5), EC (1:10), OM, total N, NO3-, B, Fe, Zn, Cu and Mn. The soil samples were characterized at T1, T2 and T3 (Table 3). All parameters were analyzed according to standard methods (M.A.P.A., 1994). Minerals and metals were determined through inductively coupled plasma-optical emission spectrometry (ICP-OES) after acid digestion.
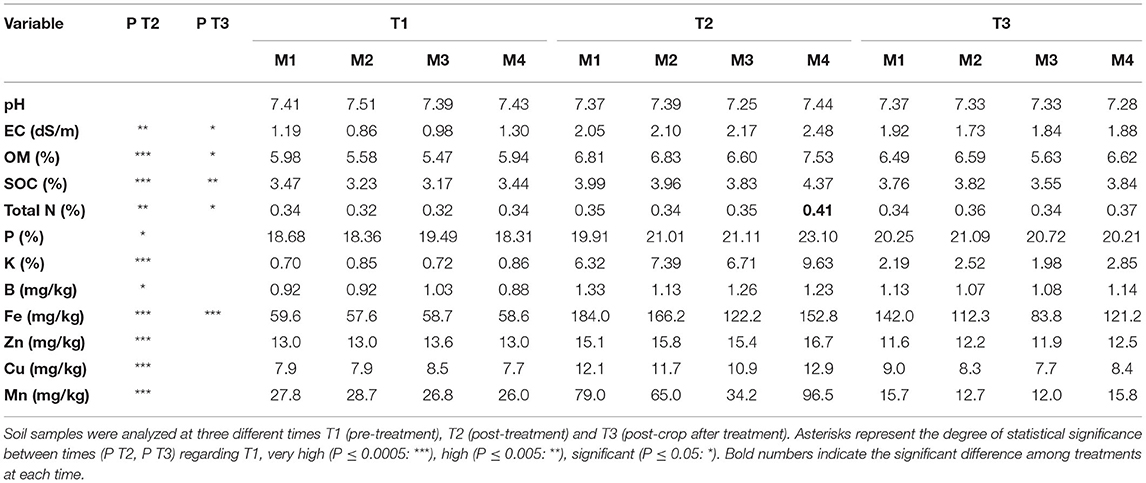
Table 3. Average values and standard errors of soil physicochemical variables (DM, dry matter; OM, organic matter; SOC, soil organic C) in each treatment (M1, M2, M3, M4).
Temperature
During biodisinfestation, temperatures were monitored inside the greenhouse and at 15 and 30 cm soil depth every 15 min. Soil temperatures were registered in one plot of each treatment with specific probes connected to a Campbell logger (CR1000x). Average values per hour were calculated to know the number of accumulated hours at each recorded temperature.
Root Galling Index (GI) and Number of Eggs
The root galling index (GI) of lettuce plants was estimated according to the scale developed by Bridge and Page (1980). In this scale, 0 represents a healthy root without galls and 10 is the maximum degree of galling, when the root functioning is lost. For this purpose, the roots were carefully washed under tap water and visually assessed. This scale helped to evaluate the degree of crop damage caused by this pathogen.
The same samples were used to quantify the number of eggs per gram of fresh root according to Hussey and Barker (1973). In short, clean fresh roots were weight and blended in NaClO 0.05%. The solution was sieved through 75 and 25μm and the part retained in 25 μm mesh was collected to quantify the number of eggs under a magnifying glass (40X) LEICA S9i (Leica Microsystems, Heerbrugg, Switzerland). The number of eggs per gram root might be a good indicator of survival ability that might vary according to environmental conditions (Kokalis-Burelle et al., 2013).
Presence of M. incognita and Soil Nematode Community Structure
Part of the composing soil samples (600 g) was stored at 4°C for a maximum of 2 weeks before nematode extraction. This procedure was performed following the Baermann-Funnel technique (Barker, 1985). Fresh and non-processed samples were divided into three sub-replicates of 200 g each and nematodes were visually identified at the family level with an optical microscope LEICA DM6000B (200X) and counted on 100 μl of extraction.
The effect of biodisinfestation treatments on the pathogen population was assessed by quantifying the number of M. incognita juveniles in the samples and translating into the number of juveniles per 100 g dry soil. Considering the efforts of the visual quantification, together with M. incognita, the other juveniles present in the samples were also identified at the family level to categorize them according to their feeding habits (bacterivores: Ba; fungivores: Fu; herbivores: He; predators: Pr; omnivores: Om). Soil nematodes are complex organisms of the soil food web and the population survey might help in the assessment of soil status (Stockdale and Watson, 2009; Lu et al., 2020). Thus, the impact of biodisinfestations on the nematode community structure was assessed through the analysis of the relative presence of the different trophic groups.
Soil Microbiological Properties
Fresh soil samples (200 g) were sieved to <2 mm and stored at 4°C for a maximum of 1 month. In this trial, the following soil microbiological parameters were analyzed at the three sampling times (T1, T2, and T3): soil respiration rate (SR), microbial biomass carbon (MBC), water-soluble organic carbon (WSOC) and the community-level physiological profiles (CLPP) of the heterotrophic bacteria through Biolog Ecoplates™.
Soil Respiration Rate (SR)
Microbial activity was assessed by measuring soil respiration (SR) rate according to the ISO standard 16072:2002. Briefly, soil samples (20 g) were incubated in hermetic jars containing NaOH 0.2 N, for 72 h at 30°C and CO2 emission rate was quantified by titration with HCl. The soil respiration rate is commonly used in soil health assessment (Bünemann et al., 2018; Guo, 2021).
Microbial Biomass Carbon (MBC) and Water-Soluble Organic Carbon (WSOC)
Microbial biomass carbon (MBC) and water-soluble organic carbon (WSOC) were measured as fertility indicators to study soil quality change from management (Guo, 2021; Li et al., 2021). The MBC concentration was measured according to Vance et al. (1987) by chloroform fumigation method on organic C extractable by 0.5M K2S04. The WSOC was measured with the same procedure but without fumigation and extracted with deionized water. In both analyses, the resulting product was measured at 445 nm in spectrophotometer (Shimadzu UV-1800, Shimadzu Corporation, Kyoto, Japan). MBC might help understanding the number of nutrients and elements held in the microbial pool but part of this biomass might be dead, dormant, or active. WSOC indicated the available C organic fraction in the soil as a result of microbial activity. Both are active soil C fractions and sensitive indicators of soil organic C dynamics controlled by physical, chemical and biological processes (Scaglia and Adani, 2009; Li et al., 2021).
