Polyhydroxy Fullerenes Enhance Antibacterial and Electrocatalytic Activity of Silver Nanoparticles
Abstract
:1. Introduction
1.1. Metallic Nanoparticles Synthesis: Reducing and Stabilizing Agents
1.2. Antimicrobial and Electrocatalytic Properties of AgNPs
1.3. Potential for Enhancement of Electronic Properties of AgNPs
2. Materials and Methods
2.1. Synthesis and Characterization of Citrate-AgNPs and PHF-AgNPs
2.2. Antibacterial Assays
2.3. Cyclic Voltammetry Tests
3. Results and Discussions
3.1. Characterization of Citrate-AgNPs and PHF-AgNPs
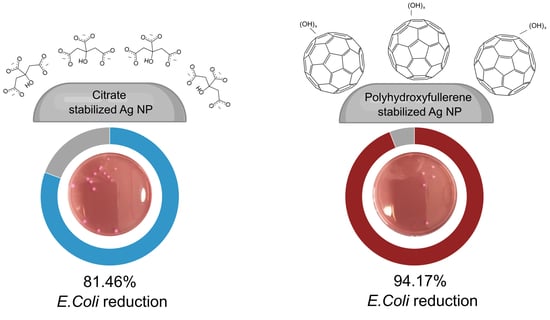
3.2. Antibacterial Activity
3.3. Cyclic Voltammetry Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Qu, Y.; Yu, Q.; Chen, H. Gold nanoparticle layer: A versatile nanostructured platform for biomedical applications. Mater. Chem. Front. 2018, 2, 2175–2190. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Dong, X.; Ji, X.; Wu, H.; Zhao, L.; Li, J.; Yang, W. Shape control of silver nanoparticles by stepwise citrate reduction. J. Phys. Chem. C 2009, 113, 6573–6576. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, S336–S343. [Google Scholar] [CrossRef]
- Gakiya-Teruya, M.; Palomino-Marcelo, L.; Rodriguez-Reyes, J.C.F. Synthesis of highly concentrated suspensions of silver nanoparticles by two versions of the chemical reduction method. Methods Protoc. 2019, 2, 3. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ramalingam, C. Silver nanoparticle antimicrobial activity explained by membrane rupture and reactive oxygen generation. Environ. Chem. Lett. 2016, 14, 477–485. [Google Scholar] [CrossRef]
- Yin, W.; Liu, M.; Zhao, T.L.; Qian, F.J.; Li, H.; Yao, Q.Z.; Fu, S.Q.; Zhou, G.T. Removal and recovery of silver nanoparticles by hierarchical mesoporous calcite: Performance, mechanism, and sustainable application. Environ. Res. 2020, 187, 109699. [Google Scholar] [CrossRef]
- Ajitha, B.; Kumar Reddy, Y.A.; Reddy, P.S.; Jeon, H.J.; Ahn, C.W. Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Swolana, D.; Kępa, M.; Idzik, D.; Dziedzic, A.; Kabała-Dzik, A.; Wąsik, T.J.; Wojtyczka, R.D. The Antibacterial Effect of Silver Nanoparticles on Staphylococcus Epidermidis Strains with Different Biofilm-Forming Ability. Nanomaterials 2020, 10, 1010. [Google Scholar] [CrossRef]
- Okazaki, J.; Komasa, S.; Sekino, T.; Sekino, T.; Tahara, Y. Antimicrobial Efficacy of Silver Nanoparticles against Candida Albicans. Materials 2022, 15, 5666. [Google Scholar] [CrossRef]
- Liu, M.; Deng, Z.; Zhang, D.; Zhang, H. Synthetic Conditions, Physical Properties, and Antibacterial Activities of Silver Nanoparticles with Exopolysaccharides of a Medicinal Fungus. Materials 2022, 15, 5620. [Google Scholar] [CrossRef]
- Xiliang, Q.; Yang, C.; Tiesong, L.; Peng, H.; Jun, W.; Ping, L.; Xiaolong, G. Large-Scale synthesis of silver nanoparticles by aqueous reduction for low-temperature sintering bonding. J. Nanomater. 2014, 2014, 594873. [Google Scholar] [CrossRef]
- Kim, C.; Jeon, H.S.; Eom, T.; Jee, M.S.; Kim, H.; Friend, C.M.; Min, B.K.; Hwang, Y.J. Achieving Selective and Efficient Electrocatalytic Activity for CO2 Reduction Using Immobilized Silver Nanoparticles. J. Am. Chem. Soc. 2014, 137, 13844–13850. [Google Scholar] [CrossRef]
- Poolnapol, L.; Kao-Ian, W.; Somwangthanaroj, A.; Mahlendorf, F.; Nguyen, M.T.; Yonezawa, T.; Kheawhom, S. Silver Decorated Reduced Graphene Oxide as Electrocatalyst for Zinc–Air Batteries. Energies 2020, 13, 462. [Google Scholar] [CrossRef]
- Chala, S.A.; Tsai, M.C.; Su, W.N.; Ibrahim, K.B.; Duma, A.D.; Yeh, M.H.; Wen, C.Y.; Yu, C.H.; Chan, T.S.; Dai, H.; et al. Site Activity and Population Engineering of NiRu-Layered Double Hydroxide Nanosheets Decorated with Silver Nanoparticles for Oxygen Evolution and Reduction Reactions. ACS Catal. 2019, 9, 117–129. [Google Scholar] [CrossRef]
