Preliminary Assessment of Tara Gum as a Wall Material: Physicochemical, Structural, Thermal, and Rheological Analyses of Different Drying Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
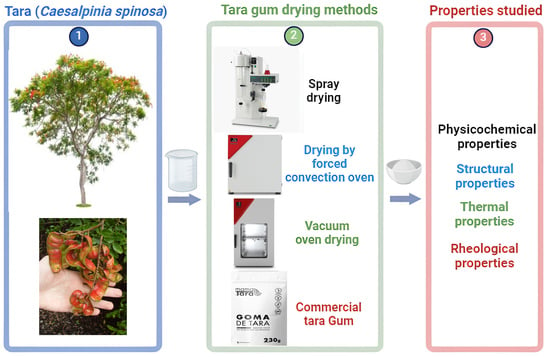
2.2. Obtaining the Tara Gum
2.3. Yield
2.4. Moisture
2.5. Water Activity (Aw)
2.6. Hygroscopicity
2.7. Bulk Density
2.8. Colour Analysis
2.9. ζ-Potential
2.10. Particle Size and Polydispersity
2.11. SEM-EDS Analysis
2.12. FTIR Analysis
2.13. Thermal Analysis
2.14. Rheological Analysis
2.15. Statistical Analysis
3. Results and Discussions
3.1. Physical and Chemical Properties of Tara Gum
3.2. SEM-EDS Analysis
3.3. FTIR Analysis
3.4. Thermal Analysis
3.5. Rheological Properties
3.6. Rheological Analysis
3.7. Result Overview
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taheri, A.; Jafari, S.M. Gum-based nanocarriers for the protection and delivery of food bioactive compounds. Adv. Colloid Interface Sci. 2019, 269, 277–295. [Google Scholar] [CrossRef]
- Halahlah, A.; Piironen, V.; Mikkonen, K.S.; Ho, T.M. Polysaccharides as wall materials in spray-dried microencapsulation of bioactive compounds: Physicochemical properties and characterization. Crit. Rev. Food Sci. Nutr. 2022, 63, 6983–7015. [Google Scholar] [CrossRef]
- Coimbra, P.P.S.; Cardoso, F.d.S.N.; Goncalves, E.C.B.d.A. Spray-drying wall materials: Relationship with bioactive compounds. Crit. Rev. Food Sci. Nutr. 2021, 61, 2809–2826. [Google Scholar] [CrossRef]
- Mukherjee, K.; Dutta, P.; Badwaik, H.R.; Saha, A.; Das, A.; Giri, T.K. Food Industry applications of Tara Gum and its modified forms. Food Hydrocoll. Health 2022, 3, 100107. [Google Scholar] [CrossRef]
- Chaudhari, B.B.; Annapure, U.S. Physiochemical and rheological characterization of Pithecellobium dulce (Roxb.) benth gum exudate as a potential wall material for the encapsulation of rosemary oil. Carbohydr. Polym. Technol. Appl. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Santos, M.B.; dos Santos, C.H.C.; de Carvalho, M.G.; de Carvalho, C.W.P.; Garcia-Rojas, E.E. Physicochemical, thermal and rheological properties of synthesized carboxymethyl tara gum (Caesalpinia spinosa). Int. J. Biol. Macromol. 2019, 134, 595–603. [Google Scholar] [CrossRef]
- Murga-Orrillo, H.; Lobo, F.D.A.; Santos Silva Amorim, R.; Fernandes Silva Dionisio, L.; Nuñez Bustamante, E.; Chu-Koo, F.W.; López, L.A.A.; Arévalo-Hernández, C.O.; Abanto-Rodriguez, C. Increased Production of Tara (Caesalpinia spinosa) by Edaphoclimatic Variation in the Altitudinal Gradient of the Peruvian Andes. Agronomy 2023, 13, 646. [Google Scholar] [CrossRef]
- Santander, S.; Aoki, M.; Hernandez, J.; Pombo, M.; Moins-Teisserenc, H.; Mooney, N.; Fiorentino, S. Galactomannan from Caesalpinia spinosa induces phenotypic and functional maturation of human dendritic cells. Int. Immunopharmacol. 2011, 11, 652–660. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Samborska, K.; Poozesh, S.; Barańska, A.; Sobulska, M.; Jedlińska, A.; Arpagaus, C.; Malekjani, N.; Jafari, S.M. Innovations in spray drying process for food and pharma industries. J. Food Eng. 2022, 110960. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Gordon, M.H. Influence of polysaccharides and storage during processing on the properties of mango seed kernel extract (microencapsulation). Food Chem. 2012, 134, 1453–1460. [Google Scholar] [CrossRef]
