Molecular Aspects and Therapeutic Implications of Herbal Compounds Targeting Different Types of Cancer
Abstract
:1. Introduction
2. Pathophysiology of Cancer
3. Types of Cancer and Their Targets for the Treatments
3.1. Bladder Cancer
3.2. Breast Cancer
3.3. Colorectal Cancer (CRC)
3.4. Kidney Cancer
3.5. Lung Cancer
3.6. Lymphoma
3.7. Melanoma Cancer
3.8. Oral and Oropharyngeal Cancer
3.9. Pancreatic Cancer
3.10. Prostate Cancer
3.11. Thyroid Cancer
3.12. Uterine Cancer
3.13. Adenoid Cystic Carcinoma (ACC)
3.14. Amyloidosis Cancer
3.15. Anal Cancer
3.16. Astrocytoma Cancer
3.17. Bone Cancer
4. Natural Compounds with Anti-Cancer Properties
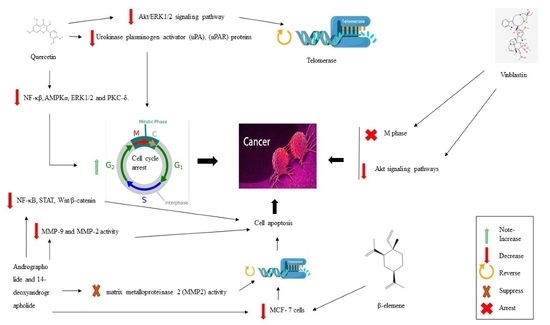
4.1. Quercetin
4.2. Andrographolide and 14-Deoxyandrographolide
4.3. Vinblastine
4.4. β-Elemene
4.5. Curcumin
4.6. Salinosporamide A
4.7. Chalcones
4.8. Baicalein
4.9. Neferine
4.10. 9-Methoxy Ellipticine
4.11. Rutin, Scopoletin, Kaempferol
4.12. Ginsenosides
4.13. Aloe-Emodin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Meacham, C.E.; Morrison, S.J. Tumour Heterogeneity and Cancer Cell Plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer Heterogeneity: Implications for Targeted Therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.M.; et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl. Cancer Inst. 2015, 1, 107. [Google Scholar] [CrossRef]
- Schottenfeld, D.; Fraumeni, J.F., Jr. Cancer Epidemiology and Prevention; Oxford University Press: Madison Avenue, NY, USA, 2006. [Google Scholar]
- Yoo, K.Y.; Shin, H.R. Cancer Epidemiology and Prevention. Korean J. Epidemiol. 2003, 25, 1–15. [Google Scholar]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Toma, M.M.; Gitea, D.; et al. Current Perspective on the Natural Compounds and Drug Delivery Techniques in Glioblastoma Multiforme. Cancers 2021, 13, 2765. [Google Scholar] [CrossRef]
- Kaplan, W. Background Paper 6.5 Cancer and Cancer Therapeutics. World Health Organ. Ed Prior. Med. Eur. World Update 2013, 6.5-1–6.5-62. Available online: https://www.iccp-portal.org/system/files/resources/s20252en.pdf (accessed on 5 December 2022).
- Rosenberg, S.A.; Restifo, N.P. Adoptive Cell Transfer as Personalized Immunotherapy for Human Cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Nie, F.; Yu, X.; Huang, M.; Wang, Y.; Xie, M.; Ma, H.; Wang, Z.; De, W.; Sun, M. Long Noncoding RNA ZFAS1 Promotes Gastric Cancer Cells Proliferation by Epigenetically Repressing KLF2 and NKD2 Expression. Oncotarget 2017, 8, 38227. [Google Scholar] [CrossRef] [Green Version]
- Newton, H.B. Neurological Complications of Systemic Cancer. Am. Fam. Physician 1999, 59, 878. [Google Scholar]
- Arboix, A. Cerebrovascular Disease in the Cancer Patient. Rev. Neurol. 2000, 31, 1250–1252. [Google Scholar]
- De Bruin, M.L.; Dorresteijn, L.D.; van’t Veer, M.B.; Krol, A.D.; Van der Pal, H.J.; Kappelle, A.C.; Boogerd, W.; Aleman, B.M.; Van Leeuwen, F.E. Increased Risk of Stroke and Transient Ischemic Attack in 5-Year Survivors of Hodgkin Lymphoma. JNCI J. Natl. Cancer Inst. 2009, 101, 928–937. [Google Scholar] [CrossRef] [Green Version]
- Ruocco, M.R.; Avagliano, A.; Granato, G.; Vigliar, E.; Masone, S.; Montagnani, S.; Arcucci, A. Metabolic Flexibility in Melanoma: A Potential Therapeutic Target. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 59, pp. 187–207. [Google Scholar]
- De Luca, A.; Maiello, M.R.; D’Alessio, A.; Frezzetti, D.; Gallo, M.; Carotenuto, M.; Normanno, N. Pharmacokinetic Drug Evaluation of Palbociclib for the Treatment of Breast Cancer. Expert Opin. Drug Metab. Toxicol. 2018, 14, 891–900. [Google Scholar] [CrossRef]
- De Castro Sant’Anna, C.; Junior, A.G.F.; Soares, P.; Tuji, F.; Paschoal, E.; Chaves, L.C.; Burbano, R.R. Molecular Biology as a Tool for the Treatment of Cancer. Clin. Exp. Med. 2018, 18, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Karati, D.; Kumar, D. Exploring the structural and functional requirements of Phyto-compounds and their synthetic scaffolds as anticancer agents: Medicinal chemistry perspective. Pharmacol. Res.-Mod. Chin. Med. 2022, 4, 100123. [Google Scholar] [CrossRef]
- Achi, I.T.; Sarbadhikary, P.; George, B.P.; Abrahamse, H. Multi-Target Potential of Berberine as an Antineoplastic and Antimetastatic Agent: A Special Focus on Lung Cancer Treatment. Cells 2022, 11, 3433. [Google Scholar] [CrossRef]
- Foresto, E.; Gilardi, P.; Ibarra, L.E.; Cogno, I.S. Light-activated green drugs: How we can use them in photodynamic therapy and mass-produce them with biotechnological tools. Phytomed. Plus 2021, 1, 100044. [Google Scholar] [CrossRef]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal Plants and Cancer Chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ng, P.K.-S.; Kucherlapati, M.; Chen, F.; Liu, Y.; Tsang, Y.H.; de Velasco, G.; Jeong, K.J.; Akbani, R.; Hadjipanayis, A. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/MTOR Pathway Alterations. Cancer Cell 2017, 31, 820–832.e3. [Google Scholar] [CrossRef] [Green Version]
- Cizkova, M.; Cizeron-Clairac, G.; Vacher, S.; Susini, A.; Andrieu, C.; Lidereau, R.; Bièche, I. Gene Expression Profiling Reveals New Aspects of PIK3CA Mutation in ERalpha-Positive Breast Cancer: Major Implication of the Wnt Signaling Pathway. PLoS ONE 2010, 5, e15647. [Google Scholar] [CrossRef] [Green Version]