Physiological Profile of Heterotrophic Bacteria (Biolog Ecoplates™)
The functional heterotrophic bacterial community was determined with Biolog Ecoplates™ which allow determining the physiological profile at the community-level (CLPP). This technique consists on measuring the metabolic activity in 31 different C-sources distributed in triplicate in a 96 wells microplate (Garland and Mills, 1991; Insam, 1997). For that, soil water extracts were incubated at 30°C and absorbance at 590nm was measured every 12 h during 12 days in a microplate reader (Synergy HTX Multimode Reader, Agilent Technologies, California, USA). The microbial catabolic activity was evaluated by the average well color development (AWCD). Besides, bacterial diversity was estimated by the number of utilized substrates (NUS), those wells with absorbance values >0.25. Although this technique does not provide the genetic composition of the microbial community, it is a simple tool that gives valuable information about microbial activity and functional communities.
Statistical Analysis
In this study, time and treatment were analyzed as independent fixed factors to assess (i) the effects of biodisinfestation and the following yield and (ii) the possible differences among treatments, while the block was considered a random factor. For that, one-way analysis of variance (ANOVA) was performed among times and regarding the beginning of the assay (T1). Then, the same analysis was done at each time to see differences among treatments. Differences between means of variables were calculated with the multiple comparisons Tukey HSD test with adjustment for the p-values against experiment-wise type I error rate of α = 0.05. After one-way analysis, two-way ANOVA was carried out to detect possible interactions but no effect was found. Normality was determined using the Shapiro–Wilks test at each sampling data. In the case of non-parametrical distribution, the Kruskal-Wallis test was performed for analysis of variance followed by the post-hoc Mann-Whitney test (P = 0.05). All data were analyzed using R Studio 4.1.1.
Results
Organic Amendments and Soil Physicochemical Characterization
All the physicochemical variables measured in the organic amendments are shown in Table 1. The selected amendments had very low water content (<10%) excluding FCM and BB (85 and 74%, respectively). All by-products had acid pH, whereas FCM was close to 8. The fresh manure presented the highest EC value (5.2 mS/cm) but the lowest content of organic matter (75%). Considering total organic C, all by-products showed similar values (53–56%). RC presented the highest N content (3.77%) followed by BB and WB, while SSH and FCM had lower values (0.87 and 1.55%, respectively). Regarding the other macro and micro nutrients measured, detailed values can be found in Table 1.
After biodisinfestation treatment, the statistical analyses showed a significant increase in all of the fertility variables and in all treatments regarding the beginning of the assay (Table 3). In fact, most of them showed high statistical differences (P ≤ 0.005), P and B were not so high but statistically different (P ≤ 0.05) and no differences were found in pH values. After the first crop post-biodisinfestations, most variables remained higher than before treatments but statistical differences could only be found in Fe (P ≤ 0.0005), organic C (P = 0.002), OM, CE (P = 0.02) and total N (P = 0.03). The comparisons among treatments at each time were not significant. Only the total N after biodisinfestation was significantly higher in treatment M4 (P ≤ 0.02). Despite the generalized lack of statistical differences among treatments, M4 showed the greatest values in all variables after biodisinfestation, except for Fe and B.
Temperature
During the seven weeks of biodisinfestation treatments, the temperature range registered inside the greenhouse was 12.9–56.9°C (Table 4). The maximum temperatures recorded in the soils differed between treatments and depths. At 15 cm depth, M2 achieved the highest temperature (48.2°C) followed by M4 (46.8°C), whereas the maximum temperatures recorded in M1 and M3 were below 45°C. All treatments registered more than 500 h above 35°C at 15cm depth but only M2 and M4 accumulated more than 100 h above 41°C (178 and 135 h, respectively).
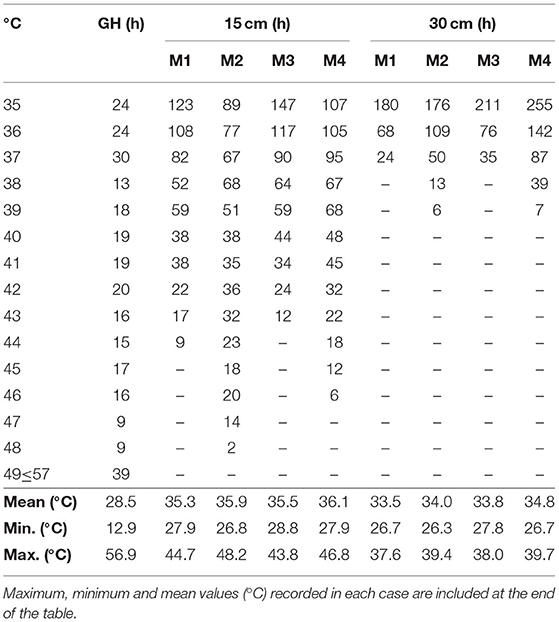
Table 4. Number of hours accumulated during 6 weeks of biodisinfestation treatments at the different temperatures registered in the different soil depths (15 and 30 cm) in one plot assigned to each treatment (M1, M2, M3, M4) and inside the greenhouse (GH).
In the deeper soil layer, at 30 cm depth, M2 and M4 also registered the highest temperatures, 39.4 and 39.7°C, respectively, while M1 and M3 remained below 38°C. All treatments recorded more than 300 h above 35°C, excepting M1 (272 h). M4 was the only treatment with more than 500 h above 35°C at both soil depths.
Root Galling Index and Number of Eggs in Roots
Whereas, root Galling Index (GI) was assessed in both yields, before and after biodisinfestations treatments, the number of eggs in roots could only be assessed after biodisinfestations. The plants harvested before the assay showed GI values around 5 and little variation within plots (Table 5). After biodisinfestation, GI significantly dropped (P ≤ 0.0001) to average values below Index-1 in all treatments without statistical differences among them (P = 0.93) but M3 and M4 showed lower values and less variation within plots. In fact, more healthy roots (GI = 0) were sampled in the plots assigned to these treatments (M3 and M4).