- Outuki, P.M.; de Francisco, L.M.B.; Hoscheid, J.; Bonifácio, K.L.; Barbosa, D.S.; Cardoso, M.L.C. Development of arabic and xanthan gum microparticles loaded with an extract of Eschweilera nana Miers leaves with antioxidant capacity. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 103–112. [Google Scholar] [CrossRef]
- Aguilar-Galvez, A.; Noratto, G.; Chambi, F.; Debaste, F.; Campos, D. Potential of tara (Caesalpinia spinosa) gallotannins and hydrolysates as natural antibacterial compounds. Food Chem. 2014, 156, 301–304. [Google Scholar] [CrossRef]
- Rahaiee, S.; Assadpour, E.; Faridi Esfanjani, A.; Silva, A.S.; Jafari, S.M. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid Interface Sci. 2020, 279, 102153. [Google Scholar] [CrossRef]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Galves, C.; Galli, G.; Kurozawa, L. Potato protein: Current review of structure, technological properties, and potential application on spray drying microencapsulation. Crit. Rev. Food Sci. Nutr. 2023, 63, 6564–6579. [Google Scholar] [CrossRef]
- Liang, X.; Chen, L.; McClements, D.J.; Peng, X.; Xu, Z.; Meng, M.; Jin, Z. Bioactive delivery systems based on starch and its derivatives: Assembly and application at different structural levels. Food Chem. 2024, 432, 137184. [Google Scholar] [CrossRef]
- Hamdani, A.M.; Wani, I.A.; Bhat, N.A. Sources, structure, properties and health benefits of plant gums: A review. Int. J. Biol. Macromol. 2019, 135, 46–61. [Google Scholar] [CrossRef]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef]
- Vilaró Luna, P. Obtención y Caracterización de Gomas Provenientes de Semillas de Especies Nativas del Género Prosopis. 2019. Available online: https://www.colibri.udelar.edu.uy/jspui/handle/20.500.12008/21333 (accessed on 13 March 2024).
- Al-Shammari, B.; Al-Ali, R.; Al-Sahi, A. Electrical, characterization and functional properties of extract gum (Tniqonella Foenum graecum L.) from Fenugreek seeds. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kerbala City, Iraq, 17–18 November 2019; p. 012060. [Google Scholar]
- Ligarda-Samanez, C.A.; Moscoso-Moscoso, E.; Choque-Quispe, D.; Ramos-Pacheco, B.S.; Arévalo-Quijano, J.C.; Cruz, G.D.; Huamán-Carrión, M.L.; Quispe-Quezada, U.R.; Gutiérrez-Gómez, E.; Cabel-Moscoso, D.J.; et al. Native Potato Starch and Tara Gum as Polymeric Matrices to Obtain Iron-Loaded Microcapsules from Ovine and Bovine Erythrocytes. Polymers 2023, 15, 3985. [Google Scholar] [CrossRef]
- Horwitz, W. Official methods of analysis of AOAC International. In Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2010; Volume I. [Google Scholar]
- Choque-Quispe, D.; Ligarda-Samanez, C.A.; Huamán-Rosales, E.R.; Aguirre Landa, J.P.; Agreda Cerna, H.W.; Zamalloa-Puma, M.M.; Álvarez-López, G.J.; Barboza-Palomino, G.I.; Alzamora-Flores, H.; Gamarra-Villanueva, W. Bioactive Compounds and Sensory Analysis of Freeze-Dried Prickly Pear Fruits from an Inter-Andean Valley in Peru. Molecules 2023, 28, 3862. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.É.G.; Costa, M.G.M.; de Souza, A.C.R.; de Brito, E.S.; de Medeiros, M.d.F.D.; de Azeredo, H.M. Physical properties of spray dried acerola pomace extract as affected by temperature and drying aids. LWT-Food Sci. Technol. 2009, 42, 641–645. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Huamán-Carrión, M.L.; Ramos-Pacheco, B.S.; Peralta-Guevara, D.E.; De la Cruz, G.; Martínez-Huamán, E.L.; Arévalo-Quijano, J.C.; Muñoz-Saenz, J.C. Obtaining and characterizing andean multi-floral propolis nanoencapsulates in polymeric matrices. Foods 2022, 11, 3153. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Palomino-Rincón, H.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Arévalo-Quijano, J.C.; Huamán-Carrión, M.L.; Quispe-Quezada, U.R.; Muñoz-Saenz, J.C.; Gutiérrez-Gómez, E.; Cabel-Moscoso, D.J.; et al. Bioactive Compounds and Sensory Quality in Chips of Native Potato Clones (Solanum tuberosum spp. andigena) Grown in the High Andean Region of PERU. Foods 2023, 12, 2511. [Google Scholar] [CrossRef]
- Ramos-Pacheco, B.S.; Choque-Quispe, D.; Ligarda-Samanez, C.A.; Solano-Reynoso, A.M.; Palomino-Rincón, H.; Choque-Quispe, Y.; Peralta-Guevara, D.E.; Moscoso-Moscoso, E.; Aiquipa-Pillaca, Á.S. Effect of Germination on the Physicochemical Properties, Functional Groups, Content of Bioactive Compounds, and Antioxidant Capacity of Different Varieties of Quinoa (Chenopodium quinoa Willd.) Grown in the High Andean Zone of Peru. Foods 2024, 13, 417. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Xu, J.; Shao, J.; Ding, M.; Shi, S. Preparation, characterization, and evaluation of breviscapine nanosuspension and its freeze-dried powder. Pharmaceutics 2022, 14, 923. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Huamán-Carrión, M.L.; Ramos-Pacheco, B.S.; De la Cruz, G.; Arévalo-Quijano, J.C.; Muñoz-Saenz, J.C.; Muñoz-Melgarejo, M.; Quispe-Quezada, U.R. Microencapsulation of Propolis and Honey Using Mixtures of Maltodextrin/Tara Gum and Modified Native Potato Starch/Tara Gum. Foods 2023, 12, 1873. [Google Scholar] [CrossRef]
- Jedlińska, A.; Samborska, K.; Wieczorek, A.; Wiktor, A.; Ostrowska-Ligęza, E.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Błażowski, Ł.; Tułodziecki, M. The application of dehumidified air in rapeseed and honeydew honey spray drying-Process performance and powders properties considerations. J. Food Eng. 2019, 245, 80–87. [Google Scholar] [CrossRef]
- Napiórkowska, A.; Szpicer, A.; Wojtasik-Kalinowska, I.; Perez, M.D.T.; González, H.D.; Kurek, M.A. Microencapsulation of juniper and black pepper essential oil using the coacervation method and its properties after freeze-drying. Foods 2023, 12, 4345. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Palomino-Rincón, H.; Taipe-Pardo, F.; Landa, J.P.A.; Arévalo-Quijano, J.C.; Muñoz-Saenz, J.C.; Quispe-Quezada, U.R.; Huamán-Carrión, M.L. Nanoencapsulation of Phenolic Extracts from Native Potato Clones (Solanum tuberosum spp. andigena) by Spray Drying. Molecules 2023, 28, 4961. [Google Scholar] [CrossRef]
- Ren, Z.; Huang, X.; Shi, L.; Liu, S.; Yang, S.; Hao, G.; Qiu, X.; Liu, Z.; Zhang, Y.; Zhao, Y.; et al. Characteristics and potential application of myofibrillar protein from golden threadfin bream (Nemipterus virgatus) complexed with chitosan. Int. J. Biol. Macromol. 2023, 240, 124380. [Google Scholar] [CrossRef]
- Quispe-Chambilla, L.; Pumacahua-Ramos, A.; Choque-Quispe, D.; Curro-Pérez, F.; Carrión-Sánchez, H.M.; Peralta-Guevara, D.E.; Masco-Arriola, M.L.; Palomino-Rincón, H.; Ligarda-Samanez, C.A. Rheological and functional properties of dark chocolate with partial substitution of peanuts and sacha inchi. Foods 2022, 11, 1142. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of active ingredients in food industry by spray-drying and nano spray-drying technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Bashir, M.; Haripriya, S. Assessment of physical and structural characteristics of almond gum. Int. J. Biol. Macromol. 2016, 93, 476–482. [Google Scholar] [CrossRef]