- Farazi, P.A.; DePinho, R.A. Hepatocellular Carcinoma Pathogenesis: From Genes to Environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Schwartz, M.; Mazzaferro, V. Resection and Liver Transplantation for Hepatocellular Carcinoma. In Seminars in Liver Disease; Thieme Medical Publishers, Inc., 333 Seventh Avenue: New York, NY, USA, 2005; Volume 25, pp. 181–200. [Google Scholar]
- Fukui, Y. Mechanisms behind Signet Ring Cell Carcinoma Formation. Biochem. Biophys. Res. Commun. 2014, 450, 1231–1233. [Google Scholar] [CrossRef]
- Kikushige, Y. Pathophysiology of Chronic Lymphocytic Leukemia and Human B1 Cell Development. Int. J. Hematol. 2020, 111, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yang, Y.; Gad, E.; Wenner, C.A.; Chang, A.; Larson, E.R.; Dang, Y.; Martzen, M.; Standish, L.J.; Disis, M.L. Polysaccharide Krestin Is a Novel TLR2 Agonist That Mediates Inhibition of Tumor Growth via Stimulation of CD8 T Cells and NK CellsPSK Activates TLR2. Clin. Cancer Res. 2011, 17, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pelsmaeker, S.; Devriendt, B.; De Regge, N.; Favoreel, H.W. Porcine NK Cells Stimulate Proliferation of Pseudorabies Virus-Experienced CD8+ and CD4+ CD8+ T Cells. Front. Immunol. 2019, 9, 3188. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ Biology in Cancer Progression and Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Mitra, A.P.; Datar, R.H.; Cote, R.J. Molecular Pathways in Invasive Bladder Cancer: New Insights into Mechanisms, Progression, and Target Identification. J. Clin. Oncol. 2006, 24, 5552–5564. [Google Scholar] [CrossRef]
- Benson, J.R.; Jatoi, I.; Keisch, M.; Esteva, F.J.; Makris, A.; Jordan, V.C. Early Breast Cancer. Lancet 2009, 373, 1463–1479. [Google Scholar] [CrossRef]
- Ellis, M.J.; Coop, A.; Singh, B.; Tao, Y.; Llombart-Cussac, A.; Jänicke, F.; Mauriac, L.; Quebe-Fehling, E.; Chaudri-Ross, H.A.; Evans, D.B. Letrozole Inhibits Tumor Proliferation More Effectively than Tamoxifen Independent of HER1/2 Expression Status. Cancer Res. 2003, 63, 6523–6531. [Google Scholar]
- Wigner, P.; Bijak, M.; Saluk-Bijak, J. The green anti-cancer weapon. The role of natural compounds in bladder cancer treatment. Int. J. Mol. Sci. 2021, 22, 227787. [Google Scholar] [CrossRef] [PubMed]
- Maximov, P.Y.; Lee, T.M.; Jordan, C.V. The Discovery and Development of Selective Estrogen Receptor Modulators (SERMs) for Clinical Practice. Curr. Clin. Pharmacol. 2013, 8, 135–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodsell, D.S. The Molecular Perspective: Tamoxifen and the Estrogen Receptor. Oncologist 2002, 7, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.G. The NSABP Study of Tamoxifen and Raloxifene (STAR) Trial. Expert Rev. Anticancer Ther. 2009, 9, 51–60. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R. Natural products for the management and prevention of breast cancer. Evid.-Based Complement. Altern. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef]
- Berg, M.; Danielsen, S.A.; Ahlquist, T.; Merok, M.A.; Ågesen, T.H.; Vatn, M.H.; Mala, T.; Sjo, O.H.; Bakka, A.; Moberg, I. DNA Sequence Profiles of the Colorectal Cancer Critical Gene Set KRAS-BRAF-PIK3CA-PTEN-TP53 Related to Age at Disease Onset. PLoS ONE 2010, 5, e13978. [Google Scholar] [CrossRef]
- Elbadawy, M.; Usui, T.; Yamawaki, H.; Sasaki, K. Emerging Roles of C-Myc in Cancer Stem Cell-Related Signaling and Resistance to Cancer Chemotherapy: A Potential Therapeutic Target against Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2340. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.-S.; Chen, H.-Y.; Que, Y.; Xiao, W.; Zeng, M.-S.; Zhang, X. ALKATI Interacts with C-Myc and Promotes Cancer Stem Cell-like Properties in Sarcoma. Oncogene 2020, 39, 151–163. [Google Scholar] [CrossRef]
- Nappi, A.; Berretta, M.; Romano, C.; Tafuto, S.; Cassata, A.; Casaretti, R.; Silvestro, L.; Divitiis, C.; Alessandrini, L.; Fiorica, F. Metastatic Colorectal Cancer: Role of Target Therapies and Future Perspectives. Curr. Cancer Drug Targets 2018, 18, 421–429. [Google Scholar] [CrossRef]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Weiss, R.H. Metabolomics and Metabolic Reprogramming in Kidney Cancer. In Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 38, pp. 175–182. [Google Scholar]
- Sanchez-Gastaldo, A.; Kempf, E.; Del Alba, A.G.; Duran, I. Systemic Treatment of Renal Cell Cancer: A Comprehensive Review. Cancer Treat. Rev. 2017, 60, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Kovaleva, O.V.; Samoilova, D.V.; Shitova, M.S.; Gratchev, A. Tumor Associated Macrophages in Kidney Cancer. Anal. Cell. Pathol. 2016, 2016, 9307549. [Google Scholar] [CrossRef] [Green Version]
- Porta, C.; Cosmai, L.; Leibovich, B.C.; Powles, T.; Gallieni, M.; Bex, A. The Adjuvant Treatment of Kidney Cancer: A Multidisciplinary Outlook. Nat. Rev. Nephrol. 2019, 15, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Lipworth, L.; Tarone, R.E.; McLaughlin, J.K. The Epidemiology of Renal Cell Carcinoma. J. Urol. 2006, 176, 2353–2358. [Google Scholar] [CrossRef]
