Noradrenergic modulation of intrinsic and synaptic properties of lumbar motoneurons in the neonatal rat spinal cord
1
CNRS UMR 5227, Université de Bordeaux, Bordeaux, France
2
Département de Physiologie, GRSNC, Université de Montréal, Montréal, QC, Canada
Although it is known that noradrenaline (NA) powerfully controls spinal motor networks, few data are available regarding the noradrenergic (NAergic) modulation of intrinsic and synaptic properties of neurons in motor networks. Our work explores the cellular basis of NAergic modulation in the rat motor spinal cord. We first show that lumbar motoneurons express the three classes of adrenergic receptors at birth. Using patch-clamp recordings in the newborn rat spinal cord preparation, we characterized the effects of NA and of specific agonists of the three classes of adrenoreceptors on motoneuron membrane properties. NA increases the motoneuron excitability partly via the inhibition of a KIR like current. Methoxamine (α1), clonidine (α2) and isoproterenol (β) differentially modulate the motoneuron membrane potential but also increase motoneuron excitability, these effects being respectively inhibited by the antagonists prazosin (α1), yohimbine (α2) and propranolol (β). We show that the glutamatergic synaptic drive arising from the T13-L2 network is enhanced in motoneurons by NA, methoxamine and isoproterenol. On the other hand, NA, isoproterenol and clonidine inhibit both the frequency and amplitude of miniature glutamatergic EPSCs while methoxamine increases their frequency. The T13-L2 synaptic drive is thereby differentially modulated from the other glutamatergic synapses converging onto motoneurons and enhanced by presynaptic α1 and β receptor activation. Our data thus show that the NAergic system exerts a powerful and complex neuromodulation of lumbar motor networks in the neonatal rat spinal cord.
In all vertebrates including humans, the descending noradrenergic (NAergic) pathways, originating from the pons, have been shown to initiate and facilitate the expression of spinal locomotor output (Jordan et al., 2008
) and to modulate the segmental reflexes (Kitazawa et al., 1985
; Jankowska et al., 1998
). In the chronic spinal cat, the NAergic compounds were found to be the most effective pharmacological agents in initiating locomotion (Forssberg and Grillner, 1973
; Kiehn et al., 1992
; Chau et al., 1998a
; Barbeau and Norman, 2003
). Furthermore, the NAergic precursor L-DOPA has been shown to induce coordinated air stepping in intact or spinal rat pups (Van Hartesveldt et al., 1991
). Evidence regarding the role of NAergic modulation in locomotor networks has also been provided in in vitro preparations (Merrywest et al., 2002
; Rauscent et al., 2009
). In the isolated spinal cord preparation from newborn rats, noradrenaline (NA) induces a slow non-locomotor rhythm but appears to be a potent modulator of the ongoing locomotor rhythm (Kiehn et al., 1999
; Sqalli-Houssaini and Cazalets, 2000
; Gordon and Whelan, 2006
).
All NAergic receptors are G-protein coupled receptors that can be divided into three main classes: three α1-receptors (α1A, α1B, α1C), three α2-receptors (α2A, α2B, α2C) and three β-adrenoreceptors (β1, β2, β3) (Hein, 2006
). Little information is available on the precise role of these receptors in the NAergic neuromodulation of spinal motor networks. In the spinal cat, activation of the α2 receptors by specific agonists strongly improves functional rehabilitation after spinal cord injury (SCI), while α1 receptor agonists appears less potent (Rossignol et al., 2001
). By contrast, in the neonatal rat spinal cord, α1 receptor agonists induce motor activity and boost ongoing fictive locomotion while α2 and β receptor agonists solely slow down the chemically induced fictive locomotion (Sqalli-Houssaini and Cazalets, 2000
)
Collectively, these studies underlie the important neuromodulatory role of the NAergic system in the physiology and pathophysiology of the spinal motor neuronal networks. Paradoxically, few data are available regarding the modulation of intrinsic and synaptic properties sustaining the excitatory action of NA in motor networks.
Taking advantage of the knowledge accumulated on the organization of the lumbar motor network in the isolated spinal cord preparation of the newborn rat, our study provides the first detailed analysis of the cellular basis of the NAergic modulation in lumbar motor network. Patch-clamp experiments were conducted to precisely analyze the role of the three main types of adrenoreceptors in the modulation of the membrane properties and synaptic inputs of lumbar motoneurons.
Ethical Approval
Experiments were performed using 115 Wistar rats aged 1–5 days (mean 2.4 ± 0.1 days) bred in our laboratory. All experiments were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of Bordeaux 2 University.
Isolated Spinal Cord Preparation
The animals were chilled by hypothermia until reflexes were lost, then decapitated. A laminectomy was performed to remove the spinal cord. The spinal cord was cut at the thoracic level, placed in a recording chamber (approximate volume 5–6 ml) and superfused (2 ml min−1) with oxygenated (95% O2–5% CO2) physiological saline containing (in mM): NaCl 130, KCl 3, CaCl2 2.5, MgSO4 1.3, NaH2PO4 0.58, NaHCO3 25, glucose 10 adjusted to pH 7.4 with HCl. All experiments were performed at room temperature (25°C).
Spinal Cord Partitioning
A Vaseline wall was built with a syringe at the junction between the lumbar 2 (L2) and L3 level so that the low lumbar spinal cord (L3, L4 and L5 segments) could be superfused separately from the thoracic 13 (T13)/L2 locomotor network. The water tightness of the wall was checked at the beginning and at the end of the experiment by filling the pool until a meniscus was created. If no changes in the saline level occurred after 5 min, the wall was taken to be watertight.
Extracellular Recordings and Stimulations
The motor output was recorded in the differential mode from the ventral roots using extracellular stainless steel pin electrodes insulated with Vaseline. Locomotor-like activity was induced by bath applying a mixture of serotonin (5-HT, 18–20 μM) and N-methyl DL-aspartate (NMA, 18–20 μM) on the T8-L2 segments.
Electrophysiological Methods
Blind whole-cell patch-clamp recordings from the L4-L5 motoneurons were made using glass microelectrodes (15–20 MΩ) filled with a solution containing (in mM): K-gluconate 120, KCl 20, CaCl2 0.1, MgCl2 0.1, EGTA 1, HEPES 10, GTP 0.1, cAMP 0.2, leupeptin 0.1, Na2-ATP 3, and D-mannitol 77, pH 7.3. A liquid junction potential of +15 mV was experimentally determined (Neher, 1992
) and records were corrected for this potential. Motoneurons were identified by antidromic action potentials in response to ventral root stimulation. Electrophysiological recordings were analyzed using the Axograph software program (Axograph Scientific, Sydney, Australia). To assess the motoneuron excitability, the average spike frequency was calculated by taking the mean interspike interval across a current step. To compute the mean synaptic drive, intracellular recordings were divided into single locomotor cycles based on the extracellular recordings (see Fig. 1c in Bertrand and Cazalets, 1999
). Each isolated cycle was subsequently normalized (from 0 to 100%) by resampling and the mean locomotor drive was calculated by averaging the data from at least 25 cycles.