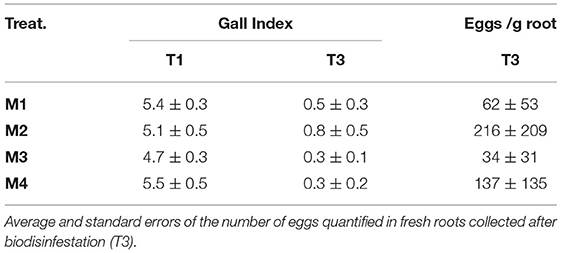
Table 5. Average values and standard errors of root galling index in the plants harvested before (T1) and after biodisinfestation treatments (T3).
Regarding the number of M. incognita eggs per gram of fresh root, fewer eggs were quantified in M1 and M3 treatments when compared to M2 and M4. Due to the extremely high variability, statistical similarity was obtained among treatments (P = 0.92) (Table 5).
M. incognita Population and Nematode Community Structure
Before the assay, high variability was found among plots in the presence of M. incognita (4–17 juveniles/100 g soil), thus no statistical differences were observed (P = 0.95) (Figure 1). Despite this great variability, a higher average value was detected in plots assigned to M4 (9 ± 3 juveniles/100 g soil). After treatments, M. incognita was only detected in one of the plots assigned to M4 but at low presence (3 ± 2). However, any juveniles of M. incognita was detected in any of the plots after the first yield post-biodisinfestations.
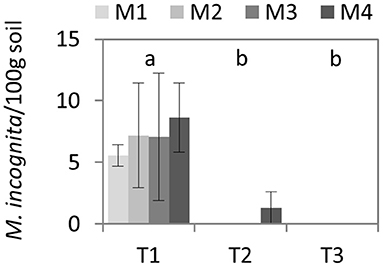
Figure 1. Average and standard errors of the number of juveniles of Meloidogyne incognita quantified in 100 g dry soil. Values of the four biodisinfestation treatments (M1, M2, M3, M4) are shown before biodisinfestation (T1), after biodisinfestation (T2) and after harvesting the first crop after treatments (T3). No statistical differences (P ≤ 0.05) were obtained among treatments. Letters indicate the significant differences among times according to Tukey HSD test (P ≤ 0.05).
Regarding the relative presence of nematodes trophic groups, herbivorous represented about half of the total nematode population in most plots before biodisinfestations but M. incognita population represented <10% in all cases. After treatments, bacterivorous nematodes dominated in all treatments (50-90%) in detriment of herbivorous and this trend continued after the first yield post-biodisinfestations (Figure 2). In this assay, biodisinfestation treatments did not show a negative impact on fungivorous group. In fact, the population remained after treatment in M4 and slightly increased after harvesting the first crop after biodisinfestations.
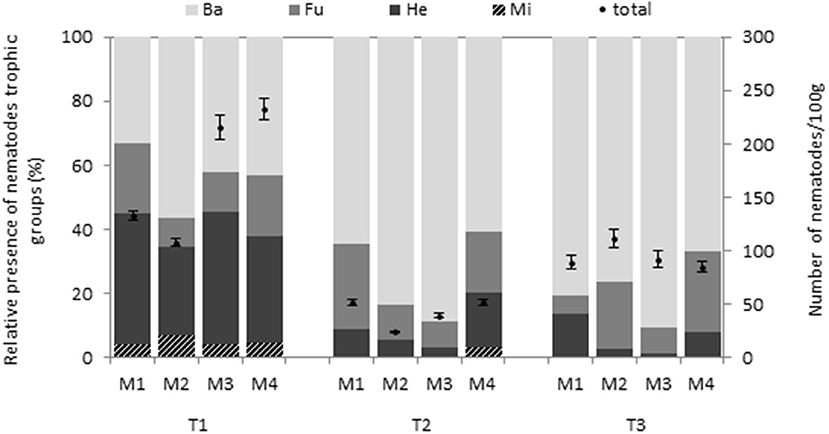
Figure 2. Bar chart represents the relative presence of the different nematode trophic groups identified in the soil samples: bacterivores (Ba), fungivores (Fu) and herbivores (He). The pathogen nematode M. incognita (Mi) was also included to discriminate it from herbivores. The total number of nematodes in 100 g of soil (dry matter) is represented by dot chart with the standard errors. Values were obtained for the four biodisinfestation treatments (M1, M2, M3, M4) at three different times: T1 (pre-treatment), T2 (post-treatment) and T3 (post-crop after treatment).
Microbiological Soil Properties
Soil Respiration Rate
Before biodisinfestation treatments, soil respiration rate was similar in all plots (P = 0.42) with very low values (0.9–1.3 mgCO2/h/kg) (Figure 3). After biodisinfestation, a significant increase was observed (P ≤ 0.0001) in all treatments but without statistical difference among them (P = 0.56), although M3 showed a lower average value (3.1 mgCO2/h/kg) considering the other treatments (4.1–4.6 mgCO2/h/kg). After harvesting the first lettuce crop, respiration rate significantly decreased in all treatments but continued to be significantly higher than at the beginning (P = 0.04). Although no statistical differences were observed among treatments at the end of the assay (P = 0.14), M1 showed a lower average value regarding the other treatments.
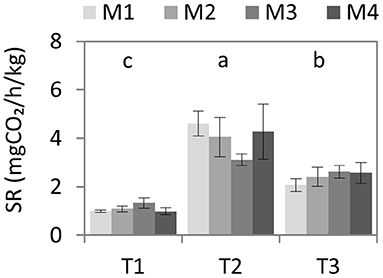
Figure 3. Average values and standard errors of soil respiration rate (mgCO2/kg/h, dry weight) after 72 h incubation time at 30°C. Values of the four biodisinfestation treatments (M1, M2, M3, M4) were measured at three different times: T1 (pre-treatment), T2 (post-treatment) and T3 (post-crop after treatment). No statistical differences (P ≤ 0.05) were obtained among treatments. Letters indicate the significant differences among times according to Tukey HSD test (P ≤ 0.05).