- Lenis, A.T.; Donin, N.M.; Johnson, D.C.; Faiena, I.; Salmasi, A.; Drakaki, A.; Belldegrun, A.; Pantuck, A.; Chamie, K. Adjuvant Therapy for High Risk Localized Kidney Cancer: Emerging Evidence and Future Clinical Trials. J. Urol. 2018, 199, 43–52. [Google Scholar] [CrossRef]
- Kang, H.G.; Lee, H.K.; Cho, K.B.; Park, S.I. A review of natural products for prevention of acute kidney injury. Medicina 2021, 57, 1266. [Google Scholar] [CrossRef]
- Pick, A.M.; Nystrom, K.K. Pazopanib for the Treatment of Metastatic Renal Cell Carcinoma. Clin. Ther. 2012, 34, 511–520. [Google Scholar] [CrossRef]
- Dorff, T.B.; Pal, S.K.; Quinn, D.I. Novel Tyrosine Kinase Inhibitors for Renal Cell Carcinoma. Expert Rev. Clin. Pharmacol. 2014, 7, 67–73. [Google Scholar] [CrossRef]
- Mulders, P.; Hawkins, R.; Nathan, P.; de Jong, I.; Osanto, S.; Porfiri, E.; Protheroe, A.; van Herpen, C.M.; Mookerjee, B.; Pike, L. Cediranib Monotherapy in Patients with Advanced Renal Cell Carcinoma: Results of a Randomised Phase II Study. Eur. J. Cancer 2012, 48, 527–537. [Google Scholar] [CrossRef]
- Gross-Goupil, M.; Massard, C.; Ravaud, A. Targeted Therapies in Metastatic Renal Cell Carcinoma: Overview of the Past Year. Curr. Urol. Rep. 2012, 13, 16–23. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; Volume 94, pp. 1623–1640. [Google Scholar]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran Jr, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung Cancer: Current Therapies and New Targeted Treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Kurban, T.G.; Ishiwata, Y.P.; Lu, T.; Fujii, K.; Kawahara, Z.; Naito, N.; Yamada, G.A. Expression and Intracytoplasmic Signal Transduction Pathway of Fibroblast Growth Factor (FGF)-10 in Human Cervical Cancer Cell Lines. J. Nippon Med. Sch. 2001, 68, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.C.; Tretiakova, M.S.; Nallasura, V.; Jagadeeswaran, R.; Husain, A.N.; Salgia, R. Downstream Signalling and Specific Inhibition of C-MET/HGF Pathway in Small Cell Lung Cancer: Implications for Tumour Invasion. Br. J. Cancer 2007, 97, 368–377. [Google Scholar] [CrossRef]
- Kinoshita, T.; Goto, T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int. J. Mol. Sci. 2019, 20, 1461. [Google Scholar] [CrossRef]
- Pockley, A.G.; Vaupel, P.; Multhoff, G. NK Cell-Based Therapeutics for Lung Cancer. Expert Opin. Biol. Ther. 2020, 20, 23–33. [Google Scholar] [CrossRef]
- Eapen, M.S.; Hansbro, P.M.; Larsson-Callerfelt, A.-K.; Jolly, M.K.; Myers, S.; Sharma, P.; Jones, B.; Rahman, M.A.; Markos, J.; Chia, C. Chronic Obstructive Pulmonary Disease and Lung Cancer: Underlying Pathophysiology and New Therapeutic Modalities. Drugs 2018, 78, 1717–1740. [Google Scholar] [CrossRef]
- Lin, M.; Luo, H.; Liang, S.; Chen, J.; Liu, A.; Niu, L.; Jiang, Y. Pembrolizumab plus Allogeneic NK Cells in Advanced Non–Small Cell Lung Cancer Patients. J. Clin. Investig. 2020, 130, 2560–2569. [Google Scholar] [CrossRef]
- Wen, T.; Song, L.; Hua, S. Perspectives and controversies regarding the use of natural products for the treatment of lung cancer. Cancer Med. 2021, 10, 2396–2422. [Google Scholar] [CrossRef]
- Adrianzen Herrera, D.; Ashai, N.; Perez-Soler, R.; Cheng, H. Nanoparticle Albumin Bound-Paclitaxel for Treatment of Advanced Non-Small Cell Lung Cancer: An Evaluation of the Clinical Evidence. Expert Opin. Pharmacother. 2019, 20, 95–102. [Google Scholar] [CrossRef]
- Chen, R.; Tao, Y.; Shan, L.; Jiang, H.; Cai, F.; Ma, L.; Yu, Y. The Efficacy and Safety of Nivolumab, Pembrolizumab, and Atezolizumab in the Treatment of Advanced Non-Small Cell Lung Cancer. Discov. Med. 2018, 26, 155–166. [Google Scholar]
- Li, S.; Young, K.H.; Medeiros, L.J. Diffuse Large B-Cell Lymphoma. Pathology 2018, 50, 74–87. [Google Scholar] [CrossRef] [Green Version]
- Leeman-Neill, R.J.; Bhagat, G. BCL6 as a Therapeutic Target for Lymphoma. Expert Opin. Ther. Targets 2018, 22, 143–152. [Google Scholar] [CrossRef]
- Taparra, K.; Liu, H.; Polley, M.-Y.; Ristow, K.; Habermann, T.M.; Ansell, S.M. Bleomycin Use in the Treatment of Hodgkin Lymphoma (HL): Toxicity and Outcomes in the Modern Era. Leuk. Lymphoma 2020, 61, 298–308. [Google Scholar] [CrossRef]
- Robak, T.; Jin, J.; Pylypenko, H.; Verhoef, G.; Siritanaratkul, N.; Drach, J.; Raderer, M.; Mayer, J.; Pereira, J.; Tumyan, G. Frontline Bortezomib, Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (VR-CAP) versus Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Transplantation-Ineligible Patients with Newly Diagnosed Mantle Cell Lymphoma: Final Overall Survival Results of a Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2018, 19, 1449–1458. [Google Scholar]
- Cicenas, J.; Tamosaitis, L.; Kvederaviciute, K.; Tarvydas, R.; Staniute, G.; Kalyan, K.; Meskinyte-Kausiliene, E.; Stankevicius, V.; Valius, M. KRAS, NRAS and BRAF Mutations in Colorectal Cancer and Melanoma. Med. Oncol. 2017, 34, 26. [Google Scholar] [CrossRef]
- Chou, C.-K.; Liu, R.-T.; Kang, H.-Y. MicroRNA-146b: A Novel Biomarker and Therapeutic Target for Human Papillary Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 636. [Google Scholar] [CrossRef]