Miniature excitatory postsynaptic currents (mEPSCs) were recorded using glass microelectrodes filled with a solution containing (in mM): 150 CsCl, 1 EGTA, 10 HEPES, 0.1 CaCl2, 4.6 MgCl2, 2 ATP, and 0.5 GTP. A liquid junction potential of +7 mV was measured in these experimental conditions (Neher, 1992
) and post hoc corrections of the membrane potential values were performed in accordance. Due to the very small amplitude of the mEPSCs (around 6 pA, see Section “Results”), recordings were filtered at 300 Hz. Sequences of at least 5 min of synaptic activity were recorded at a holding potential of −75 mV. The traces obtained were analyzed with an algorithm developed in the software program Labview (National Instruments, Austin, TX, USA). The threshold of mEPSCs was set by eye and varied depending on the RMS noise level of the recordings. Automatically screened mEPSCs were accepted or rejected based on visual inspection. Statistical analysis was performed using the Kolmogorov–Smirnov (KS) test for distribution differences. Significant differences in the mean amplitude and frequency were tested using the Student’s paired t-test.
Series resistance (mean value: 28 ± 1 MΩ, n = 99 neurons) was monitored throughout the experiments and was not compensated. Data were discarded if series resistance varied more than ±20% of the initial value.
Drugs
Fresh drug solutions of NA and of the NAergic agonists and antagonists were prepared daily and protected from light exposure. All other drugs were prepared at stock solutions, aliquoted and frozen until use. 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) was diluted in DMSO with a final concentration of less than 1‰.
Immunohistochemistry
Wistar rats (n = 2, postnatal day 5) were deeply anesthetized with sodium pentobarbital and perfused through the ascending aorta with freshly prepared fixative containing 4% paraformaldehyde (PFA) in phosphate buffer (PB 0.1 M). Spinal cords were dissected out and postfixed in the fixative solution for approximately 4 h. They were thoroughly rinsed in 0.1 M PB and cryoprotected in 30% sucrose overnight. Spinal cords were sectioned to isolate lumbar segments (L3-L5) and coronal sections (25 μm thick) were cut on a freezing microtome (Leica SM2000R, Germany). Free-floating sections were processed for double immunofluorescence using polyclonal rabbit antibodies against (1) the alpha 1a adrenergic receptor (1:200, Sigma, Canada), (2) the alpha 2a adrenergic receptor (1:200, Sigma, Canada) and (3) the beta 1 adrenergic receptor (1:250, Santa Cruz, CA, USA), and the goat anti-choline acetyltransferase (ChAT, 1:200, Millipore, CA, USA). Sections were then put in goat anti-rabbit IgGs conjugated to FITC (1:200) and donkey anti-goat IgGs conjugated to Texas red (1:200, Jackson Immunoresearch Inc.). To verify the specificity of the antibodies, controls were done by omitting primary antibodies and, in each case, no visible staining was detected. The pre-absorption of NA antibodies with their immunizing peptide also showed their specificity (Milner et al., 1998
; Fauser et al., 2004
; Queiroz et al., 2008
).
Statistical Analysis
Statistical analyses were performed on raw data. For samples less than 10 neurons, statistical analyses were performed using non-parametric tests. Wilcoxon matched pairs or Mann–Whitney tests were used to compare two series of data. Kruskal–Wallis or Friedman one-way analysis of variance (ANOVA) were carried out to test for significant effects between the different drugs for unpaired or paired observations, respectively. Pairwise comparisons were performed using Dunn’s post-tests. The level of significance was set at p < 0.05. All data are expressed as mean ± standard error of the mean (SEM) in the text and figures. Asterisks in the figures indicate positive significance levels and the numbers in histogram bars or in parenthesis refers to the number of neurons examined.
Lumbar Motoneurons Express the Three Main Classes of Adrenoreceptors at Birth
Previous studies using radioligand binding have shown that the α1-receptors are detectable in motoneurons as early as P1–P5 while the α2a receptors are transiently expressed in rat motoneurons at high level during the embryonic and early postnatal periods. In contrast, few data are available concerning the expression of the β receptors in motoneurons (for review see Rekling et al., 2000
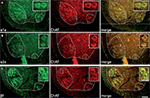
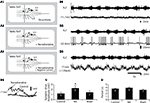
). Immunohistochemical labeling was then performed to determine whether the α1a, α2a and β1 receptors are expressed in lumbar motoneurons in early postnatal developmental stages. Immunolabeling of lumbar spinal cord sections revealed an α1a (Figure 1
A), α2a (Figure 1
B) and β1 positive (Figure 1
C, green immunofluorescence) immunoreactivity in large body neurons within lamina IX (dashed line in Figure 1
). To determine whether these labeled cells were motoneurons, we characterized the phenotype of the NAergic immunopositive neurons by immunohistochemical staining of ChAT (Figure 1
, red immunofluorescence). The merge panels in Figure 1
show that the large cell bodies in lamina IX were double stained for NAergic receptor subtypes and ChAT, therefore indicating that lumbar motoneurons express the α1a, α2a and β1 receptors at birth.
Figure 1. Expression of adrenoreceptor subtypes in cholinergic neurons in transverse sections of the lumbar spinal cord. Double labeling of large neuronal cell bodies in lamina IX (dashed lines) for alpha 1a (A); alpha 2a (B); beta 1 (C) noradrenergic receptors (green) and choline acetyltransferase (ChAT, red). The merge panels show that a high proportion of cholinergic neurons in lamina IX are immunopositive for the three different noradrenergic receptors. Scale bars 100 μm. The area boxed in the different panels is shown at higher magnification in the insets (scale bar 30 μm).
Noradrenergic Modulation of the Membrane Properties of the L4-L5 Lumbar Motoneurons
To precisely assess the action of NA, we used two different concentrations of NA (5 and 50 μM) since it was previously suggested in the neonatal rat spinal cord that NA mediated excitatory or inhibitory effects on motor networks through the differential activation of α1 and α2 adrenoreceptors depending on its concentration (Sqalli-Houssaini and Cazalets, 2000
). Furthermore we investigated the role of the different adrenoreceptors in the NAergic neuromodulation using specific agonists of the three classes of adrenoreceptors: methoxamine (α1), clonidine (α2) and isoproterenol (β).