Microbial Biomass Carbon (MBC) and Water-Soluble Organic Carbon (WSOC)
After biodisinfestation, no significant difference was observed in MBC concerning the beginning of the assay (P = 1) and no statistical difference was detected among treatments neither before (P = 0.78) nor after biodisinfestation (P = 0.2) (Figure 4). However, a significant increase was observed after harvesting the first crop after biodisinfestation in all treatments regarding the previous results (P = 0.03) and the beginning of the assay (P = 0.04). When comparing MBC values, all treatments showed greater values at the end of the assay (205–242 mgC/kg) than at the beginning (179–203 mgC/kg). In the case of M1, the values dropped after biodisinfestation but reached the initial values at the end of the assay.
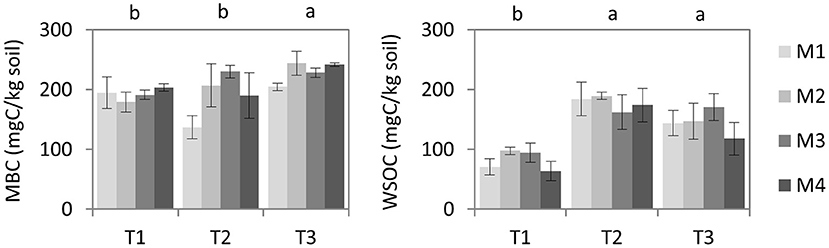
Figure 4. Average values and standard errors of soil microbial biomass carbon (MBC) and water-soluble organic carbon (WSOC). These parameters were measured in the four biodisinfestation treatments (M1, M2, M3, M4) at three different times: T1 (pre-treatment), T2 (post-treatment) and T3 (post-crop after treatment). Letters indicate the significant differences among times according to Tukey HSD test (P ≤ 0.05).
A large increase in WSOC was observed after biodisinfestation regarding the initial measurements (P ≤ 0.0001) and these values barely decreased after the first crop cycle after biodisinfestations, with no statistical difference considering post-treatments (P = 0.11), but significant difference was observed regarding the first analysis (P = 0.001) (Figure 4). Despite the lack of statistical differences among treatments at any of the studied moments, M3 showed the highest average value (171 mgC/kg) and M4 the lowest (118 mgC/kg) at the end of the assay.
Physiological Profile of Heterotrophic Bacteria (Biolog Ecoplates™)
The results obtained through Biolog EcoPlatesTM showed a significant decrease in both AWCD and NUS after biodisinfestation treatments (P ≤ 0.0001) (Figure 5). While AWDC values recovered the initial levels at the end of the assay (P = 0.92), NUS was still lower after the first yield post-treatments regarding the initial values (P = 0.01). However, a trend to recover initial NUS values could be detected. In fact, most of the substrates (29–30) were used in all treatments after the first yield post-biodisinfestation. No statistical differences were detected among treatments at any of the studied moments.
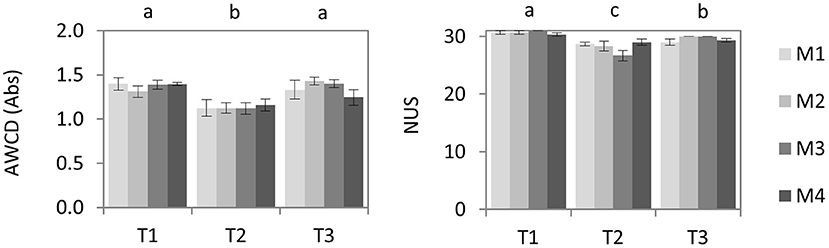
Figure 5. Mean values and standard errors of average well color development (AWCD) and number of used substrates (NUS) in Biolog Ecoplates™ after 12 days incubation at 30°C. Values of each biodisinfestation treatment (M1, M2, M3, M4) at three different times: T1 (pre-treatment), T2 (post-treatment) and T3 (post-crop after treatment). No statistical differences (P ≤ 0.05) were obtained among treatments. Letters indicate the significant differences among times according to Tukey HSD test (P ≤ 0.05).
Discussion
This study reaffirmed the success of biosolarization treatments in humid temperate regions with low solar radiation and supported the hypothesis of the importance of the other mechanisms as drivers in biodisinfestation processes. These mechanisms, even the rise in temperatures, are highly related to the microbial activity that might be conditioned by the organic amendments features. In general, the four treatments showed similar positive results proving the effectiveness of the combination of animal fresh manure with agro-industrial by-products in both soilborne pathogen control and soil fertility improvement. Although no statistical differences were observed among treatments in most properties, some trends and differences might be observed in the analyzed variables indicating differences in microbial processes. In this assay, different temperature ranges were recorded in the four treatments. This variation was more attributed to the treatment than to the environmental factors due to the plot distribution in the greenhouse. The plots amended with treatment M2 registered the maximum temperatures at 15 cm soil depth (48.2°C), followed by M4 (46.8°C). Whereas, in plots assigned to M1 and M3, lower maximum temperatures were registered (44.7 and 43.8°C, respectively). However, all treatments showed similar disease reduction, which confirmed that high temperatures (>45°C) are not necessary to obtain positive results (Ojinaga et al., 2020; Gandariasbeitia et al., 2021). Besides, the temperature effect on this soilborne pathogen is also closely related to the number of accumulated hours at sub-lethal temperatures (Wang and McSorley, 2008). In this assay, all treatments achieved more than 500 h above 35°C at 15 cm depth and more than 200 h at 30 cm depth, which was enough to damage the soilborne pathogen M. incognita causing population decline, thus disease reduction (Table 3). The treatment with the highest C/N ratio (M4), which had SSH as the only by-product, was the only one that also accumulated more than 500 h above 35°C at 30 cm depth. This means that biotransformation activity was high even at the lower soil layers in M4.