- Hussein, M.R. Ultraviolet Radiation and Skin Cancer: Molecular Mechanisms. J. Cutan. Pathol. 2005, 32, 191–205. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Jia, J.; Zheng, Y. UV-Induced Molecular Signaling Differences in Melanoma and Non-Melanoma Skin Cancer. Ultrav. Light Hum. Health Dis. Environ. 2017, 27–40. [Google Scholar] [CrossRef]
- Sample, A.; He, Y.-Y. Mechanisms and Prevention of UV-induced Melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Sarkisian, S.; Davar, D. MEK Inhibitors for the Treatment of NRAS Mutant Melanoma. Drug Des. Devel. Ther. 2018, 12, 2553. [Google Scholar] [CrossRef] [Green Version]
- Schadendorf, D.; van Akkooi, A.C.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Carr, M.J.; Khushalani, N.I. Principles of Targeted Therapy for Melanoma. Surg. Clin. 2020, 100, 175–188. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Wittig, J.A. Principles of Immunotherapy in Melanoma. Surg. Clin. 2020, 100, 161–173. [Google Scholar] [CrossRef]
- Rossi, A.; Roberto, M.; Panebianco, M.; Botticelli, A.; Mazzuca, F.; Marchetti, P. Drug Resistance of BRAF-Mutant Melanoma: Review of up-to-Date Mechanisms of Action and Promising Targeted Agents. Eur. J. Pharmacol. 2019, 862, 172621. [Google Scholar] [CrossRef]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted Agents and Immunotherapies: Optimizing Outcomes in Melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queirolo, P.; Boutros, A.; Tanda, E.; Spagnolo, F.; Quaglino, P. Immune-Checkpoint Inhibitors for the Treatment of Metastatic Melanoma: A Model of Cancer Immunotherapy. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 59, pp. 290–297. [Google Scholar]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [PubMed]
- Guha, N.; Warnakulasuriya, S.; Vlaanderen, J.; Straif, K. Betel Quid Chewing and the Risk of Oral and Oropharyngeal Cancers: A Meta-analysis with Implications for Cancer Control. Int. J. Cancer 2014, 135, 1433–1443. [Google Scholar] [CrossRef]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral Microbiome: A New Biomarker Reservoir for Oral and Oropharyngeal Cancers. Theranostics 2017, 7, 4313. [Google Scholar] [CrossRef]
- Eckardt, A.; Sinikovic, B.; Hofele, C.; Bremer, M.; Reuter, C. Preoperative Paclitaxel/Carboplatin Radiochemotherapy for Stage III/IV ResectableOral and Oropharyngeal Cancer: Seven-Year Follow-up of a Phase II Trial. Oncology 2007, 73, 198–203. [Google Scholar] [CrossRef]
- Driessen, C.M.L.; De Boer, J.P.; Gelderblom, H.; Rasch, C.R.N.; De Jong, M.A.; Verbist, B.M.; Melchers, W.J.G.; Tesselaar, M.E.T.; van der Graaf, W.T.A.; Kaanders, J. Induction Chemotherapy with Docetaxel/Cisplatin/5-Fluorouracil Followed by Randomization to Two Cisplatin-Based Concomitant Chemoradiotherapy Schedules in Patients with Locally Advanced Head and Neck Cancer (CONDOR Study)(Dutch Head and Neck Society 08-01): A Randomized Phase II Study. Eur. J. Cancer 2016, 52, 77–84. [Google Scholar]
- Gautam, S.K.; Kumar, S.; Cannon, A.; Hall, B.; Bhatia, R.; Nasser, M.W.; Mahapatra, S.; Batra, S.K.; Jain, M. MUC4 Mucin-a Therapeutic Target for Pancreatic Ductal Adenocarcinoma. Expert Opin. Ther. Targets 2017, 21, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Fanchon, L.M.; Russell, J.; Pillarsetty, N.; O’Donoghue, I.; Gangangari, K.; Yu, K.H.; Humm, J.L. Comparing the Intra-Tumoral Distribution of Gemcitabine, 5-Fluorouracil, and Capecitabine in a Murine Model of Pancreatic Ductal Adenocarcinoma. PLoS ONE 2020, 15, e0231745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, S.-Y.; Gleave, M.E.; Beltran, H. Towards Precision Oncology in Advanced Prostate Cancer. Nat. Rev. Urol. 2019, 16, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Braglia, L.; Zavatti, M.; Vinceti, M.; Martelli, A.M.; Marmiroli, S. Deregulated PTEN/PI3K/AKT/MTOR Signaling in Prostate Cancer: Still a Potential Druggable Target? Biochim. Biophys. Acta BBA-Mol. Cell Res. 2020, 1867, 118731. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J. Glycosylation Is a Global Target for Androgen Control in Prostate Cancer Cells. Endocr. Relat. Cancer 2017, 24, R49–R64. [Google Scholar] [CrossRef]
- Ali, A.; Hoyle, A.P.; Parker, C.C.; Brawley, C.D.; Cook, A.; Amos, C.; Calvert, J.; Hassan, D.; Mason, M.D.; Attard, G. Bone Metastatic Burden as a Predictor of Survival Benefit from Prostate Radiotherapy in Newly-Diagnosed Metastatic Prostate Cancer. JAMA Oncol. 2021, 7, 555–563. [Google Scholar] [CrossRef]
- Kim, K.; Parise, R.A.; Holleran, J.L.; Lewis, L.D.; Appleman, L.; van Erp, N.; Morris, M.J.; Beumer, J.H. Simultaneous Quantitation of Abiraterone, Enzalutamide, N-Desmethyl Enzalutamide, and Bicalutamide in Human Plasma by LC–MS/MS. J. Pharm. Biomed. Anal. 2017, 138, 197–205. [Google Scholar] [CrossRef]
- Pilon, D.; Behl, A.S.; Ellis, L.A.; Robitaille, M.-N.; Lefebvre, P.; Dawson, N.A. Assessment of Real-World Central Nervous System Events in Patients with Advanced Prostate Cancer Using Abiraterone Acetate, Bicalutamide, Enzalutamide, or Chemotherapy. Am. Health Drug Benefits 2017, 10, 143. [Google Scholar]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizio, A.; Limonta, P. Natural compounds in prostate cancer prevention and treatment: Mechanisms of action and molecular targets. Cells 2020, 9, 460. [Google Scholar] [CrossRef] [Green Version]