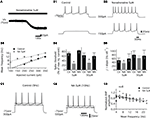
First, we tested the effects of NA on motoneuronal excitability in the presence of blockers of fast inhibitory (strychnine and bicuculline (10% of experiments) or gabazine, 1 μM, Barriere et al., 2008
) and excitatory (CNQX and AP5, 5 μM) synaptic transmission. In these conditions, NA induced a strong inward current in motoneurons held at −75 mV associated with a significant increase in the input membrane resistance computed from current–voltage curves, when bath-applied at both 5 μM (Figure 2
A and Table 1
) and 50 μM (Table 1
). When switched to current clamp conditions, NA depolarized all motoneurons tested (n = 16) beyond spike threshold at both low and high doses (data not shown). To determine whether NA modifies the instantaneous frequency–current (f–I) relationships of motoneurons, we applied a series of depolarizing current steps before (Figure 2
B1) and after (Figure 2
B2) bath applying NA in cells held at their control resting membrane potential by injecting hyperpolarizing bias current. As shown in Figure 2
B3 computed from the motoneuron presented in Figures 2
B1,B2, NA increased the spike frequency and decreased the spike threshold of the motoneuron. To quantify the NA-induced changes in lumbar motoneuron excitability, the spike threshold was expressed as the percentage of the maximum injected current needed to trigger a spike during a series of depolarizing current steps in normal saline and in the presence of NA. Figure 2
B4 shows that both 5 and 50 μM NA significantly and reversibly decreased spike threshold compared to control conditions. We then computed the slope of the f–I relationship for all motoneurons tested. The average f–I slope was significantly and reversibly decreased in the presence of 5 μM NA (Figure 2
B5, see also Figure 2
B3) and tended to be reduced when 50 μM NA was added to the saline (Figure 2
B5).
Table 1. Mean current induced by the bath-application of the different noradrenergic compounds recorded from motoneurones held at −75 mV and mean input resistance in control conditions and in the presence of the drugs.
Figure 2. Effects of noradrenaline (NA) on the membrane properties of the lumbar motoneurons. (A) Representative trace showing the NA-induced inward current in motoneurons held at −75 mV (Vh). (B) A representative cell recording showing that NA increases the excitability and the spike frequency (B1,B2) in response to depolarizing current pulses. (B3) Plot of the mean spike frequency as a function of the injected current from the cell shown in (B1,B2). (B4) The spike threshold computed as the percentage of the maximum injected current needed to evoke a spike during a series of depolarizing current steps in control conditions (Ctl), in the presence of NA and after a 30-min wash-out (Wh). (B5) Summary histograms of the slope of the frequency current (f–I) relationship in control conditions (Ctl), in the presence of NA and after a 30-min wash-out (Wh). (C) Representative traces of the spike AHP indicated by dashed lines in control (C1) and in the presence of NA (C2) at similar firing rates. Plot of normalized AHP amplitude as a function of the mean spiking frequency during a series of depolarizing steps in control conditions and in the presence of NA (C3). The dash lines in (B3,C3) correspond to the linear fits.
Spike afterhyperpolarization (AHP) plays a fundamental role in controlling the firing frequency and patterning of activity in motoneurons and is consequently targeted by almost all neuromodulatory systems (for examples see Bayliss et al., 1995
; Chevallier et al., 2006
; Han et al., 2007
). For each motoneuron, the relationship between the AHP amplitude and the firing frequency was well fitted using linear equation (mean r = 0.98 ± 0.01, n = 8) in control conditions. We sought whether NA modifies this relationship. As seen in the representative motoneuron recordings of Figures 2
C1,C2, at similar firing rate values, the AHP amplitude was not modified by the addition of NA (Figure 2
C2) compared to control conditions (Figure 2
C1). In the presence of NA, the relationship between the AHP amplitude and the firing rate was well fitted by linear equation (mean r = 0.97 ± 0.01). The pooled data plot of Figure 2
C3 shows the AHP amplitude, normalized in individual motoneuron tested by the maximum AHP amplitude computed in control conditions, and expressed as a function of the mean spike frequency measured during a series of depolarizing steps. The linear fit equation was −0.024x + 1 in control condition and −0.02x + 1 with 5 μM NA, n = 8 and −0.018x + 1 in control condition and −0.017x + 0.9, n = 5 with 50 μM NA in the bath (data not shown). The slopes and the intercepts of these linear fits were not significantly different between control condition and in the presence of NA. To validate this method, we applied short depolarizing pulses (4 ms) to evoke a single spike followed by an AHP in the absence or presence of NA. The amplitude and the duration of the AHP were not significantly changed after administration of NA (4.6 ± 0.9 mV and 200 ± 20 ms, respectively, in control conditions and 4.8 ± 0.9 mV and 231 ± 22 ms in NA; n = 6). Altogether these results indicate that NA modified the f–I relationship without affecting the AHP amplitude in the lumbar motoneurons of neonatal rats.
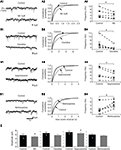
Lumbar motoneurons were previously shown to possess both the hyperpolarization-activated mixed-cation current IH and the inwardly rectifying K+ current KIR (Takahashi, 1990
; Kjaerulff and Kiehn, 2001
). Thus, we analyzed the effects of NA on IH and KIR currents.. Membrane potential was stepped from a holding potential of −75 to −155 mV (10 steps, 8 mV increment, 500 ms step duration) before (Figure 3
A1) and during application of NA (Figure 3
A2). The instantaneous current was measured immediately after the capacitive transient (filled circle in Figures 3
A1,A2) and the steady-state current at the end of the 500 ms hyperpolarizing pulse (open circle in Figures 3
A1,A2). The difference (filled triangle in Figure 3
A3) between the steady state current and the instantaneous current was defined as IH on the basis of its voltage dependence (Bayliss et al., 1994
; Kjaerulff and Kiehn, 2001
). Lumbar motoneurons exhibited a small inward current activated by hyperpolarization in control conditions (Figure 3
A1). Fifty micromolar NA did not alter the current–voltage (I–V) curve measured from series of hyperpolarizing pulses (Figures 3
A2,A3). The amplitude of IH measured at −150 mV (n = 13 motoneurons) was not significantly different between control conditions and in 5 or 50 μM NA (−34.4 ± 13 and −18.7 ± 24 pA, respectively). The modulation of KIR by NA was also studied using voltage ramps from −55 to −135 mV (Figure 3
B1). The control I–V relationship was subtracted from the NA I–V relationship to obtain the I–V relationship of the NA-suppressed current. Superfusion of NA (5 μM) inhibited an outward current at membrane potentials near rest that became inward around −110 mV (Figure 3
B2). The mean reversal potential measured in 5 μM (Erev, −104.4 ± 1.4 mV, n = 10) or 50 μM (−97.6 ± 3.1 mV, n = 6) NA was close to the K+ equilibrium potential (−99 mV) calculated with the Nernst equation. The chord conductance (G) of the current (I) measured at −80 mV (Vm) using the formula G = I/(Vm − Erev) as 3.5 ± 0.7 nS in 5 μM NA (n = 10) and 2.3 ± 1 nS in 50 μM NA (n = 6).