- Bouaziz, F.; Koubaa, M.; Neifar, M.; Zouari-Ellouzi, S.; Besbes, S.; Chaari, F.; Kamoun, A.; Chaabouni, M.; Chaabouni, S.E.; Ghorbel, R.E. Feasibility of using almond gum as coating agent to improve the quality of fried potato chips: Evaluation of sensorial properties. LWT-Food Sci. Technol. 2016, 65, 800–807. [Google Scholar] [CrossRef]
- Vijeth, S.; Heggannavar, G.B.; Kariduraganavar, M.Y. Encapsulating wall materials for micro-/nanocapsules. Microencapsul.-Process. Technol. Ind. Appl. 2019, 1–19. [Google Scholar] [CrossRef]
- Zotarelli, M.F.; da Silva, V.M.; Durigon, A.; Hubinger, M.D.; Laurindo, J.B. Production of mango powder by spray drying and cast-tape drying. Powder Technol. 2017, 305, 447–454. [Google Scholar] [CrossRef]
- Schuck, P.; Jeantet, R.; Dolivet, A. Analytical Methods for Food and Dairy Powders; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Rosland Abel, S.E.; Yusof, Y.A.; Chin, N.L.; Chang, L.S.; Mohd Ghazali, H.; Manaf, Y.N. Characterisation of physicochemical properties of gum arabic powder at various particle sizes. Food Res. 2020, 4, 107–115. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, B. Agglomerated xanthan gum powder used as a food thickener: Effect of sugar binders on physical, microstructural, and rheological properties. Powder Technol. 2020, 362, 301–306. [Google Scholar] [CrossRef]
- Goff, H.D.; Guo, Q. The role of hydrocolloids in the development of food structure. Handb. Food Struct. Dev. 2019, 18, 1. [Google Scholar]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Schramm, L.L. Emulsions, Foams, Suspensions, and Aerosols: Microscience and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Ligarda-Samanez, C.A.; Moscoso-Moscoso, E.; Choque-Quispe, D.; Palomino-Rincón, H.; Martínez-Huamán, E.L.; Huamán-Carrión, M.L.; Peralta-Guevara, D.E.; Aroni-Huamán, J.; Arévalo-Quijano, J.C.; Palomino-Rincón, W.; et al. Microencapsulation of Erythrocytes Extracted from Cavia porcellus Blood in Matrices of Tara Gum and Native Potato Starch. Foods 2022, 11, 2107. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta potential of food matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Huamaní-Meléndez, V.J.; Mauro, M.A.; Darros-Barbosa, R. Physicochemical and rheological properties of aqueous Tara gum solutions. Food Hydrocoll. 2021, 111, 106195. [Google Scholar] [CrossRef]
- Sikora, M.; Kowalski, S.; Tomasik, P. Binary hydrocolloids from starches and xanthan gum. Food Hydrocoll. 2008, 22, 943–952. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Wall Materials for Encapsulating Bioactive Compounds via Spray-Drying: A Review. Polymers 2023, 15, 2659. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R. The drying of foods and its effect on the physical-chemical, sensorial and nutritional properties. Int. J. Food Eng. 2018, 2, 93–100. [Google Scholar] [CrossRef]
- Labuschagne, P. Impact of wall material physicochemical characteristics on the stability of encapsulated phytochemicals: A review. Food Res. Int. 2018, 107, 227–247. [Google Scholar] [CrossRef]
- Cui, S.W. Food Carbohydrates: Chemistry, Physical Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Pachuau, L.; Lalhlenmawia, H.; Mazumder, B. Characteristics and composition of Albizia procera (Roxb.) Benth gum. Ind. Crops Prod. 2012, 40, 90–95. [Google Scholar] [CrossRef]
