Breaking New Ground: Exploring the Promising Role of Solid-State Fermentation in Harnessing Natural Biostimulants for Sustainable Agriculture
Abstract
:1. Introduction
2. Materials and Methods
Methodology
3. Relevant Sections
3.1. Definition and Types of Biostimulants
3.2. Advantages of Natural Biostimulants over Conventional Ones
3.2.1. Sustainability and Environmental Impact
3.2.2. Security
3.2.3. Broad Spectrum of Activity
3.2.4. Positive Interactions
3.2.5. Regulatory Compliance
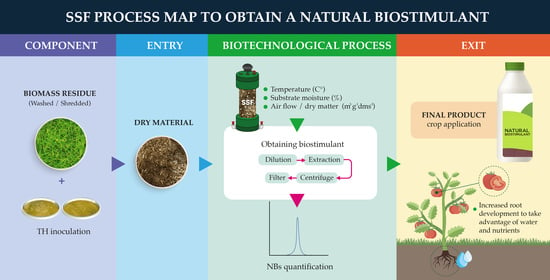
3.3. Production Processes of NBs by SSF
3.3.1. Substrate Selection in NB Production by SSF
3.3.2. Substrate Pretreatment
3.3.3. Microorganisms for NB Production by SSF and Inoculation
3.3.4. Control of SSF Conditions
3.3.5. SSF Bioreactors in NB Production
4. Methods of NB Production
4.1. Microorganisms Used in NB Production
4.2. Characteristics of SSF for NB Production
4.3. Effect of the NBs on Crops
4.3.1. Improvement of Plant Growth and Development
4.3.2. Increased Resistance to Adverse Conditions
4.3.3. Effect of NBs on Improving Crop Quality
4.3.4. Optimization of Nutrient Use Efficiency
4.3.5. Effect NBs on Agricultural Productivity
4.4. Limitations and Challenges of NBs by SSF
4.4.1. Standardization Issues in NB Production by SSF
4.4.2. Challenges in the Application of NBs from SSF in Sustainable Agriculture
4.4.3. Factors Limiting the Effectiveness of Natural Biostimulants Produced by SSF in Different Crops
5. Conclusions and Future Research Perspectives
5.1. Conclusions
5.2. Future Research Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Natural biostimulants | (NBs) |
| Solid-state fermentation | (SSF) |
| The European Biostimulants Industry Council | (EBIC) |
| Humic substances | (HS) |
| Hormone-containing products | (HCP) |
| Amino-acid-containing products | (AACP) |
| Indole-3-acetic acid | (IAA) |
| Abscisic acid | (ABA) |
References
- 2.4.1 Agricultural Sustainability|Sustainable Development Goals|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/sustainable-development-goals/indicators/241/en/ (accessed on 23 May 2023).
- Sumberg, J.; Giller, K.E. What Is ‘Conventional’ Agriculture? Glob. Food Secur. 2022, 32, 100617. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a Biostimulant on the Heat Tolerance Associated with Photosynthetic Capacity, Membrane Thermostability, and Polyphenol Production of Perennial Ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Wong, W.S.; Tan, S.N.; Ge, L.; Chen, X.; Letham, D.S.; Yong, J.W.H. The Importance of Phytohormones and Microbes in Biostimulants: Mass Spectrometric Evidence and Their Positive Effects on Plant Growth. Acta Hortic. 2016, 1148, 49–60. [Google Scholar] [CrossRef]
- Boivin, S.; Fonouni-Farde, C.; Frugier, F. How Auxin and Cytokinin Phytohormones Modulate Root Microbe Interactions. Front. Plant Sci. 2016, 7, 1240. [Google Scholar] [CrossRef] [Green Version]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Sauer, M.; Robert, S.; Kleine-Vehn, J. Auxin: Simply Complicated. J. Exp. Bot. 2013, 64, 2565–2577. [Google Scholar] [CrossRef] [Green Version]
- Rady, M.M.; Desoky, E.-S.M.; Elrys, A.S.; Boghdady, M.S. Can Licorice Root Extract Be Used as an Effective Natural Biostimulant for Salt-Stressed Common Bean Plants? S. Afr. J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Zaman, M.; Pharis, R.P. Phytohormonal Basis for the Plant Growth Promoting Action of Naturally Occurring Biostimulators. J. Sci. Food Agric. 2014, 94, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.M.; Szafrańska, K. Biostimulators: A New Trend towards Solving an Old Problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar]
- Hellequin, E.; Monard, C.; Chorin, M.; Le bris, N.; Daburon, V.; Klarzynski, O.; Binet, F. Responses of Active Soil Microorganisms Facing to a Soil Biostimulant Input Compared to Plant Legacy Effects. Sci. Rep. 2020, 10, 13727. [Google Scholar] [CrossRef]
- EBIC—The European Biostimulants Industry Council. Available online: https://biostimulants.eu/ (accessed on 8 March 2022).