Figure 3. Effects of noradrenaline (NA) on IH and KIR currents. (A) Sample current traces obtained in response to a family of hyperpolarizing voltage steps, under control conditions (A1) and in the presence of NA (A2). The current–voltage (I–V) relationships for IH (▲) computed from the traces in (A1,A2) and obtained by subtracting the instantaneous current (●) from the steady state (○) current (A3). (B) I–V relationships during voltage ramps in control saline (black line) and in the presence of NA (grey trace) (B1). The NA-suppressed current was isolated by subtracting currents of I–V relationships obtained in the presence of NA from that obtained in control conditions (B2). (C) Current responses evoked by a series of voltage steps (10 steps, −8 mV increment) from a holding potential of −75 mV (VH) in control conditions (C1) and in the presence of 20 μM ZD7288 (C3). In the corresponding I–V relationships (C2,C4), IH I–V relationships (▲) computed from the traces in C1 and C3 results and obtained from subtracting the instantaneous current (●) from the steady state (○) current. (D). Instantaneous I–V curves derived from current responses generated by a series of voltage steps from −75 mV in the presence of 20 μM ZD7288 (ZD; –♦–) and after application of 5 μM NA (◊) (D1). (D2) NA-suppressed current calculated by subtraction of instantaneous I–V curves in (D1). The reversal potential for this current is indicated (Erev). Note the pronounced reduction of the outward part of the current. (E) Instantaneous I–V relationships in the presence of 20 μM ZD7288 (ZD, –♦–), ZD7288 + 500 μM Ba2+ (ZD + Ba2+;  ) and after application of 5 μM NA (ZD + Ba2+ + NA; ◊). (F). Instantaneous I–V relationships in the presence of 20 μM ZD7288 (ZD, –♦–), ZD7288 + 1 mM Cs+ (ZD + Cs+;
) and after application of 5 μM NA (ZD + Ba2+ + NA; ◊). (F). Instantaneous I–V relationships in the presence of 20 μM ZD7288 (ZD, –♦–), ZD7288 + 1 mM Cs+ (ZD + Cs+;  ) and after application of 5 μM NA (ZD + Cs+ + NA; ◊). (G). Histograms of the mean chord conductance of the suppressed current isolated by subtracting currents of instantaneous I–V curves obtained in the presence of the different compounds from that obtained in control conditions.
) and after application of 5 μM NA (ZD + Cs+ + NA; ◊). (G). Histograms of the mean chord conductance of the suppressed current isolated by subtracting currents of instantaneous I–V curves obtained in the presence of the different compounds from that obtained in control conditions.
 ) and after application of 5 μM NA (ZD + Ba2+ + NA; ◊). (F). Instantaneous I–V relationships in the presence of 20 μM ZD7288 (ZD, –♦–), ZD7288 + 1 mM Cs+ (ZD + Cs+;
) and after application of 5 μM NA (ZD + Ba2+ + NA; ◊). (F). Instantaneous I–V relationships in the presence of 20 μM ZD7288 (ZD, –♦–), ZD7288 + 1 mM Cs+ (ZD + Cs+;  ) and after application of 5 μM NA (ZD + Cs+ + NA; ◊). (G). Histograms of the mean chord conductance of the suppressed current isolated by subtracting currents of instantaneous I–V curves obtained in the presence of the different compounds from that obtained in control conditions.
) and after application of 5 μM NA (ZD + Cs+ + NA; ◊). (G). Histograms of the mean chord conductance of the suppressed current isolated by subtracting currents of instantaneous I–V curves obtained in the presence of the different compounds from that obtained in control conditions.As the voltage ramps protocol may activate both IH and KIR currents, we tested the effects of NA in the presence of ZD7288 a blocker of IH current. Since inwardly rectifying currents conduct more inward current (at membrane potentials hyperpolarized relative to the reversal potential, Erev) than outward current (at membrane potentials depolarized relative to Erev), we examined the chord conductance of the suppressed currents. The chord conductances were obtained by subtracting the instantaneous I–V curves obtained in the presence of the different pharmacological compounds from the curves obtained in control, at membrane potentials equidistant from the reversal potential at Vm = Erev − 25 (Erev − 25; for inward current) versus Vm = Erev + 25 (Erev + 25; for outward current; see Figure 3
D2) (Bertrand et al., 2003b
).
In motoneurons in which IH current was present in control conditions during series of hyperpolarizing pulses (Figures 3
C1,C2), 20 μM ZD7288 abolished a slow inward rectification (Figures 3
C3,G) and unmasked a fast inward rectification (Figures 3
C3,C4). The application of NA in the presence of ZD7288 reduced the instantaneous current over the entire voltage range tested (Figures 3
D1,D2). It is noticeable that as previously shown for serotonin (Kjaerulff and Kiehn, 2001
) the reduction was stronger for the outward part of the current (Figures 3
D2,G). KIR channels present the characteristic to be blocked in a voltage-independent manner by external Ba2+ and in a voltage-dependent manner, with no effect on outward current, by external Cs+ (Sodickson and Bean, 1996
; Bertrand et al., 2003a
). To assess whether NA modulates such a KIR current in lumbar motoneurons, the effects of NA were then investigated in the presence of ZD7288 in combination with Ba2+ or Cs+. Addition of Ba2+ (500 μM) during voltage steps series in the presence of ZD7288 revealed a block of both outward and inward part of the instantaneous I–V curves (Figure 3
E). The chord conductances of the Ba2+-sensitive current were characteristic of an inwardly rectifying current with significant different values measured at Erev − 25 and Erev + 25 (Figure 3
G). When NA was added to the ZD72288 + Ba2+ containing saline, no further effects were observed on the instantaneous I–V curves (Figures 3
E,G). As shown in Figures 3
F,G, Cs+ strongly reduced the inward component of the endogenous current and elicited a smaller decrease on its outward portion. In contrast to the Ba2+ block that occluded the NA effects with no voltage dependency, NA still decreased the outward portion of the Cs+-insensitive current but failed to affect its inward component in the presence of Cs+ and ZD7288 (Figures 3
F,G). In summary, this series of experiments indicate that KIR current is under NAergic neuromodulatory control in lumbar motoneurons. All effects of NA were fully reversible after a wash-out period of at least 30 min (data not shown).