- Adsare, S.R.; Annapure, U.S. Microencapsulation of curcumin using coconut milk whey and Gum Arabic. J. Food Eng. 2021, 298, 110502. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Calderas, F.; Nunez Ramírez, D.; Herrera-Valencia, E.; Bernad Bernad, M.; Manero, O. Spray drying egg using either maltodextrin or nopal mucilage as stabilizer agents. J. Food Sci. Technol. 2017, 54, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Yaghoubi Hamgini, E.; Jafari, S.M. Chapter 9—Spray drying of starches and gums. In Spray Drying for the Food Industry; Jafari, S.M., Samborska, K., Eds.; Woodhead Publishing: Sawston, UK, 2024; pp. 243–274. [Google Scholar]
- Fathi, F.; Ebrahimi, S.N.; Matos, L.C.; Oliveira, M.B.P.P.; Alves, R.C. Emerging drying techniques for food safety and quality: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1125–1160. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Akcicek, A.; Bozkurt, F.; Akgül, C.; Karasu, S. Encapsulation of olive pomace extract in rocket seed gum and chia seed gum nanoparticles: Characterization, antioxidant activity and oxidative stability. Foods 2021, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Yahoum, M.M.; Toumi, S.; Tahraoui, H.; Lefnaoui, S.; Kebir, M.; Amrane, A.; Assadi, A.A.; Zhang, J.; Mouni, L. Formulation and Evaluation of Xanthan Gum Microspheres for the Sustained Release of Metformin Hydrochloride. Micromachines 2023, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.; Kumar, A.; Singh, K. Synthesis, characterization and in vitro release behavior of carboxymethyl xanthan. Int. J. Biol. Macromol. 2012, 51, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef]
- Pant, K.; Thakur, M.; Chopra, H.K.; Nanda, V. Encapsulated bee propolis powder: Drying process optimization and physicochemical characterization. LWT 2022, 155, 112956. [Google Scholar] [CrossRef]
- Jafari, Y.; Sabahi, H.; Rahaie, M. Stability and loading properties of curcumin encapsulated in Chlorella vulgaris. Food Chem. 2016, 211, 700–706. [Google Scholar] [CrossRef]
- Castro-López, C.; Espinoza-González, C.; Ramos-González, R.; Boone-Villa, V.D.; Aguilar-González, M.A.; Martínez-Ávila, G.C.; Aguilar, C.N.; Ventura-Sobrevilla, J.M. Spray-drying encapsulation of microwave-assisted extracted polyphenols from Moringa oleifera: Influence of tragacanth, locust bean, and carboxymethyl-cellulose formulations. Food Res. Int. 2021, 144, 110291. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, T.; Li, J.; Pan, B.; Hu, Q.; Duan, M.; Zhang, X. Study on the Effect of Spray Drying Process on the Quality of Microalgal Biomass: A Comprehensive Biocomposition Analysis of Spray-Dried S. acuminatus Biomass. BioEnergy Res. 2022, 15, 320–333. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compean Martínez, I.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2020, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Pashazadeh, H.; Zannou, O.; Ghellam, M.; Koca, I.; Galanakis, C.M.; Aldawoud, T.M.S. Optimization and Encapsulation of Phenolic Compounds Extracted from Maize Waste by Freeze-Drying, Spray-Drying, and Microwave-Drying Using Maltodextrin. Foods 2021, 10, 1396. [Google Scholar] [CrossRef] [PubMed]
- Kurozawa, L.E.; Park, K.J.; Hubinger, M.D. Effect of maltodextrin and gum arabic on water sorption and glass transition temperature of spray dried chicken meat hydrolysate protein. J. Food Eng. 2009, 91, 287–296. [Google Scholar] [CrossRef]