Before the assay, root galling was not severe (5) but enough to inhibit root system development and reduce nutrient uptake capacity (Bridge and Page, 1980) causing important yield loss. However, in the crop harvested after biodisinfestations these values dropped below 1 in all treatments and yield considerably recovered (data not shown). Despite the lack of data on the number of eggs in roots before biodisinfestations, the average values found after treatments showed low reproductive capacity (Meyer et al., 2015). In the roots sampled in plots assigned to M1 and M3 fewer eggs were quantified but no statistical differences were detected among treatments due to the considerable variability within the greenhouse tunnels (Table 5). The effect of biodisinfestations on M. incognita was also assessed through the presence of juveniles in soil samples. Before treatments, similar population values were observed in all plots (around 400 juveniles/100 g dry soil) (Figure 1). This population density was considerable but represented <10% of the total nematode population (Figure 2). After biodisinfestation, all treatments showed great effectiveness in reducing the pathogen population and this was more evident at the end of the assay, after the first yield post biodisinfestations (Figure 1). The presence of few galls in roots sampled after biodisinfestations denoted the survival of some juveniles of M. incognita but at a very low rate and it was undetectable in soil samples. It is important to consider that morphological identification may underestimate the populations. Although PCR-based methods are more accurate, many reference taxa and the genomic sequences are required. However, visual identification through morphological features is a costless method and may give enough information for community assessment (Seesao et al., 2017).
Considering the effect on the whole nematode community, the herbivorous population decreased more than 50% after biodisinfestations while the bacterivorous took advantage of the enrichment conditions to overgrow. This trend continued even after transplanting the first crop. The high presence of bacterivorous after biodisinfestations was an indicator of the increase in soil bacteria populations due to enrichment conditions. In general, biodisinfestations suppose a great disturbance in the microbial community and few groups are benefited from these conditions. Decrease in biodiversity but increase in activity is very common in these treatments and the opportunistic populations might change according to the organic amendment features and the environmental conditions generated during biodisinfestations (Liu et al., 2016; Fernández-Bayo et al., 2019). In this assay, the increase in microbial activity after treatments was observed through the higher values in soil respiration rate and WSOC but without a significant increase in MBC. These were indicators of high activity in organic matter biodegradation during the biodisinfestations by opportunistic populations while part of the population was loosed. After the first lettuce yield, 6 weeks after biodisinfestations, the opposite happened. The microbial activity decreased, while the populations seemed to recover. Although SR and WSOC were still significantly higher considering the beginning of the assay, the values decreased regarding the sampling after treatments, while MBC increased. These observations agreed with the results obtained in BiologEco Plates™. Both AWCD and NUS significantly decrease after biodisinfestations in all treatments, which indicated a decrease in diversity. In the same way, after harvesting the first crop, both variables significantly increased. AWCD recovered the initial values at the end of the assay and most substrates were used by the four treatments (29-30). The analysis of the physiological profile of heterotrophic bacteria through these microplates can be an easy and reliable method to detect changes in degrading bacteria populations. These results also showed a recovery trend of soil microbial communities at the end of the assay. Although biosolarization treatments suppose a great disturbance in soil microbiota, this effect is transitional and the incorporation of organic matter into the soil contribute to improve soil health once the biodisinfestation phase passes (Rosskopf et al., 2020).
Regarding the soil physicochemical properties, all fertility variables considerably improved in all treatments after biodisinfestation and this trend remained after the first yield post-treatments. Important fertility variables like EC, OM, total N and organic C continued being significantly higher regarding the beginning of the assay. In the case of Fe, the concentration values were still very high at the end of the assay (83 ± 16 – 142 ± 15 mg/kg). High concentrations of Fe and Mn after treatments were indicators of soil reduction, which also might have biocidal effect. (Fernández-Bayo et al., 2018). While the increase in Mn concentration might be attributed to the incorporation of FCM, the high Fe values could only be attributed to the biodisinfestation processes because all the organic amendments contained very low Fe concentrations (<0.11 mg/kg). The general increase in metals concentrations (Fe, Zn, Cu, and Mn) might be primarily caused by a reductive environment, i.e., anaerobic conditions (Fernández-Bayo et al., 2018). These reductive conditions could contribute on the release of VFA (e.g., acetic, butyric, formic and propionic acids) which have nematicidal effect (Oka, 2010). The total N content of each treatment was not reflected in the quantity measured after treatment. In fact, the treatments with less quantity of total N was M4 and it showed the highest increase in the total N measured in the soil after treatment and after harvesting the next lettuce crop. This increase might be related with the high C/N rate and the lignocellulosic nature of the C-source that influenced the microbiological processes. This fact was also observed by Shrestha et al. (2021b), who also demonstrated that most of the inorganic N measured in the soils after disinfestation treatments was primarily NO2-N and NO3-N which improve yield performance.
Although all treatments seemed to be similar without statistical differences among them, the treatment M4 showed higher average values in most of the fertility variables after biodisinfestation and after the following crop. Moreover, the only statistical difference found among treatments was detected in total N content in M4 after treatments. This mixture had FCM combined with SSH as the only by-product, thus in higher quantity (1 kg/m2). Curiously, this treatment provided the lowest N input to the soil (555 kgN/ha) but the highest C/N ratio (34). Cellulose, hemicellulose and lignin are the main components of SSH. These biopolymers are complex structures of polysaccharides mainly degraded by thermophilic actinomycetes and thermophilic fungi. The result of the degradation is closely related to humus production and it depends on several factors, like C/N ratio, temperature, pH, moisture, etc. (Yang et al., 2021). Mesophilic temperatures and C/N ratios >25 promote degradation of lignocellulose materials (Gaind et al., 2005). Furthermore, M4 was the only treatment that registered more than 500 h above 35°C at both soil layers, despite being the second treatment regarding the maximum temperatures achieved. This effect could be the result of the increase in microbial activity during biodisinfestation even in the lower soil layers. This led to high mineralization of soil nutrients in plots treated with M4, which showed the highest average values in most fertility variables (OM, SOC, total N, K, B, Zn, and Mn) even after the first lettuce yield (Table 4).