- Hebenstreit, D.; Pichler, R.; Heidegger, I. Drug-Drug Interactions in Prostate Cancer Treatment. Clin. Genitourin. Cancer 2020, 18, e71–e82. [Google Scholar] [CrossRef]
- Swami, U.; McFarland, T.R.; Nussenzveig, R.; Agarwal, N. Advanced Prostate Cancer: Treatment Advances and Future Directions. Trends Cancer 2020, 6, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansouri, L.; Gurney, H. Clinical Concepts for Cabazitaxel in the Management of Metastatic Castration-resistant Prostate Cancer. Asia-Pacific J. Clin. Oncol. 2019, 15, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazari, S.; Majd, A.; Heydari, I.; Tehrani, M.R.M.; Nekouian, R. Association of Serum and Tumor Tissue MicroRNA Profile with Aggressiveness of Papillary Thyroid Carcinoma in an Iranian Population. J. Mol. Biomark. Diagn. 2021, 12, 1–5. [Google Scholar]
- Gruber, J.J.; Colevas, A.D. Differentiated Thyroid Cancer: Focus on Emerging Treatments for Radioactive Iodine-Refractory Patients. Oncologist 2015, 20, 113–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancker, O.V.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. Multikinase Inhibitor Treatment in Thyroid Cancer. Int. J. Mol. Sci. 2019, 21, 10. [Google Scholar] [CrossRef] [Green Version]
- Cabanillas, M.E.; Ryder, M.; Jimenez, C. Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond. Endocr. Rev. 2019, 40, 1573–1604. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [Green Version]
- Dillon, P.M.; Chakraborty, S.; Moskaluk, C.A.; Joshi, P.J.; Thomas, C.Y. Adenoid Cystic Carcinoma: A Review of Recent Advances, Molecular Targets, and Clinical Trials. Head Neck 2016, 38, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Yarbrough, W.G.; Panaccione, A.; Chang, M.T.; Ivanov, S.V. Clinical and Molecular Insights into Adenoid Cystic Carcinoma: Neural Crest-like Stemness as a Target. Laryngoscope Investig. Otolaryngol. 2016, 1, 60–77. [Google Scholar] [CrossRef]
- Chae, Y.K.; Chung, S.Y.; Davis, A.A.; Carneiro, B.A.; Chandra, S.; Kaplan, J.; Kalyan, A.; Giles, F.J. Adenoid Cystic Carcinoma: Current Therapy and Potential Therapeutic Advances Based on Genomic Profiling. Oncotarget 2015, 6, 37117. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.K.; Åman, P.; Stenman, G. IGF2/IGF1R Signaling as a Therapeutic Target in MYB-Positive Adenoid Cystic Carcinomas and Other Fusion Gene-Driven Tumors. Cells 2019, 8, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morassi, M.; Scavuzzo, A.; Cobelli, M.; Liserre, B.; Arias, J.A.; Di Biasi, B. Late Intracranial Metastasis from Adenoid-Cystic Carcinoma of the Parotid Gland: Imaging, Histologic and Molecular Features. Curr. Probl. Cancer 2020, 44, 100564. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotto, R.; Heymach, J.V.; Glisson, B.S. MYB-Fusions and Other Potential Actionable Targets in Adenoid Cystic Carcinoma. Curr. Opin. Oncol. 2016, 28, 195–200. [Google Scholar] [CrossRef]
- Yan, K.; Yesensky, J.; Hasina, R.; Agrawal, N. Genomics of Mucoepidermoid and Adenoid Cystic Carcinomas. Laryngoscope Investig. Otolaryngol. 2018, 3, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Sidiqi, M.H.; Gertz, M.A. Daratumumab for the Treatment of AL Amyloidosis. Leuk. Lymphoma 2019, 60, 295–301. [Google Scholar] [CrossRef]
- Pearson, K.T.; Vota, S. Amyloidosis and Its Management: Amyloid Neuropathies. Curr. Probl. Cancer 2016, 40, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Scheinberg, M.; Waddington-Cruz, M.; Heitner, S.B.; Karam, C.; Drachman, B.; Khella, S.; Whelan, C.; Obici, L. Inotersen for the Treatment of Adults with Polyneuropathy Caused by Hereditary Transthyretin-Mediated Amyloidosis. Expert Rev. Clin. Pharmacol. 2019, 12, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzowska-Firych, J.; Lucas, G.; Lucas, C.; Lucas, N.; Pietrzyk, Ł. An Overview of Human Papillomavirus (HPV) as an Etiological Factor of the Anal Cancer. J. Infect. Public Health 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Symer, M.M.; Yeo, H.L. Recent Advances in the Management of Anal Cancer. F1000Research 2018, 7, F1000 Faculty Rev-1572. [Google Scholar] [CrossRef]
- Sclafani, F.; Rao, S. Systemic Therapies for Advanced Squamous Cell Anal Cancer. Curr. Oncol. Rep. 2018, 20, 53. [Google Scholar] [CrossRef]
- Wang, C.-C.J.; Palefsky, J.M. HPV-Associated Anal Cancer in the HIV/AIDS Patient. HIVAIDS-Assoc. Viral Oncog. 2019, 177, 183–209. [Google Scholar]