To assess the role of the three classes of adrenoreceptors in the modulation of the motoneuronal membrane properties, the same paradigms were performed in the presence of the three different agonists. Sqalli-Houssaini and Cazalets (2000)
previously showed using extracellular recordings that 50 μM methoxamine, 100 μM isoproterenol and 100 μM clonidine slow down the locomotor rhythm. Based on these results, we determined the concentration of each agonist that consistently and reproducibly elicited changes in motoneuron membrane potential. We found that 80 μM methoxamine and 50 μM isoproterenol triggered an inward current in motoneurons held at −75 mV (Figure 4
A and Table 1
). In two neurons (data not shown), we observed that 100 or 500 μM clonidine caused no detectable variations in the motoneuron membrane potential while 1 mM clonidine induced a consistent outward current (Figure 4
A and Table 1
). Despite the fact that their effects on the membrane potential of the motoneurons differ, the three agonists increased the membrane input resistance of lumbar motoneurons (Table 1
). These changes in membrane input resistance were significantly different in the presence of methoxamine while they only showed a tendency to be increased in the presence of clonidine or isoproterenol (see Table 1
). We then tested the changes induced by methoxamine (Figure 4
B1), clonidine (Figure 4
B2) and isoproterenol (Figure 4
B3) on the motoneuron excitability. All three compounds reproduced the NA action, i.e., increased the firing frequency and decreased the spike threshold of lumbar motoneurons (Figures 4
B1–B3). All three NAergic agonists increased the excitability of all motoneurons tested by significantly reducing their spike threshold (Figure 4
C1). The slope of the f–I relationship computed during a series of depolarizing steps was significantly decreased in the presence of isoproterenol and also tended to decline in the presence of methoxamine and clonidine (Figure 4
C2). In the presence of the different NAergic agonists, the correlation coefficient of the linear fit of the relationship between the AHP amplitude and the firing rate in each neuron was not significantly different compared to control conditions (r = 0.98 ± 0.1 in control and 0.97 ± 0.1, n = 22 in the presence of the agonists). When the normalized AHP amplitude was expressed as a function of the mean frequency, the slope and the intercepts of the linear fit of this relationship were not significantly modified in the presence of clonidine for the population of motoneurons tested (Figure 4
C3; control −0.025x + 1; clonidine −0.025x + 1 n = 7), or of isoproterenol (data not shown, control −0.018x + 1; isoproterenol−0.022x + 1, n = 7) or of methoxamine (data not shown, control −0.03x + 1.2; methoxamine −0.033x + 1.2, n = 8). As illustrated in the presence of isoproterenol (Figure 4
D), none of the three adrenoreceptors agonists altered the I–V curve obtained from membrane voltage steps (10 steps, 8 mV increment). The amplitude of the IH current measured at −150 mV was −17 ± 4 pA in control, −21 ± 10 pA n = 7 with isoproterenol; −18.9 ± 13 in control, −10.2 ± 7 pA n = 8 with methoxamine and −26.3 ± 16 pA in control and −24.5 ± 5 pA n = 7 with clonidine. In contrast, voltage ramps revealed that methoxamine and clonidine suppressed a current with an I–V curve computed by the subtraction of I–V relationships obtained in control condition and in the presence of one of the agonist (Figure 4
E, example for methoxamine). In the presence of methoxamine or clonidine, the mean Erev of the suppressed current was −101 ± 2 and −94 ± 2 mV and the mean chord conductance measured at −80 mV was 2.7 ± 1 nS, n = 8 and 2.2 ± 0.5 nS, n = 7, respectively. Interestingly, we found that isoproterenol had a small inhibitory effect on the current expressed during voltage ramp in 50% of the motoneurons tested (Erev = −96 ± 2 mV and chord conductance at −80 mV = 1.1 ± 0.4 nS, n = 4) and failed to affect it in the remaining 50% (n = 4; data not shown). This depressing effect of the different NAergic agonists on inward rectifying currents was still observed in the presence of the IH blocker ZD7288 (n = 2 with methoxamine, n = 2 with clonidine and n = 1 with isoproterenol; data not shown).
Figure 4. Effects of the adrenoreceptor agonists on the membrane properties of the lumbar motoneurons. (A) Representative traces of the effects of methoxamine, clonidine and isoproterenol on the membrane potential of motoneurons held at −75 mV (Vh). (B) Representative traces of both the spike frequency and the threshold for spike generation during depolarizing current pulses (current value between the traces) in the absence and presence of methoxamine (B1), clonidine (B2) and isoproterenol (B3). (C,C1) Summary histograms of the spike threshold computed as the percentage of the maximum injected current needed to evoke spiking in the presence of methoxamine (Meth), clonidine (Clo) and isoproterenol (Iso) compared to control condition (Ctl). (C2) Summary histograms of the slopes of the frequency current (f–I) relationship in the absence (filled bars) or presence of the noradrenergic agonists (grey bars). (C3) Plot of the normalized AHP amplitude as a function of mean spiking frequency during series of depolarizing steps in the absence or presence of clonidine. The dashed lines correspond to the linear fits (D). Sample current traces obtained in response to a family of hyperpolarizing voltage steps (first step −155 mV, 8 mV increment) under control conditions and in the presence of isoproterenol (D1). The current–voltage (I–V) relationships (▲) computed from the traces in D1 results from subtracting the instantaneous (●) from the steady state (○) current (D2). (E) I–V relationships obtained during voltage ramps in control saline (black trace) and in the presence of methoxamine (grey trace) (E1). The methoxamine-suppressed current was isolated by subtracting currents of I–V relationship in the presence of the agonist from that in control condition (E2).
We then checked the specificity of the NAergic agonists by using specific antagonists. In a first step, we investigated whether prazosin (α1 antagonist), yohimbine (α2 antagonist) and propranolol (β?antagonist) induced changes in the membrane properties targeted by the NAergic agonists in lumbar motoneurons. None of these antagonists, which were first bath-applied alone to the in vitro spinal cord, triggered significant inward or outward currents in motoneurons held at −75 mV (Figure 5
A and Table 1
) and caused no significant changes in motoneuron input membrane resistance (Table 1
). Motoneuron excitability as well as the slopes of the f–I relationship were also not altered by the antagonists (Figure 5
B middle panels and 5C1-2). Prazosin (Figure 5
D1) and yohimbine (data not shown) did not change the I–V relationships during voltage ramps from −55 to −135 mV. In the presence of prazosin or yohimbine (dashed bars in Figure 5
D2), the mean chord conductance of the current, measured at −80 mV and computed by the subtraction of I–V relationships obtained in normal saline and in the presence of each antagonist, was small or even non-existent. The chord conductance of the current modulated by prazosin or yohimbine was significantly smaller than the methoxamine or clonidine-suppressed current reported above (open bars in Figures 5
D2 and 4
E). In the presence of propranolol, a small inhibitory effect on the I–V relationship was observed during voltage ramps (data not shown; Figure 5
D2).
Figure 5. Effects of adrenoreceptor antagonists on adrenoreceptor agonist-induced changes in membrane properties. (A) Representative traces showing the effects of different noradrenergic antagonists (α1 prazosin; α2 yohimbine; β propranolol) alone or in combination with their corresponding agonist (α1 methoxamine; α2 clonidine; β isoproterenol) on the current recorded from motoneurons held at −75 mV (Vh). (B) Representative traces showing the spike frequency and the threshold for spike generation during depolarizing current pulses (current value between the traces) in the presence of the antagonists alone or the antagonist–agonist combinations (C). (C1) Plot of the spike threshold (computed as the percentage of the maximum injected current needed to evoke spiking) in the presence of the three different antagonists (hatched bars; prazosin: Praz, yohimbine: Yoh and propranolol: Prop) and in the presence of the antagonist–agonist combinations (grey bars; prazosin plus methoxamine: Praz + Meth, yohimbine plus clonidine: Yoh + Clo and propranolol plus isoproterenol: Prop + Iso) compared to control condition (Ctl, black bars). (C2) Summary histograms of the slopes of the frequency current (f–I) relationship in control (filled bars), in the presence of the antagonists (hatched bars) and during co-applications of the antagonist and agonist (grey bars) (D). I–V relationships obtained during voltage ramps in control conditions (black line), in the presence of prazosin (dashed line) and in the presence of both prazosin and methoxamine (grey line) (D1). The current modulated by the noradrenergic antagonists was isolated by subtracting the current seen in the I–V relationship in the presence of the antagonist from that in control condition. Similarly, the current modulated by the antagonist–agonist combinations was isolated by subtracting the current of the I–V relationship in the presence of both antagonist and agonist from that in the presence of the antagonist alone. (D2) Summary histograms of the chord conductance of the current extracted from voltage ramps in the presence of the agonist alone (open bars), the antagonist alone (hatched bars), and in the presence of both antagonist and agonist (grey bars).