- Descamps, N.; Palzer, S.; Roos, Y.H.; Fitzpatrick, J.J. Glass transition and flowability/caking behaviour of maltodextrin DE 21. J. Food Eng. 2013, 119, 809–813. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Pozo, L.M.F.; Ramos-Pacheco, B.S.; Palomino-Rincón, H.; Gutiérrez, R.J.G.; Peralta-Guevara, D.E. Effect of Inlet Air Temperature and Quinoa Starch/Gum Arabic Ratio on Nanoencapsulation of Bioactive Compounds from Andean Potato Cultivars by Spray-Drying. Molecules 2023, 28, 7875. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, L.; Brito-De La Fuente, E.; Torrestiana-Sanchez, B.; Katthain, R. Rheological properties of the mucilage gum (Opuntia ficus indica). Food Hydrocoll. 2000, 14, 417–424. [Google Scholar] [CrossRef]
- Hussain, R.; Singh, A.; Vatankhah, H.; Ramaswamy, H.S. Effects of locust bean gum on the structural and rheological properties of resistant corn starch. J. Food Sci. Technol. 2017, 54, 650–658. [Google Scholar] [CrossRef]
- Dolez, P.I.; Mlynarek, J. 22—Smart materials for personal protective equipment: Tendencies and recent developments. In Smart Textiles and Their Applications; Koncar, V., Ed.; Woodhead Publishing: Oxford, UK, 2016; pp. 497–517. [Google Scholar]
- Wagner, N.J.; Brady, J.F. Shear thickening in colloidal dispersions. Phys. Today 2009, 62, 27–32. [Google Scholar] [CrossRef]
- Rapp, B.E. Microfluidics: Modeling, Mechanics and Mathematics; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Xu, Y. Stabbing Resistance of Soft Ballistic Body Armour Impregnated with Shear Thickening Fluid; The University of Manchester: Manchester, UK, 2017. [Google Scholar]
- Dewar, R.J.; Joyce, M.J. The thixotropic and rheopectic behaviour of maize starch and maltodextrin thickeners used in dysphagia therapy. Carbohydr. Polym. 2006, 65, 296–305. [Google Scholar] [CrossRef]
- Díaz, R. Reología aplicada a sistemas alimentarios. Editor. Grupo Compás. 2018, 18, 8080. [Google Scholar]
- Gao, X.; Huang, L.; Xiu, J.; Yi, L.; Zhao, Y. Evaluation of Viscosity Changes and Rheological Properties of Diutan Gum, Xanthan Gum, and Scleroglucan in Extreme Reservoirs. Polymers 2023, 15, 4338. [Google Scholar] [CrossRef] [PubMed]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Palomino-Rincón, H.; Ramos-Pacheco, B.S.; Moscoso-Moscoso, E.; Huamán-Carrión, M.L.; Peralta-Guevara, D.E.; Obregón-Yupanqui, M.E.; Aroni-Huamán, J.; Bravo-Franco, E.Y.; et al. Modified Polymeric Biosorbents from Rumex acetosella for the Removal of Heavy Metals in Wastewater. Polymers 2022, 14, 2191. [Google Scholar] [CrossRef] [PubMed]
- Vítězová, M.; Jančiková, S.; Dordević, D.; Vítěz, T.; Elbl, J.; Hanišáková, N.; Jampílek, J.; Kushkevych, I. The possibility of using spent coffee grounds to improve wastewater treatment due to respiration activity of microorganisms. Appl. Sci. 2019, 9, 3155. [Google Scholar] [CrossRef]








| Model | Equation | Parameters |
|---|---|---|
| Power Law | , | |
| Bingham Plastic | , | |
| Herschel–Bulkley | , , |
| Properties | GA | GE | GV | GC | ||||
|---|---|---|---|---|---|---|---|---|
| * | * | * | * | |||||
| Yield (%) | 12.18 ± 0. 45 | a | 42.38 ± 0.44 | b | 45.21 ± 0.60 | c | 51.37 ± 0.19 | d |
| Moisture (%) | 8.63 ± 0. 35 | a | 11.42 ± 0.40 | bc | 12.55 ± 0.48 | c | 10.86 ± 0.53 | b |
| Aw | 0.37 ± 0.01 | a | 0.39 ± 0.01 | b | 0.41 ± 0.01 | b | 0.40 ± 0.01 | b |