- Castaneda, S.A.; Romak, L.B. Radiotherapy for Anal Cancer: Intensity-Modulated Radiotherapy and Future Directions. Surg. Oncol. Clin. 2017, 26, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, M.; Praticò, A.D.; Serra, A.; Maiolino, L.; Cocuzza, S.; Di Mauro, P.; Licciardello, L.; Milone, P.; Privitera, G.; Belfiore, G. Childhood Neurofibromatosis Type 2 (NF2) and Related Disorders: From Bench to Bedside and Biologically Targeted Therapies. Acta Otorhinolaryngol. Ital. 2016, 36, 345. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, N.; Seider, M.I.; Gururangan, S.; Mruthyunjaya, P. Everolimus to Treat Aggressive Retinal Astrocytic Hamartoma in Tuberous Sclerosis Complex. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2017, 21, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Landon, C.D.; Park, J.-Y.; Needham, D.; Dewhirst, M.W. Nanoscale Drug Delivery and Hyperthermia: The Materials Design and Preclinical and Clinical Testing of Low Temperature-Sensitive Liposomes Used in Combination with Mild Hyperthermia in the Treatment of Local Cancer. Open Nanomed. J. 2011, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wu, S.; Wu, X.; Zhong, J.; Lv, A.; Jiao, J.; Chen, Z. Blocking Mammalian Target of Rapamycin Alleviates Bone Cancer Pain and Morphine Tolerance via Μ-opioid Receptor. Int. J. Cancer 2016, 138, 2013–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, J.M. Delgado-Calle, Sclerostin: An emerging target for the treatment of Cancer-induced bone disease. Curr. Osteoporos. Rep. 2017, 15, 532–541. [Google Scholar] [CrossRef]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Valdiani, A.; Ofoghi, H.; Akbarizare, M.; Talei, D. Andrographis Paniculata Extract as an Immunity Modulator against Cancer via Telomerase Inhibition. 3 Biotech 2022, 12, 319. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, S.; Shukla, A.; Oh, S.H.; Kim, H.M. Andrographolide and Analogues in Cancer Prevention. Front. Biosci.-Elite 2015, 7, 293–304. [Google Scholar] [CrossRef]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I. Anticancer Potential of Alkaloids: A Key Emphasis to Colchicine, Vinblastine, Vincristine, Vindesine, Vinorelbine and Vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic Studies on Cancer Cell Interaction and Its Chemosensitization Effect. Front. Pharmacol. 2017, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanda, S.; Ramachandra, T.V. Phytochemical and Pharmacological Importance of Turmeric (Curcuma Longa): A Review. Res. Rev. J. Pharmacol. 2019, 9, 16–23. [Google Scholar]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical Effects of Curcumin in Enhancing Cancer Therapy: A Systematic Review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Gulder, T.A.; Moore, B.S. Salinosporamide Natural Products: Potent 20 S Proteasome Inhibitors as Promising Cancer Chemotherapeutics. Angew. Chem. Int. Ed. 2010, 49, 9346–9367. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The Fascinating Effects of Baicalein on Cancer: A Review. Int. J. Mol. Sci. 2016, 17, 1681. [Google Scholar] [CrossRef] [Green Version]
- Manogaran, P.; Beeraka, N.M.; Padma, V.V. The Cytoprotective and Anti-Cancer Potential of Bisbenzylisoquinoline Alkaloids from Nelumbo Nucifera. Curr. Top. Med. Chem. 2019, 19, 2940–2957. [Google Scholar] [CrossRef]
- Asokan, S.M.; Mariappan, R.; Muthusamy, S.; Velmurugan, B.K. Pharmacological Benefits of Neferine-A Comprehensive Review. Life Sci. 2018, 199, 60–70. [Google Scholar] [CrossRef]
- El-shiekh, R.A.; Al-Mahdy, D.A.; Hifnawy, M.S.; Tzanova, T.; Evain-Bana, E.; Philippot, S.; Bagrel, D.; Abdelsattar, E.A. Chemical and Biological Investigation of Ochrosia Elliptica Labill. Cultivated in Egypt. Rec. Nat. Prod. 2017, 11, 552–557. [Google Scholar] [CrossRef]
- Sharma, K.; Pachauri, S.D.; Khandelwal, K.; Ahmad, H.; Arya, A.; Biala, P.; Agrawal, S.; Pandey, R.R.; Srivastava, A.; Srivastav, A. Anticancer Effects of Extracts from the Fruit of MorindaCitrifolia (Noni) in Breast Cancer Cell Lines. Drug Res. 2016, 66, 141–147. [Google Scholar]
- Wang, J.-H.; Nao, J.-F.; Zhang, M.; He, P. 20 (s)-Ginsenoside Rg3 Promotes Apoptosis in Human Ovarian Cancer HO-8910 Cells through PI3K/Akt and XIAP Pathways. Tumor. Biol. 2014, 35, 11985–11994. [Google Scholar] [CrossRef] [PubMed]
- Gatter, K.; Pezzella, F. Diffuse Large B-Cell Lymphoma. Diagn. Histopathol. 2010, 16, 69–81. [Google Scholar] [CrossRef]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]






| Agent | Features |
|---|---|
| Bevacizumab (Phase III) | VEGF-A binding/inhibition |
| Ziv-aflibercept196 Phase I | VEGF binding/inhibiting agent |
| Cabozantinib (Phase II) | VEGFR-2 inhibitor |
| Pazopanib (Phase II) | inhibitor of several tyrosine kinases |
| Tamoxifen (Phase II) | An antagonist of estrogen receptors |
| Buparlisib (Phase II) | Pi3K inhibitor |
| Dovitinib (Phase II) | FGFR and veGFR inhibitor |
| MeK162 (Phase II) | MeK inhibitor |
| MGAH22 (Phase I) | HeR2-targeting antibody |
| Afatinib (Phase II) | eGFR and HeR2 inhibitor |
| AZD5312 (Phase I) | Androgen receptor antisense inhibitor |
| Everolimus (Phase I) (Phase II) | mTOR inhibitor (mTORC1 and mTORC2) |
| Rapamycin (Phase I) (Phase II) | mTOR inhibitor |
| ABi-009 (albumin-bound rapamycin) (Phase I) (Phase II) | mTOR inhibitor |
| ALT-801 (Phase I) (Phase II) | p53/HLA-A2-expressing tumor cells |
| HS-410 (Phase I) (Phase II) | immune activator along with BCG |
| ALT-803 (Phase I) (Phase II) | immune activator through iL-15 |
| Ipilimumab (Phase II) | CTLA-4 antibody |
| MeDi4736 (Phase I) | PDL1 antibody antagonist |
| Tremelimumab (Phase I) | CTLA-4antibodyDownregulationofT-reg cells |
| AGS15e (Phase I) | Slitrk6 targeting immunotherapy |