In a second step, we tested whether the antagonists abolished the actions of their respective agonists. As shown in Figure 5
A and in Table 1
, methoxamine and isoproterenol, when added to a prazosin or propranolol-containing medium, respectively, failed to induce any detectable inward current and a significant modification of the membrane input resistance. In the presence of yohimbine, clonidine still induced an outward current in motoneurons (Figure 5
A2) but with an intensity that was almost 50% less than in control condition (Table 1
). This reduced outward current was not associated with significant changes in the membrane input resistance (Table 1
). When co-applied with their corresponding antagonists, methoxamine (Figure 5
B1), clonidine (Figure 5
B2) or isoproterenol (Figure 5
B3) no longer caused an increase in motoneuron excitability. As evident in the summary plot in Figure 5
C1, spike threshold was not significantly altered by the different antagonist–agonist combinations. The significant change in the f–I relationship (Figure 4
C2) was no longer observed when isoproterenol was bath-applied with propranolol (Figure 5
C2). A similar effect on the f–I relationship was observed when methoxamine or clonidine were superfused in the presence of prazosin and yohimbine, respectively (Figure 5
C2). Finally, methoxamine bath-applied with prazosin (Figure 5
D1) or clonidine with yohimbine (data not shown) did not alter the current expressed during voltage ramp (Figure 5
D2). The chord conductance of the current computed by subtraction of voltage ramps in the presence of the antagonist alone (hatched bars in Figure 5
D2) and in the presence of both the antagonist and the agonist (grey bars in Figure 5
D2) was significantly smaller than the one computed in the presence of methoxamine or clonidine alone (open bars in Figure 5
D2). The chord conductance of the current observed during the co-application of isoproterenol and propranolol was further reduced, but not significantly, compared to the current suppression caused by isoproterenol alone (Figure 5
D2).
Altogether these data suggest that (1) the membrane potential of the motoneurons could be differentially modulated depending on the type of adrenoreceptors activated (2) the NAergic system increased the excitability of the lumbar motoneurons partly via the inhibition a KIR-like current and (3) the three different agonists, methoxamine, clonidine and isoproterenol, specifically activate α1-, α2- and β-receptors, respectively, in lumbar motoneurons.
All the effects of the adrenoreceptor agonists described herein were not reversible after a 30-min period of wash-out with normal saline. When it was possible to obtain it, a partial wash was however observed when the wash-out period was extended to 1 h (data not shown).
Noradrenergic Modulation of the Synaptic Inputs Received by the Lumbar Motoneurons
In the isolated spinal cord preparation of the newborn rat, the T13-L2 network sends a biphasic monosynaptic drive to the lumbar motoneurons consisting of alternating excitatory (glutamatergic) and inhibitory (glycinergic) synaptic inputs (Cazalets, 2000
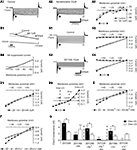
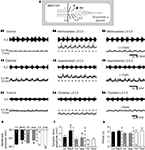
). Using the partitioned spinal cord preparation, experiments were conducted to analyze whether the NAergic system could modulate the synaptic inputs conveyed by the T13-L2 network to the L3-L5 motoneurons. A Vaseline wall was then built at the L2 level to specifically activate the T13-L2 network independently from the caudal motoneurons. Fictive locomotion was induced by bath-applying a mixture of NMA/5HT on the thoracic and upper lumbar segments and recorded extracellularly from the L2 ventral root and intracellularly from lumbar motoneurons (Figure 6
A). Strychnine (1 μM) was added to the L3-L5 compartment to isolate the glutamatergic synaptic drive (Figure 6
A1). In control conditions, the glutamatergic synaptic drive consists of small rhythmic depolarizations that could be in phase or out of phase with the extracellular ventral root recordings as an extensor and a flexor center are present in T13-L2 segments (Cazalets et al., 1995
; Butt et al., 2002
; Dougherty and Kiehn, 2009
) (Figure 6
B1 see also Figures 7
B1,C1,D1). The addition of NA (5 μM) to the L3-L5 compartment induced a strong depolarization of the recorded cells that reached the spike threshold generation in the majority of the cells tested (seven of nine neurons; mean membrane potential −73.5 ± 1 mV in control and −58.2 ± 2 mV in the presence of NA, n = 9). The spiking activity could be restricted to the depolarizing phase of the locomotor drive as illustrated in Figure 6
B2 (four neurons of seven) or sustained (three neurons of seven; data not shown). To analyze the variation of the excitatory synaptic drive induced by NA, hyperpolarizing bias currents were injected into the cells to return to their control membrane potential value (Figure 6
B3). Figure 6
B4 shows a representative trace of the mean synaptic drive (see Section “Materials and Methods”) computed in control condition and in the presence of NA. NA strongly and reversibly enhanced the amplitude of the synaptic drive conveyed by the T13-L2 locomotor network to the caudal lumbar motoneurons (Figure 6
C). This enhancing action is not associated with any change in the period of the fictive locomotor rhythm (Figure 6
D). When the NA concentration was raised to 50 μM on the caudal lumbar segments, the motoneuronal membrane potential was very difficult to stabilize and to maintain at control value. Therefore accurate measurement of the synaptic drive could not be performed (data not shown).
Figure 6. Effects of NA on the glutamatergic synaptic drive. (A) Schema of the experimental procedure. Vaseline wall (grey bar) was built at the L2 level. NMA/5HT was bath applied to the rostral compartment whereas strychnine alone (A1) or in combination with noradrenaline (NA) (A2,A3) was superfused onto the caudal compartment. (B) Representative traces recorded from a left L5 motoneuron (lL5) that was rhythmically depolarized out of phase with the lL2 ventral root activity (B1). In the presence of NA, the motoneuron was strongly depolarized and spiking activity was superposed on the depolarizing phases (B2). The injection of hyperpolarizing bias current (B3) revealed a strong amplification of the synaptic drive received by the motoneuron in the presence of NA. (B4) Mean synaptic drive computed from the traces in (B1,B3). Summary plots of the mean synaptic drive amplitude (C) and of the rhythm period (D) in the presence or absence of NA.
Using the same protocol, we subsequently examined the effects of the three adrenoreceptor agonists on the excitatory synaptic drive received by the lumbar motoneurons (Figure 7
A). In these experimental conditions, methoxamine bath-applied on the L3-L5 segments induced a significant depolarization of the motoneurons (Figure 7
E) that reached the threshold for spike generation (compare Figures 7
B1,B2). When motoneurons were held at their control membrane potential by injecting hyperpolarizing bias current (Figure 7
B3), we observed a strong potentiation of the locomotor synaptic drive in the presence of the α1 agonist (Figures 7
B3,F). When added to the L3-L5 compartment, the β agonist, isoproterenol significantly depolarized the lumbar motoneurons (Figures 7
C1,C2,E) and slightly but significantly enhanced the synaptic inputs received by the motoneurons during fictive locomotion (Figure 7
C3). In contrast, clonidine elicited an hyperpolarization of the motoneurons (Figures 7
D1,D2,E). When depolarizing bias current was injected into the cells to return to their control membrane potential value (Figure 7
D3), no significant change of the synaptic drive was observed (Figure 7
F). The modifications of the synaptic drive amplitude did not rely on an effect on the T13-L2 locomotor network as the period of the rhythm was not modified by the bath-application of the adrenoreceptor agonists on the caudal lumbar segments (Figure 7
G).