Despite the lack of statistical differences among treatments in the biological variables, some observations were made on plots treated with M4 at the end of the assay. Higher average values in SR and MBC might indicate that the microbial activity and population remained high. The nematode community was barely affected by the treatment and fungivorous relative presence slightly increased. The growth of the fungivorous group denoted the presence of fungi, usually highly affected in biodisinfestations. This was also observed in M2, both treatments M2 and M4 contained the highest amount of SSH (0.8 and 1 kg dry weight/m2, respectively). Fungi play a key role in polysaccharide degradation and lignocellulosic amendment. Therefore, the incorporation of SSH in biodisinfestation treatments might contribute to strengthen fungal populations and equilibrate soil food web after biodisinfestations. Besides, fungi and fungivorous nematodes might have antagonistic effect against plant-parasitic nematodes competing for the same ecological niches (Oka, 2010; Gandariasbeitia et al., 2021). According to Liu et al. (2016) the organic C input and the quality of organic materials condition the response of nematode communities and chemically-complex biomass help to support the community structure and greater species richness. All these reinforced the importance of the organic amendment features in biotransformation processes and biodisinfestation mechanisms. Considering that certain microorganisms play a determinant role in these processes, some authors incorporated Trichoderma spp. and other antagonistic microorganisms along with the organic amendments in biodisinfestation assays but with different results (Shrestha et al., 2020; Khadka and Miller, 2021). Perhaps, the incorporation of these microorganisms could be more effective after the biodisinfestation treatment. The combination of both approaches could be interesting to assess in further studies.
In conclusion, this trial proved that biodisinfestation treatments using sunflower seed husk with fresh cow manure might be a valuable alternative to implement in crop management in temperate areas under greenhouse conditions. Although the four treatments showed similar positive effects on both, control of the soilborne pathogen M. incognita and improvement in soil fertility, the treatment with SSH as the only by-product added with FCM, thus in higher quantity, showed greater values in most micro- and macronutrients measured. Besides, the lignocellulosic nature of SSH boosted certain microbiological processes that improved soil temperatures at lower layers and contributed on a balanced soil microbiota and nutrient availability. The microbial community within the soil is responsible for maintaining nutrients and this is provided by a rich and balanced soil food web which is affected in biodisinfestation treatments. The use of SSH demonstrated to generate less impact on the microbial community and even improved the soil food web after biodisinfestation regarding the other treatments with SSH in less quantity and combined with other by-products. In summary, SSH might be a promising alternative not only in biodisinfestation treatments against soilborne pathogens but also as organic amendment to improve soil fertility in both terms, improvement of physicochemical and biological properties. This organic by-product is affordable, available and can be incorporated in other crops to improve soil health making ecosystems more resilient to stress in a sustainable way.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
MG, JL-P, and SL contributed to the conception and design of the study. MG and BJ conducted the assay and samplings. MG and JL-P performed the laboratory analyses. MG organized the database and performed the statistical analysis and wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research has been financially supported by FEDER funds (project INIA RTA 2015-00060-C04-04 Comprehensive revaluation of by-products based on their potential uses: obtainment of biofumigants and fertilizers and project MICINN/AEI PID2019-106148RR-C44 Integration of revalorized agro-food by-products into an enhanced circular economy model: new applications for horticultural and extensive crops in commercial facilities) and by the Department of Environment, Territorial Planning, Agriculture and Fisheries of the Basque Government (projects REVABIO, REVAL and REVAL 2.0). MG was the recipient of a predoctoral contract (project reference INIA-2017-0043) of the State Training Subprogram of the Spanish Ministry of Economy, Industry and Competitiveness (MICINN).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors want to thank the family of farmers that allowed us to perform this assay.
References
Avato, P., D'Addabbo, T., Leonetti, P., and Argentieri, M. P. (2013). Nematicidal potential of Brassicaceae. Phytochem. Rev. 12, 791–802. doi: 10.1007/s11101-013-9303-7
Barker, K. R. (1985). “Nematode extraction and bioassays”, in An Advanced Treatise on Meloidogyne: Methodology, Vol. 2, ed. K. R. Barker, C. C. Carter, and J. N. Sasser (Raleigh, USA: North Carolina State University Graphics), 19–35.
Blok, W. J., Lamers, J. G., Termorshuizen, A. J., and Bollen, G. J. (2000). Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology. 90, 253–259. doi: 10.1094/PHYTO.2000.90.3.253
Bongers, T. (1990). The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia. 83, 14–19. doi: 10.1007/BF00324627
Bongers, T., and Ferris, H. (1999). Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. E14, 224–228. doi: 10.1016/S0169-5347(98)01583-3
Bridge, J., and Page, S. L. J. (1980). Estimation of root-knot nematode infestation levels on roots using a rating chart. Trop. Pest Manag. 26, 296–298. doi: 10.1080/09670878009414416
Bünemann, E. K., Bongiorno, G., Bai, Z., Creamer, R. E., Deyn, D. e., and de Goede, G. (2018). Soil quality—a critical review. Soil Biol. Biochem. 120, 105–125. doi: 10.1016/j.soilbio.2018.01.030
Butler, D. M., Ownley, B. H., Dee, M. E., Eichler Inwood, S. E., McCarty, D.G., Shrestha, U., et al. (2014). Low carbon amendment rates during anaerobic soil disinfestation (ASD) at moderate soil temperatures do not decrease viability of Sclerotinia sclerotiorum sclerotia or fusarium root rot of common bean. Acta Hortic. 1044, 203–208. doi: 10.17660/ActaHortic.2014.1044.23
Cesarz, S., Reich, P. B., Scheu, S., Ruess, L., Schaefer, M., and Eisenhauer, N. (2015). Nematode functional guilds, not trophic groups, reflect shifts in soil food webs and processes in response to interacting global change factors. Pedobiologia. 58, 23–32. doi: 10.1016/j.pedobi.2015.01.001
Chitwood, B. G. (1949). Root-knot nematodes - Part 1. A revision of the genus Meloidogyne Goeldi, 1887. Proc. Helminthol. Soc. Washington. 16, 90–104.
FAO (2021). “Food and Agriculture Organization of the United Nation (FAO),” in World Food and Agriculture: Statistical Yearbook 2021. Rome, Italy.