| MK-3745 (pembrolizumab) (Phase I) (Phase II) | PDL1 |
| Ad/HeR2/Neu vaccine (Phase I) | vaccination/immune activation |
| SAR566658 (Phase I) | Anti-CA6-DM4 immunotherapy |
| Lenalidomide (Phase I) | Immunomodulation |
| MPDL3280A (Phase I) | Anti-PDL1 immunotherapy |
| Eribulin mesylate (Phase I) (Phase II) | Microtubule formation/mitosis |
| Abraxane (Phase I) (Phase II) | Protein-bound paclitaxel—mitosis |
| Tesetaxel (Phase II) | Tubulin stabilization—antimitotic |
| ASG-22Ce (Phase I) | Inhibition of tubulin formation in cancer cells by targeting cells expressing adhesion molecule nectin-4 with monomethyl auristatin e |
| Amrubicin (Phase II) | Anthracycline targeting topoisomerase ii |
| Gemcitabine | Nucleoside analog targeting S phase |
| 5-Fluoro-2-deoxycytidine with Tetrahydrouridine (Phase II) | inhibition of DNA methylation/cytosine deamination |
| Romidepsin (Phase I) | HDAC inhibitor |
| BBi608 (Phase I) (Phase II) | Cancer cell stemness |
| Ganetespib (Phase I) | inhibition of HSP90 |
| OGX-427 (Phase II) | HSP27 inhibitor |
| Veliparib (Phase I) | PARP inhibitor |
| Gefitinib (Phase II) | Inhibit EGFR TKI |
| Etunimab (Phase II) | Inhibit EGFR TKI |
| Erlotinib (Phase II) | Blocks EGFR |
| Trastuzumab (Phase II) | Blocks ErbB2 |
| Lapatinip | Reversed inhibition of EGFR and ErbB2 |
| Sunitinib (Phase II) | Inhibition of VEGFR1–3, PDGFR, C-Kit, and Flt3 |
| Pazopanib (Phase II) | Inhibition of VEGFR1–3, PDGFR, and C-Kit |
| Sorafenib (Phase II) | Inhibition of VEGFR2/3, PDGFR, Raf, C-Kit, and Flt3 |
| Ad CMV-TP53 (Phase I) | Delivery of functional TP53 into cells |
| Bevacizymab (Phase II) | Inhibits VEGF antibody |
| Aflibercept (Phase II) | VEGF binding to endothelial cells and blocking VEGFR interaction |
| Curcumin | decrease VEGF binding, c-MYC |
| Sulforaphane | decrease VEGF binding, c-MYC |
| Resveratrol | decrease c-MYC |
| Quercetin | decrease VEGF binding, c-MYC |
| Target Agent | Process of Intervention |
|---|---|
| Trastuzumab | Suppresses downstream signaling involved in normal cell proliferation, motility, anti-apoptosis, along with malignant cell invasiveness and angiogenesis |
| Pertuzumab | Prevents dimerization among HER2 and further HER family members, particularly HER3, and stimulates ADCC (antibody-dependent cellular cytotoxicity), 16 whereas trastuzumab only averts dimerization among HER2 and other HER family members, particularly HER3 |
| Lapatinib | Hinder receptor phosphorylation and inhibit downstream pathways that affect tumor cell proliferation and survival. |
| T-DM1 (Trastuzumabemtansine) | Transmit the microtubule-inhibitory drug to HER2-positive cancer cells, reducing systemic toxicity along with improving anticancer efficacy |
| Everolimus | Suppress mTOR activation while also efficiently inhibiting upstream signal transmission, which is important for tumor cell development. |
| Ipatasertib | Inhibits AKT |
| Veliparib | PARP1 and PARP2 inhibitors |
| Talazoparib | Inhibits PARP |
| Olaparib | PARP inhibitor with potential anticancer efficacy in BRCA1/2-mutated breast cancer patients |
| Palbociclib, abemaciclib, and ribociclib | CDK4/6 inhibitors |
| Atezolizummab and durvalumab pembrolizumab | Inhibit the PD-1 receptor-mediated negative immune regulatory signal |
| Bevacizumab | Inhibits VEGF |
| Curcumin | regulating p53gene expressions |
| EGCG | upregulation of p21 |
| Genistein | activation of ERK |
| Lycopene | activation of ERK, Akt, and p70S6 kinases |
| Name of Drugs | Targets | Reference |
|---|---|---|
| Sorafenib | VEGFR 1–3, C-Kit, PDGFR | [52] |
| Sunitinib | VEGFR 1–3, C-Kit, PDGFR and Fit-3 | [50] |
| Bevacizumab | VEGF | [50] |
| Pazopanib | VEGFR 1–3, C-Kit and PDGFR | [53] |
| Temsirolimus | mTOR | [50] |
| Everolimus | mTOR | [50] |
| Axitinib | VEGFR1–3 | [50] |
| Nivolumab | PD1 | [50] |
| Cabozamtinib | MET, RET and VEGFR2 | [50] |
| Lenvatinib | VEGFR1–3, PDGFRβ, RET, FGFR1–4 and KIT | [50] |
| Regorafenib | VEGFR | [54] |
| Cediranib | VEGFR 1–3 | [55] |
| Dovitinib | VEGFR and mTOR | [56] |
| Quercetin | reducing the lipid ROS | [51] |
| Luteolin | increase p53gene and decrease the PUMA-α | [51] |
| Kaempferol | suppressed TNF-α, activate NF-κB | [51] |
| Drugs | Drugs Mechanism | Tumors Name Which Is Treatable | References |
|---|---|---|---|
| Lirilumab | In CT phase I, halt KIR signaling | Solid-form tumors (Squamous cell carcinoma) | [65] |
| Paclitaxel | Work as Chemo and immune-cytokines | Melanoma, NSCLC | [66] |
| Pembrolizumab | Work against programmed death-ligand 1 by reprogramming NK cells. | NSCLC | [67] |
| Nvolumab | NK cell activation in CT phase II, activity against programmed death-ligand 1. | NSCLC | [65] |
| Curcumin | inhibiting PI3 K/Akt pathway | - | [64] |
| β-elemene | inhibiting PI3 K/Akt pathway | - | [64] |
| Name of Drugs | Main Target | Reference |
|---|---|---|
| Ipilimumab | CTLA-4 | [81,82] |
| Perbrolizumab | PD-1 | |
| Nivolumab | PD-1 | |
| Vemurafenib | BRAF | |
| Trametinib | MEK and PD-1 | |
| Dabrafenib | BRAF | |
| Cobimetinib | MEK and PD-1 | |
| Quercetin | inhibit STAT3 | [83] |
| kaempferol | inhibit STAT3 | [83] |