Figure 7. Effects of the NAergic agonists on the glutamatergic synaptic drive. (A) Schema of the experimental procedure. NMA/5HT was added to the saline superfused on the rostral compartment to elicit fictive locomotion recorded extracellularly from the L2 ventral roots. Strychnine was bath applied alone or in combination with one of the agonist on the caudal compartment of the spinal cord and motoneurons were recorded in the left part of the cord. Representative motoneurons recorded from the right or left L5 segment (rL5 or lL5) in control condition (B1,C1,D1) and in the presence of methoxamine (B2), isoproterenol (C2) or clonidine (D2). Hyperpolarizing (B3,C3) or depolarizing (D3) bias current (value in parenthesis) was injected into neurons to returned to their control membrane potential in the presence of the different noradrenergic agonists. The dash lines in (B2,C2,D2) correspond to the control membrane potential value of the neurons before the agonist application. Summary plot of the mean membrane potential (E), the mean synaptic drive amplitude (F) and the rhythm period (G) in the presence of methoxamine (black bars), isoproterenol (grey bars) and clonidine (white bars).
Presynaptic Control of the Glutamatergic Transmission
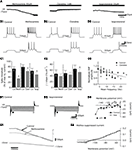
To determine whether the NAergic modulation of the excitatory synaptic drive described herein could partly rely on presynaptic mechanisms, we conducted mEPSCs recordings experiments from motoneurons. For this purpose, the motoneurons were synaptically isolated with tetrodotoxin (TTX; 0.5 μM) and the inhibitory synaptic inputs were blocked by adding 1 μM strychnine (glycinergic antagonist) and 1 μM gabazine (GABAergic antagonist) to the saline. mEPSCs were blocked by the NMDA and AMPA receptors antagonists AP5 and CNQX (5 μM, n = 3 data not shown) suggesting that they were due to the release of glutamate and the activation of ionotropic glutamate receptors. The control mean mEPSC amplitude was 6.3 ± 0.3 pA (n = 26 motoneurons). As shown in the representative traces in Figure 8
A1, 5 μM NA decreased mEPSC frequency and shifted the distribution of inter-event interval to the right (p < 0.05, KS test; Figure 7
A2). Figure 8
A3 shows that for each individual neuron (n = 5), NA significantly decreased the mean mEPSC frequency. The mean mEPSC frequency was reduced from 5.3 ± 1 Hz in control condition to 3.6 ± 1 Hz in the presence of NA. The pooled data also showed that the mean mEPSC amplitude was significantly altered (Figure 8
E). The same inhibitory effect on both mEPSC frequency and amplitude was observed using 50 μM NA. The mean mEPSC frequency and amplitude were respectively 7.2 ± 2 Hz and 6.4 ± 0.5 pA in normal saline and 5 ± 1.4 Hz and 5.6 ± 0.9 pA (n = 3, data not shown) in the presence of 50 μM NA. Next, we analyzed the effects of the three NAergic agonists on mEPSCs. We observed that clonidine and isoproterenol mimicked the inhibitory action of NA when superfused on the spinal cord. The bath-application of clonidine (Figure 8
B1) or isoproterenol (Figure 8
C1) decreased the mEPSC occurrence and significantly shifted the distribution of inter-event interval to the right (Figures 8
B2,C2). The mean mEPSC frequency was significantly decreased from 6.1 ± 1 Hz in normal saline to 3.9 ± 1 Hz (n = 5) in the presence of clonidine and from 7.2 ± 2 to 5.3 ± 1 Hz (n = 5) in the presence of isoproterenol. We observed a significant decrease of the mEPSC amplitude with isoproterenol and a substantial but not significant reduction in the presence of clonidine (Figure 8
E). In contrast, methoxamine elicited an increase in the frequency of mEPSCs (Figure 8
D1) and significantly shifted the distribution of inter-event interval to the left (Figure 8
D2). The mean mEPSC frequency was significantly increased from 4 ± 0.7 Hz in control conditions to 5.2 ± 0.5 Hz (n = 5) in the presence of methoxamine (Figure 8
D3) while the mean mEPSC amplitude was not significantly reduced (Figure 8
E).
Figure 8. Effects of noradrenaline and its agonists on mEPSCs. (A) Examples of mEPSCs recorded under control condition at −75 mV and in the presence of 5 μM NA (A1). Cumulative distributions of intervals between mEPSCs (A2) for the motoneuron in (A1). Line plots illustrating the actions of NA on the frequency of mEPSCs in each neuron of the sample (A3). (B,C,D) As for (A) but in the presence of clonidine (B), isoproterenol (C) and methoxamine (D). (E) Summary histogram of the mean amplitude of the mEPSCs in control conditions (black bars) and in the presence of the various NAergic agents (grey bars).
The present work provides the first detailed analysis of the spinal cellular targets of the NAergic pathways in the lumbar motor spinal cord and reveals a very complex neuromodulatory system.
Modulation of Intrinsic Properties
Voltage clamp recordings from neurons with extended dendritic tree, as motoneurons, should be interpreted with caution. In this study, we compare control conditions to different pharmacological conditions. Although we undoubtedly did not clamp the whole dendritic tree of the motoneurons, we reported a strong inhibitory effect on the KIR current by the NAergic agents during voltage ramps or pulses. The quantification of this effect is certainly reduced due to sub-optimal voltage clamp conditions, but its occurrence remains. Although the NAergic agents differentially modulate the membrane potential of the motoneurons, they all increased their excitability and input membrane resistance and reduced a KIR-like conductance. These results are in agreement with previous studies showing that membrane depolarization associated with an input resistance increase underlie the NAergic-induced increase in hypoglossal and cervical motoneuron excitability (Kitazawa et al., 1985
; Bayliss et al., 1997
). Lumbar motoneurons, however, exhibit a specific NAergic neuromodulatory profile since AHP and IH current are not targeted by the NAergic system in these neurons. This contrasts with data obtained from hypoglossal motoneurons in which NA reduces the AHP amplitude (Parkis et al., 1995
) and clonidine inhibits the IH current (Parkis and Berger, 1997
). These differences between hypoglossal and lumbar locomotor motoneurons could result from distinct NAergic receptor sub-type expression and/or intracellular coupling (Rekling et al., 2000
). The KIR channels contribute to the resting membrane potential and neuronal excitability and for these reasons, they constitute the main targets of numerous G-protein coupled receptors (see for examples Bertrand et al., 2003a
,b
; Derjean et al., 2003
; Chevallier et al., 2008
). In the neonatal rat spinal cord, it has been shown that serotonin as NA increases the motoneuron excitability partly via the inhibition of KIR-like current (Kjaerulff and Kiehn, 2001
). A question now arises over how NA changes the slope of the f–I relationship without affecting the AHP amplitude. NA was reported to increase the excitability of spinal lumbar neurons by hyperpolarizing the spike threshold probably via a facilitation of the activation of Na+ channels (Fedirchuk and Dai, 2004
). Serotonin has been shown to facilitate an L-type calcium current in lumbar motoneurons (Li et al., 2007
). The modulation of such calcium channels by NA could maybe also account for the changes observed in the f–I relationships slope.