| Hygroscopicity (%) | 10.51 ± 0. 41 | a | 12.29 ± 0.43 | b | 13.41 ± 0.36 | c | 11.42 ± 0.16 | d |
| Bulk Density (g/mL) | 0.43 ± 0.01 | a | 0.71 ± 0.01 | b | 0.68 ± 0.01 | c | 0.76 ± 0.01 | d |
| L* | 91.02 ± 0.01 | a | 77.82 ± 0.01 | b | 79.62 ± 0.01 | c | 89.75 ± 0.01 | d |
| a* | 0.06 ± 0.01 | a | −0.33 ± 0.05 | b | −0.17 ± 0.04 | c | −0.41 ± 0.01 | b |
| b* | 3.52 ± 0.05 | a | 4.23 ± 0.05 | b | 6.01 ± 0.05 | c | 9.83 ± 0.02 | d |
| ζ-Potential (mV) | −16.57 ± 0.31 | a | −22.29 ± 0.38 | b | −22.97 ± 0.32 | bc | −23.77 ± 0.29 | c |
| Particle Size (µm) | 3.46 ± 0.01 | a | 30.94 ± 0.79 | b | 115.80 ± 1.64 | c | 139.60 ± 0.89 | d |
| Polydispersity | 1.77 ± 0.01 | a | 2.88 ± 0.09 | b | 3.09 ± 0.05 | c | 1.54 ± 0.02 | d |
| Model | Parameters | GA | GE | GV | GC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 °C | 60 °C | 80 °C | 40 °C | 60 °C | 80 °C | 40 °C | 60 °C | 80 °C | 40 °C | 60 °C | 80 °C | ||
| Power Law | k (×10−4 Pa·sn) | 9.9045 | 0.7980 | 0.3872 | 14.20 | 5.2710 | 0.9830 | 14.90 | 3.3455 | 1.1252 | 0.0024 | 16.10 | 4.8530 |
| 1.1344 | 1.6180 | 1.7884 | 1.1073 | 1.2642 | 1.6141 | 1.0908 | 1.3583 | 1.5944 | 1.0684 | 1.0866 | 1.3152 | ||
| R2 | 0.9739 | 0.9854 | 0.9913 | 0.9912 | 0.9816 | 0.9884 | 0.9902 | 0.9796 | 0.9938 | 0.9965 | 0.9967 | 0.9926 | |
| Bingham Plastic | (Pa) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (Pa·sn) | 0.0020 | 0.002 | 0.0023 | 0.0025 | 0.0021 | 0.0024 | 0.0024 | 0.0022 | 0.0025 | 0.0034 | 0.0026 | 0.0025 | |
| R2 | 0.9626 | 0.8578 | 0.8081 | 0.9826 | 0.9472 | 0.8628 | 0.9828 | 0.9244 | 0.8726 | 0.9944 | 0.9880 | 0.9422 | |
| Herschel–Bulkley | (Pa) | 0.0093 | 0.0166 | 0.0119 | 0.0034 | 0.0185 | 0.0215 | 0 | 0.0221 | 0.0219 | 0.0027 | 0.0035 | 0.0141 |
| (×10−4 Pa·sn) | 6.8532 | 0.2850 | 0.1993 | 0.0013 | 2.2715 | 0.3197 | 0.0017 | 1.1315 | 0.3804 | 0.0023 | 14.60 | 2.8672 | |
| 1.2010 | 1.8081 | 1.9070 | 1.1250 | 1.4179 | 1.8217 | 1.0672 | 1.5573 | 1.7947 | 1.0780 | 1.1040 | 1.4111 | ||
| R2 | 0.9743 | 1 | 1 | 0.9912 | 1 | 1 | 0.9892 | 1 | 0.9967 | 0.9966 | 0.9938 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscoso-Moscoso, E.; Ligarda-Samanez, C.A.; Choque-Quispe, D.; Huamán-Carrión, M.L.; Arévalo-Quijano, J.C.; De la Cruz, G.; Luciano-Alipio, R.; Calsina Ponce, W.C.; Sucari-León, R.; Quispe-Quezada, U.R.; et al. Preliminary Assessment of Tara Gum as a Wall Material: Physicochemical, Structural, Thermal, and Rheological Analyses of Different Drying Methods. Polymers 2024, 16, 838. https://doi.org/10.3390/polym16060838
Moscoso-Moscoso E, Ligarda-Samanez CA, Choque-Quispe D, Huamán-Carrión ML, Arévalo-Quijano JC, De la Cruz G, Luciano-Alipio R, Calsina Ponce WC, Sucari-León R, Quispe-Quezada UR, et al. Preliminary Assessment of Tara Gum as a Wall Material: Physicochemical, Structural, Thermal, and Rheological Analyses of Different Drying Methods. Polymers. 2024; 16(6):838. https://doi.org/10.3390/polym16060838
Chicago/Turabian StyleMoscoso-Moscoso, Elibet, Carlos A. Ligarda-Samanez, David Choque-Quispe, Mary L. Huamán-Carrión, José C. Arévalo-Quijano, Germán De la Cruz, Rober Luciano-Alipio, Wilber Cesar Calsina Ponce, Reynaldo Sucari-León, Uriel R. Quispe-Quezada, and et al. 2024. "Preliminary Assessment of Tara Gum as a Wall Material: Physicochemical, Structural, Thermal, and Rheological Analyses of Different Drying Methods" Polymers 16, no. 6: 838. https://doi.org/10.3390/polym16060838