| Name of Drugs | Targets | References |
|---|---|---|
| Bicalutamide | CYP3A4, CYP2C9, CYP2D, CPY2C19, Binding of plasma protein | [97] |
| Abiraterone | CYP2C8, CYP1A2, CYP3D6 | [97] |
| Enzalutamide | CYP3A4, CTP2C9, CYP2C19, Pgp, BCRP, OATPs | [97] |
| Abiraterone Acetate | CYP2C8, CYP3D, CYP1A2 | [97,98] |
| Docetaxel | Binding of plasma protein | [87,97,98] |
| Cabazitaxel | CYP3A4, CYP2C8, BCRP, OATP1B1, OATP1B3, UGT, stabilize tubulin | [97,99] |
| Drugs | Drugs Targets | Treatment for Various Cancer Types | References |
|---|---|---|---|
| Axitinib | TKI, VEGFR1–3 | ATC, MTC, and DTC | [102,103] |
| Lenvatinib | TKI, VEGFR1–3, FGFR1–4, RET, PDGFR | ATC, MTC, and DTC | |
| Cabozantinib | TKI, VEGFR2, MET, FLT3, RET, c-kit | DTC and MTC | |
| Dabrafenib | STKI, BRAF V600E, MEK1 &2 | ATC and DTC | |
| Everolimus | m-TOR | RCC, TS, SEGA | |
| Pazopanib | TKI, VEGFR1–3, FGFR1–4, RET, PDGFR, c-kit | ATC, MTC, and DTC | |
| Larotrectinib | NTRK | NTRK-fused thyroid cancer | |
| Sorafenib | TKI, VEGFR1–3, FGFR1–4, RET, PDGFR, c-kit, BRAF | ATC, MTC, and DTC | |
| Sunitinib | TKI, VEGFR1–3, FGFR1–4, RET, PDGFR, c-kit, CSF-1R | DTC and MTC | |
| Vandetanib | TKI, VEGFR2–3, RET, EGFR | Only MTC | |
| Vemurafenib | BRAF V600E | Only PTC |
| Name of Drugs | Main Targets of Drugs | Main Pathways of Tumor Formation | References |
|---|---|---|---|
| Everolimus | mTOR | P53, MYB-NF-κB, MYBL1-NFIB fusion, DNA methylation, TGF-β, C-Kit fusion | [110,111] |
| MK-2206 | AKT | ||
| Nelfinavir | |||
| Gefitinib | EGFR | ||
| Sorafenib | PDGFR/VEGFR | ||
| Axitinib | |||
| Dovitinib | FGFR | ||
| Vorinostat | HDAC |
| Sr.No. | Component | Process of Intervention | Target Cell | Reference |
|---|---|---|---|---|
| 1. | Quercetin | Repressed Akt/ERK ½ pathways, inactive NF-κB, COX-2 | AMPK α, PI3K-Akt, Akt/ERK ½, NF-κβ, COX-2, ARP-1, RPMI8226 | [125] |
| 2. | Andrographolide, 14-deoxyandrographolide | Inactive AKT/REK signaling, dominant MAPK | AKT/REK, MMP2, MAPK, MMP-7, MMP-9, MMP-2 | [126,127] |
| 3. | Vinblastine | hinder the PI3K pathways and CDK | PI3K pathways | [128] |
| 4. | β-elemene | resist leukemia (DNR/K562) and GS cell lines (ADR/SGC7901), downregulate Akt signaling pathways, block ABCB1 transporters efflux portion, which over-expressed in KB-C2 cells, resist MCF-7 cells, PTEN expression and Pgp expression | DNR/K562, GS cell lines, ABCB1 transporters efflux portion, MCF-7 | [129] |
| 5. | Curcumin | Activation of beta-growth factor (TGF-β), by inhibiting AP 1 and Hypoxia-inducible factors HIF-(1) it stimulate VEGF expression, reduces the cells of MMPs, ICAM-1 and VCAM, increases several anti- metastatic proteins such as, non-metastatic gene NM23, tissue inhibitor metalloproteinase (TIMP 2) and E-cadherin | TGF-β, AP 1, HIF-(1), VEGF, MMPs, ICAM-1, VCAM, NM23, TIMP 2 and E-cadherin | [130,131] |
| 6. | Salinisporamide A | inhibit activation | NF-κB | [132] |
| 7. | Chalcones | activity against HCT116, MCF-7 and 143B cancer cell line | HCT116, MCF-7 and 143B cancer cell line | [133] |
| 8. | Baicalein | Inhibit Akt phosphorylation, activate caspase-3, caspase-9, upregulate Bax expression and downregulate Bcl-2 expression | ERK/p38 MAPK pathway, MCF-7 cell, T24 cells vimentin, N-cadherin, ZEB2, ZEB1, I-kappa-B (IKB)-β, nuclear translocation of p65 and p50 | [134] |
| 9. | Neferine | downregulating the PI3K/Akt/mTOR ace-survival signalling pathway, as well as PI3K CIII independent autophagy and ROS-mediated Beclin-1 in human lung A549 adenocarcinoma cells | Bcl2, cycline D1, NF-κB, PI3K/Akt/mTOR signaling, PRAP cleavage, p21, p27, Atg 7, Lc3b, JNK/MAPK protein and Bcl2 protein | [135,136] |
| 10. | 9-methoxy ellipticine | Inhibit MCF-7 | Cdc25 phosphatase | [137] |
| 11. | Rutin, scopoletin, kaempferol | Reduce the MDA-MB-231cells and MCF-7 cells | MEK and ERK pathways | [138] |
| 12. | Ginsenosides | Inhibition of cell viability and cell apoptosis are encouraged by ginsenoside Rg3 in human ovarian cancer HO8910 cells | HO8910, HCT-116 and SW-480 | [139,140] |
| 13. | Aloe-emodin | Reduced hoGG1, hMHT11, and apurinic endonuclease in H460 cells expression | H460 cells, hoGG1, hMHT11 | [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Sharma, L.; Nandy, S.K.; Payal, N.; Yadav, S.; Vargas-De-La-Cruz, C.; Anwer, M.K.; Khan, H.; Behl, T.; Bungau, S.G. Molecular Aspects and Therapeutic Implications of Herbal Compounds Targeting Different Types of Cancer. Molecules 2023, 28, 750. https://doi.org/10.3390/molecules28020750
Sharma A, Sharma L, Nandy SK, Payal N, Yadav S, Vargas-De-La-Cruz C, Anwer MK, Khan H, Behl T, Bungau SG. Molecular Aspects and Therapeutic Implications of Herbal Compounds Targeting Different Types of Cancer. Molecules. 2023; 28(2):750. https://doi.org/10.3390/molecules28020750
Chicago/Turabian StyleSharma, Aditi, Lalit Sharma, Shouvik Kumar Nandy, Nazrana Payal, Shivam Yadav, Celia Vargas-De-La-Cruz, Md. Khalid Anwer, Haroon Khan, Tapan Behl, and Simona Gabriela Bungau. 2023. "Molecular Aspects and Therapeutic Implications of Herbal Compounds Targeting Different Types of Cancer" Molecules 28, no. 2: 750. https://doi.org/10.3390/molecules28020750