- Guldi, D.M. Fullerenes: Three dimensional electron acceptor materials. Chem. Commun. 2000, 33, 321–327. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Zhao, F.; Xu, Z.; Dong, S. The direct electron transfer of glucose oxidase and glucose biosensor based on carbon nanotubes/chitosan matrix. Biosens. Bioelectron. 2005, 21, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, X.; Huang, B.; Li, J.; Wang, E. An interfacial electron transfer relay center for accelerating the hydrogen evolution reaction. J. Mater. Chem. A Mater. 2019, 7, 18304–18310. [Google Scholar] [CrossRef]
- Krishna, V.; Yanes, D.; Imaram, W.; Angerhofer, A.; Koopman, B.; Moudgil, B. Mechanism of enhanced photocatalysis with polyhydroxy fullerenes. Appl. Catal. B 2008, 79, 376–381. [Google Scholar] [CrossRef]
- Krishna, V.; Pumprueg, S.; Lee, S.H.; Zhao, J.; Sigmung, W.; Koopman, B.; Moundgil, B.M. Photocatalytic Disinfection with Titanium Dioxide Coated Multi-Wall Carbon Nanotubes. Process Saf. Environ. Prot. 2005, 83, 393–397. [Google Scholar] [CrossRef]
- Krishna, V.; Noguchi, N.; Koopman, B.; Moudgil, B. Enhancement of titanium dioxide photocatalysis by water-soluble fullerenes. J. Colloid Interface Sci. 2006, 304, 166–171. [Google Scholar] [CrossRef]
- Kokubo, K.; Espejo Cabello, M.K.; Sato, N.; Uetake, Y.; Sakurai, H. Gold Nanoparticles Stabilized by Molecular Fullerenols. ChemNanoMat 2020, 6, 524–528. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Gakiya-Teruya, M.; Palomino-marcelo, L.; Pierce, S.; Angeles-Boza, A.M.; Krishna, V.; Rodriguez-Reyes, J.C.F. Enhanced antimicrobial activity of silver nanoparticles conjugated with synthetic peptide by click chemistry. J. Nanoparticle Res. 2020, 22, 90. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Zielińska, A.; Skwarek, E.; Zaleska, A.; Gazda, M.; Hupka, J. Preparation of silver nanoparticles with controlled particle size. Procedia Chem. 2009, 1, 1560–1566. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.; Kolla, P.; Zhao, Y.; Fong, H.; Smirnova, A.L. Lignin-derived electrospun carbon nanofiber mats with supercritically deposited Ag nanoparticles for oxygen reduction reaction in alkaline fuel cells. Electrochim. Acta 2014, 130, 431–438. [Google Scholar] [CrossRef]
- Vijaya, J.J.; Jayaprakash, N.; Kombaiah, K.; Kaviyarasu, K.; Kennedy, L.J.; Ramalingam, R.J.; Al-Lohedan, H.A.; Mansoor-Ali, V.M.; Maaza, M. Bioreduction potentials of dried root of Zingiber officinale for a simple green synthesis of silver nanoparticles: Antibacterial studies. J. Photochem. Photobiol. B Biol. 2017, 177, 62–68. [Google Scholar] [CrossRef]
- Rangayasami, A.; Kannan, K.; Murugesan, S.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Influence of nanotechnology to combat against COVID-19 for global health emergency: A review. Sensors Int. 2021, 2, 100079. [Google Scholar] [CrossRef]




| Sample | Average | Standard Deviation | % Reduction |
|---|---|---|---|
| Control | 160.0 | 35.6 | -- |
| PHF | 115.7 | 61.2 | 27.71% |
| Citrate-AgNPs | 29.7 | 18.0 | 81.46% |
| PHF-AgNPs | 9.3 | 3.8 | 94.17% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomino, L.; Chipoco Haro, D.A.; Gakiya-Teruya, M.; Zhou, F.; La Rosa-Toro, A.; Krishna, V.; Rodriguez-Reyes, J.C.F. Polyhydroxy Fullerenes Enhance Antibacterial and Electrocatalytic Activity of Silver Nanoparticles. Nanomaterials 2022, 12, 3321. https://doi.org/10.3390/nano12193321
Palomino L, Chipoco Haro DA, Gakiya-Teruya M, Zhou F, La Rosa-Toro A, Krishna V, Rodriguez-Reyes JCF. Polyhydroxy Fullerenes Enhance Antibacterial and Electrocatalytic Activity of Silver Nanoparticles. Nanomaterials. 2022; 12(19):3321. https://doi.org/10.3390/nano12193321
Chicago/Turabian StylePalomino, Luis, Danae A. Chipoco Haro, Miguel Gakiya-Teruya, Feng Zhou, Adolfo La Rosa-Toro, Vijay Krishna, and Juan Carlos F. Rodriguez-Reyes. 2022. "Polyhydroxy Fullerenes Enhance Antibacterial and Electrocatalytic Activity of Silver Nanoparticles" Nanomaterials 12, no. 19: 3321. https://doi.org/10.3390/nano12193321