Specificity of the NAergic Agonists
As previously mentioned, we investigated the threshold dose at which the agonist elicited significant changes in the motoneuron membrane potential. Using this criterion, some NAergic agonists were used at relatively high concentrations. We show, however, that the specific antagonists of NAergic receptors inhibit most of the effects of the NAergic agonists on the motoneuron membrane properties. This suggests that despite the high concentrations used, methoxamine, clonidine and isoproterenol specifically activate α1, α2 and β receptors respectively. The need to use high clonidine concentration in newborn animals (see also Selvaratnam et al., 1998
) maybe linked to the weak expression of the α2 receptors at this developmental stages and to the properties of the α2 receptor sub-types in motoneurons at birth.
Modulation of Synaptic Transmission
The increase in the motor synaptic drive we reported herein could originate from a direct action on the motoneurons and/or a modulation of the glutamatergic transmission at the presynaptic level and/or on the rhythm-generating L3-L6 network of premotor interneurons (Kiehn, 2006
). To discriminate among these various levels, we have used the partitioned spinal cord preparation and mEPSC recordings.
As previously mentioned, NA induces a slow non-locomotor rhythm and slows down the NMA-induced rhythm in the neonatal rat spinal cord preparation. In the neonatal rat spinal cord preparation, NA at the concentrations used in the present study, induces a slow non-locomotor rhythm and slows down the NMA- or NMA/5HT-induced fictive locomotion (Kiehn et al., 1999
; Sqalli-Houssaini and Cazalets, 2000
). If we hypothesize that NA acts on the rhythm generating interneurons located in the L3-L6 segments, modifications of the locomotor period and/or locomotor pattern should have been observed in the intracellular and/or extracellular recordings. Such effects were no observed in this study. It has been also shown that the α1 agonist methoxamine speeds up the fictive locomotor rhythm without modifying the motor burst amplitude. On the contrary, the α2 and β agonist receptors slow down the motor rhythm while simultaneously increasing the motor burst amplitude in the case of isoproterenol (Sqalli-Houssaini and Cazalets, 2000
). If the potentiation of the T13-L2 synaptic drive solely relies on an activation of the L3-L6 CPG interneurons, we should have observed opposite effects on the locomotor period and on the synaptic drive amplitude during the superfusion of the α1 agonist versus the α2 and β agonists. In the present study however, we observed that both methoxamine and isoproterenol increased the T13-L2 synaptic drive while clonidine failed to affect it. No effects were also reported on the locomotor period with any of the agonists. Although, these results suggest that the NAergic compounds directly control the T13-L2 descending glutamatergic inputs, the partial contribution of the L3-L6 interneurons to the increase of the T13-L2 synaptic drive could not, however, be completely excluded.
All NAergic agents except methoxamine decrease both the mEPSCs frequency and amplitude. In contrast, all NAergic compounds tested, except clonidine, enhance the excitatory synaptic drive arising from the T13-L2 network in L3-L5 motoneurons isolated with Vaseline wall. mEPSCs result from the glutamate release of all active synapses converging onto motoneurons. Using the partitioned spinal cord, we isolate the T13-L2 network synaptic drive from the other glutamatergic synapses impinging onto the motoneurons. Altogether our results then suggest that the glutamatergic inputs of the motoneurons, but certainly not all, are presynaptically inhibited via the activation of α2 and β adrenoreceptors and potentiated via the activation of α1 adrenoreceptors, while the T13-L2 glutamatergic transmission is presynaptically enhanced by the activation of α1adrenoreceptors. As the T13-L2 segments are considered as the main rhythmogenic area of the lumbar cord (Cazalets, 2000
), these results suggest that this locomotor-related excitatory synaptic drive could be specifically selected and favored by the NAergic pathways. These data further extend our knowledge on the modulation of the T13-L2 synaptic drive that was shown to be inhibited by the activation of presynaptic GABAB receptors and pre-and postsynaptic GABAA receptors (Bertrand and Cazalets, 1999
).
Physiological and Pathophysiological Relevance
Numerous studies have emphasized the role of the NAergic system in motor rhythms and segmental reflex modulations in animal models and humans (as for examples: Kitazawa et al., 1985
; Chau et al., 1998a
; Jankowska et al., 1998
; Kiehn et al., 1999
; Remy-Neris et al., 1999
; Sqalli-Houssaini and Cazalets, 2000
; Fischer et al., 2001
; Barbeau and Norman, 2003
; Gabbay and Lev-Tov, 2004
; Machacek and Hochman, 2006
; Barriere et al., 2008
). Our data shed light on the complexity of the cellular bases of the NAergic modulation in the motor spinal networks. We have previously shown that NA controls the activity dependant plasticity expressed at sensorimotor synapses via the activation of GABAergic interneurons (Barriere et al., 2008
). We demonstrated in the present study that depending on the adrenoreceptor expression and localization, the NAergic system could differentially and specifically modulate at both pre- and postsynaptic level the synaptic inputs and intrinsic properties of lumbar motoneurons.
After SCI, the excitatory supraspinal pathways controlling the spinal motor networks are damaged or lost. Functional rehabilitation strategies after SCI attempt, using various methods, to stimulate the sublesioned spinal cord. In spinal cats, α2agonists are very efficient agents at initiating locomotion (Chau et al., 1998b
). In rodents models in contrast, α2 agonists appeared less potent than α1 agonists in inducing locomotor activity (Sqalli-Houssaini and Cazalets, 2000
; Lapointe et al., 2008
). In the present work, we provide part of the cellular bases accounting for the different potencies of clonidine and methoxamine in stimulating spinal motor networks in rat. We indeed showed that clonidine while it increases the motoneuron excitability globally inhibits the glutamatergic transmission received by the motoneurons in contrast to methoxamine that boosts it. It will be therefore very interesting to compare the cellular basis of the NAergic neuromodulation in intact and sublesioned lumbar motor networks and to assess the effect of methoxamine in spinal rodent models.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors warmly thank Drs. Le Ray and Simmers for critical reading of the manuscript. This work was supported by grants from the conseil regional d’Aquitaine and by a CIHR-CNRS International Scientific Exchange Award. Jean-Claude Lacaille is supported by the Canadian Institutes of Health Research (operating grant MOP-10848), Fonds de la Recherche en Santé du Québec (FRSQ; Groupe de Recherche sur le système nerveux central), and the Canada Research Chair in Cellular and Molecular Neurophysiology. Grégory Barrière was supported by a postdoctoral fellowship from IRP.