Fernández-Bayo, J.D., Hestmark, K.V., Claypool, J.T., Harrold, D.R., Randall, T.E., Achmon, Y., et al. (2019). The initial soil microbiota impacts the potential for lignocellulose degradation during soil solarization. J. Appl. Microbiol. 126, 1729–1741. doi: 10.1111/jam.14258
Fernández-Bayo, J. D., Randall, T. E., Harrold, D. R., Achmon, Y., Hestmark, K. V., and Su, J. (2018). Effect of management of organic wastes on inactivation of Brassica nigra and Fusarium oxysporum f.sp lactucae using soil biosolarization. Pest Manag. Sci. 74, 1892–1902. doi: 10.1002/ps.4891
Gaind, S., Pandey, A. K., and Lata (2005). Biodegradation study of crop residues as afected by exogenous inorganic nitrogen fungal inoculants. J. Basic Microbiol. 4, 301–310. doi: 10.1002/jobm.200410483
Gandariasbeitia, M., López-Pérez, J. A., Juaristi, B., Abaunza, L., and Larregla, S. (2021). Biodisinfestation with agricultural by-products developed long-term suppressive soils against Meloidogyne incognita in lettuce crop. Front. Sust. Food Syst. 5, 663248. doi: 10.3389/fsufs.2021.663248
Garland, J. L., and Mills, A. L. (1991). Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 57, 2351–9. doi: 10.1128/aem.57.8.2351-2359.1991
Guo, M. (2021). Soil health assessment and management: recent development in science and practices. Soil Syst. 5, 61. doi: 10.3390/soilsystems5040061
Hussey, R. S., and Barker, K. R. (1973). A comparison of methods for collecting inocula of Meloidogyne spp. including a new technique. Plant Disease Reporter 57, 1025–1028.
Insam, H. (1997). “A new set of substrates proposed for community characterization in environmental samples” in Microbial communities: functional versus structural approaches, ed. H. Insam and A. Rangger (Berlin, Germany: Springer), 259–260.
Jones, J. T., Haegeman, A., Danchin, E. G. J., Gaur, H. S., Helder, J., and Jones, M. G. K. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 14, 946–961. doi: 10.1111/mpp.12057
Katan, J. (2017). Diseases caused by soilborne pathogens: Biology, management and challenges. J. Plant Pathol. 99, 305–315. doi: 10.4454/jpp.v99i2.3862
Katan, J., and Gamliel, A. (2014). Plant health management: soil solarization. Encycl. Agric. Food Syst. 4, 460–471. doi: 10.1016/B978-0-444-52512-3.00256-4
Khadka, R. B., and Miller, S. A. (2021). Synergy of anaerobic soil disinfestation (ASD) and Trichoderma spp. in Rhizoctonia root rot suppression. Front. Sustain. Food Syst. 5, 645736. doi: 10.3389/fsufs.2021.645736
Klein, E., Katan, J., and Gamliel, A. (2012). Soil suppressiveness to Meloidogyne javanica as induced by organic amendments and solarization in greenhouse crops. Crop Prot. 39, 26–32. doi: 10.1016/j.cropro.2012.02.013
Kokalis-Burelle, N., Butler, D. M., and Rosskopf, E. N. (2013). Evaluation of cover crops with potential for use in anaerobic soil disinfestation (ASD) for susceptibility to three species of Meloidogyne. J. Nematol. 45, 272.
Lacasa, A., Lacasa, C. M., Torres, J., Martínez, V., Ros, C., and Guerrero, M. M. (2021). Agroindustrial byproducts used in biosolarization reduce the soilborne pathogens incidence in green-house pepper crops. ITEA-Inf. Tec. Econ. Agrar. 118, 181–197. doi: 10.12706/itea.2021.026
Li, Y., Xie, Z., Yu, Z., Wang, Y., Liu, C., and Wang, G. (2021). Impact of surface soil manuring on particulate carbon fractions in relevant to nutrient stoichiometry in a Mollisol profile. Soil Tillage Res. 207, 104859. doi: 10.1016/j.still.2020.104859
Liu, T., Chen, X., Hu, F., Ran, W., Shen, Q., and Li, H. (2016). Carbon-rich organic fertilizers to increase soil biodiversity: Evidence from a meta-analysis of nematode communities. Agric. Ecosyst. Environ. 232, 199–207. doi: 10.1016/j.agee.2016.07.015
Lu, Q., Liu, T., Wang, N., Dou, Z., Wang, K., and Zuo, Y. (2020). A review of soil nematodes as biological indicators for the assessment of soil health. Front. Agric. Sci. Eng. 7, 275–281. doi: 10.15302/J-FASE-2020327
M.A.P.A. (1994). Métodos oficiales de análisis de suelos y agua (Madrid, Spain: Ministry of Agriculture).
Mazzola, M., Granatstein, D. M., Elfving, D. C., and Mullinix, K. (2001). Suppression of specific apple root pathogens by Brassica napus seed meal amendment regardless of glucosinolate content. Phytopathology. 91, 673–679. doi: 10.1094/PHYTO.2001.91.7.673
Meyer, S. L., Zasada, I. A., Rupprecht, S. M., VanGessel, M.J., Hooks, C.R., Morra, M.J., et al. (2015). Mustard seed meal for management of root-knot nematode and weeds in tomato production. Horttechnology. 25, 192–202. doi: 10.21273/HORTTECH.25.2.192
Mocali, S., Landi, S., Curto, G., Dallavalle, E., Infantino, A., and Colzi, C. (2015). Resilience of soil microbial and nematode communities after biofumigant treatment with defatted seed meals. Ind. Crops Prod. 75, 79–90. doi: 10.1016/j.indcrop.2015.04.031
Ney, L., Franklin, D., Mahmud, K., Cabrera, M., Hancock, D., Habteselassie, et al. (2019). Rebuilding soil ecosystems for improved productivity in biosolarized soils. Int. J. Agron. 4, 1–10. doi: 10.1155/2019/5827585
Ojinaga, M., Gandariasbeitia, M., Orbegozo, E., Ortíz, A., Guerrero, M. M., and Lacasa, C. M. (2020). Biodisinfestation for Meloidogyne and Verticillium control in commercial protected crops in the Basque Country Atlantic area (Northern Spain). Acta Hortic. 1270, 327–336. doi: 10.17660/ActaHortic.2020.1270.40
Oka, Y. (2010). Mechanisms of nematode suppression by organic soil amendments—a review. Appl. Soil Ecol. 44, 101–115. doi: 10.1016/j.apsoil.2009.11.003
Osman, N. S., Sapawe, N., Sapuan, M. A., Fozi, M. F., Azman, M. I., and Fazry, A. H. (2018). Sunflower shell waste as an alternative animal feed. Mat Today: Proc. 5, 21905–21910. doi: 10.1016/j.matpr.2018.07.049
Perea-Moreno, M. A., Manzano-Agugliaro, F., and Perea-Moreno, A. J. (2018). Sustainable energy based on sunflower seed husk boiler for residential buildings. Sustainability. 10, 3407. doi: 10.3390/su10103407
Robertiello, A., Angelini, L., Conte, L., Sciaraffia, F., Genevini, P. L., and Pialorsi, S. (1984). Sunflower hulls as a component of feeds. AWS. 10, 257–266. doi: 10.1016/0141-4607(84)90002-7
Rosskopf, E., Di Gioia, F., Hong, J. C., Pisani, C., and Kokalis-Burelle, N. (2020). Organic amendments for pathogen and nematode control. Annu. Rev. Phytopathol. 58, 277–311. doi: 10.1146/annurev-phyto-080516-035608
Scaglia, B., and Adani, F. (2009). Biodegradability of soil water soluble organic carbon extracted from seven different soils. J. Environ. Sci. 21, 641–646. doi: 10.1016/S1001-0742(08)62319-0
Seesao, Y., Gay, M., Merlin, S., Viscogliosi, E., Aliouat-Denis, C. M., and Audebert, C. (2017). A review of methods for nematode identification. J. Microbiol. Meth. 138, 37–49. doi: 10.1016/j.mimet.2016.05.030
Serrano-Pérez, P., Rosskopf, E., De Santiago, A., and Rodríguez-Molina, A. (2017). Anaerobic soil disinfestation reduces survival and infectivity of Phytophthora nicotianae chlamydospores in pepper. Sci. Hortic. 215, 38–48. doi: 10.1016/j.scienta.2016.12.003
Shaukat, R. A., Saqib, Q. M., Khan, M. U., Chougale, M. Y., and Bae, J. (2021). (2021). Bio-waste sunflower husks powder based recycled triboelectric nanogenerator for energy harvesting. Energy Rep. 7, 724–731. doi: 10.1016/j.egyr.2021.01.036
Shea, E. A., Fernández-Bayo, J. D., Hodson, A. K., Parr, A. E., López, E., and Achmon, Y. (2022). Biosolarization restructures soil bacterial communities and decreases parasitic nematode populations. Appl. Soil Ecol. 172, 104343. doi: 10.1016/j.apsoil.2021.104343
Shinmura, A. (2000). “Causal agent and control of root rot of welsh onion, “ in PSJ Soil-Borne Disease Workshop Report, 133–143.
Shrestha, U., Dee, M. E., Piya, S., Ownley, B. H., and Butler, D. M. (2020). Soil inoculation with Trichoderma asperellum, T. harzianum or Streptomyces griseoviridis prior to anaerobic soil disinfestation (ASD) does not increase ASD efficacy against Sclerotium rolfsii germination. Appl. Soil Ecol. 147, 103383. doi: 10.1016/j.apsoil.2019.103383
Shrestha, U., Ownley, B. H., Bruce, A., Rosskopf, E. N., and Butler, D. M. (2021a). Anaerobic soil disinfestation efficacy against Fusarium oxysporum is affected by soil temperature, amendment type, rate, and C: N ratio. Phytopathology. 111, 1380–1392. doi: 10.1094/PHYTO-07-20-0276-R
Shrestha, U., Swilling, K. J., and Butler, D. M. (2021b). Amendment properties affect crop performance, leaf tissue nitrogen, and soil nitrogen availability following soil treatment by anaerobic soil disinfestation. Front. Sustain. Food Syst. 5, 299. doi: 10.3389/fsufs.2021.694820
Simmons, C. W., Higgins, B., Staley, S., Joh, L. D., Simmons, B. A., and Singer, S. W. (2016). The role of organic matter amendment level on soil heating, organic acid accumulation, and development of bacterial communities in solarized soil. Appl. Soil Ecol. 106, 37–46. doi: 10.1016/j.apsoil.2016.04.018
Stockdale, E. A., and Watson, C. A. (2009). Biological indicators of soil quality in organic farming systems. Renew. Agric. Food Syst. 24, 308–318. doi: 10.1017/S1742170509990172
Testen, A. L., Rotondo, F., Mills, M. P., Horvat, M. M., and Miller, S. A. (2021). Evaluation of agricultural byproducts and cover crops as anaerobic soil disinfestation carbon sources for managing a soilborne disease complex in high tunnel tomatoes. Front. Sustain. Food Syst. 5, 145. doi: 10.3389/fsufs.2021.645197
Vance, E. D., Brookes, P. C., and Jenkinson, D. S. (1987). An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem, 19, 703–707. doi: 10.1016/0038-0717(87)90052-6
Wang, K. H., and McSorley, R. (2008). Exposure time to lethal temperatures for Meloidogyne incognita suppression and its implication for soil solarization. J. Nematol. 40, 7.
Keywords: biosolarization, ASD, commercial field, Meloidogyne incognita, nematode community, organic amendments
Citation: Gandariasbeitia M, López-Pérez JA, Juaristi B and Larregla S (2022) Sunflower Seed Husk as Promising By-Product for Soil Biodisinfestation Treatments and Fertility Improvement in Protected Lettuce Crop. Front. Sustain. Food Syst. 6:901654. doi: 10.3389/fsufs.2022.901654
Received: 22 March 2022; Accepted: 10 June 2022;
Published: 11 July 2022.
Edited by:
Snehasish Mishra, KIIT University, IndiaReviewed by:
Valeria Reginatto, University of São Paulo, BrazilUtsala Shrestha, The University of Tennessee, Knoxville, United States
Copyright © 2022 Gandariasbeitia, López-Pérez, Juaristi and Larregla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maite Gandariasbeitia, mgandariasbeitia@neiker.eus
 Maite Gandariasbeitia
Maite Gandariasbeitia José Antonio López-Pérez2
José Antonio López-Pérez2  Santiago Larregla
Santiago Larregla