- Biological Products Industry Alliance|Advancing Knowledge About Biopesticides & Biostimulants. Available online: https://www.bpia.org/ (accessed on 23 April 2023).
- do Prado, D.Z.; Okino-Delgado, C.H.; Zanutto-Elgui, M.R.; da Silva, R.B.G.; Pereira, M.S.; Jahn, L.; Ludwig-Müller, J.; da Silva, M.R.; Velini, E.D.; Fleuri, L.F. Screening of Aspergillus, Bacillus and Trichoderma Strains and Influence of Substrates on Auxin and Phytases Production through Solid-State Fermentation. Biocatal. Agric. Biotechnol. 2019, 19, 101165. [Google Scholar] [CrossRef]
- Ghoreishi, G.; Barrena, R.; Font, X. Using Green Waste as Substrate to Produce Biostimulant and Biopesticide Products through Solid-State Fermentation. Waste Manag. 2023, 159, 84–92. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology Principles of Solid State Fermentation. Mod. Solid State Ferment. 2013, 23–74. [Google Scholar] [CrossRef]
- Sanchez-Montesinos, B.; Dianez, F.; Moreno-Gavira, A.; Gea, F.J.; Santos, M. Role of Trichoderma Aggressivum f. Europaeumas Plant-Growth Promoter in Horticulture. Agronomy 2020, 10, 1004. [Google Scholar] [CrossRef]
- Do Prado, D.Z.; Oliveira, S.L.; Okino-Delgado, C.H.; Auer, S.; Ludwig-Müller, J.; da Silva, M.R.; da Costa Fernandes, C.J.; Carbonari, C.A.; Zambuzzi, W.F.; Fleuri, L.F. Aspergillus Flavipes as a Novel Biostimulant for Rooting-Enhancement of Eucalyptus. J. Clean. Prod. 2019, 234, 681–689. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The Opportunity of Valorizing Agricultural Waste, Through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An Overview of Plant-Based Natural Biostimulants for Sustainable Horticulture with a Particular Focus on Moringa Leaf Extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Li, Y.; Cui, Y.; Liu, R.; Li, Y.; Chen, Q.; Gu, Y.; Zhao, K.; Xiang, Q.; Xu, K.; et al. An Indoleacetic Acid-Producing Ochrobactrum Sp. MGJ11 Counteracts Cadmium Effect on Soybean by Promoting Plant Growth. J. Appl. Microbiol. 2017, 122, 987–996. [Google Scholar] [CrossRef]
- Karnwal, A.; Dohroo, A. Effect of Maize Root Exudates on Indole-3-Acetic Acid Production by Rice Endophytic Bacteria under Influence of L-Tryptophan. F1000Research 2018, 7, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, T.C.V.; Tran, D.T.; Le, T.G.; Nguyen, Q.T. Characterization of Endogenous Auxins and Gibberellins Produced by Chlorella sorokiniana TH01 under Phototrophic and Mixtrophic Cultivation Modes toward Applications in Microalgal Biorefinery and Crop Research. J. Chem. 2020, 2020, e4910621. [Google Scholar] [CrossRef]
- Ma, D.; Liu, B.; Ge, L.; Weng, Y.; Cao, X.; Liu, F.; Mao, P.; Ma, X. Identification and Characterization of Regulatory Pathways Involved in Early Flowering in the New Leaves of Alfalfa (Medicago sativa L.) by Transcriptome Analysis. BMC Plant Biol. 2021, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Rusmin, D.; Basmal, J.; Kusumawati, R.; Darwati, I. Improving the Growth of Clove Seedlings by the Application of Seaweed Waste as Organic Fertilizers. IOP Conf. Ser. Earth Environ. Sci. 2020, 418, 012029. [Google Scholar] [CrossRef]
- Jain, P.; Farooq, B.; Lamba, S.; Koul, B. Foliar Spray of Moringa Oleifera Lam. Leaf Extracts (MLE) Enhances the Stevioside, Zeatin and Mineral Contents in Stevia Rebaudiana Betoni. S. Afr. J. Bot. 2020, 132, 249–257. [Google Scholar] [CrossRef]
- Basra, S.M.A.; Lovatt, C.J. Exogenous Applications of Moringa Leaf Extract and Cytokinins Improve Plant Growth, Yield, and Fruit Quality of Cherry Tomato. HortTechnology 2016, 26, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, B.; Wong, J.W.C.; Selvam, A.; Sekaran, G. Influence of Microbial Diversity and Plant Growth Hormones in Compost and Vermicompost from Fermented Tannery Waste. Bioresour. Technol. 2016, 217, 200–204. [Google Scholar] [CrossRef]
- Ravindran, B.; Contreras-Ramos, S.M.; Sekaran, G. Changes in Earthworm Gut Associated Enzymes and Microbial Diversity on the Treatment of Fermented Tannery Waste Using Epigeic Earthworm Eudrilus Eugeniae. Ecol. Eng. 2015, 74, 394–401. [Google Scholar] [CrossRef]
- Ali, H.M.; Khan, H.Z.; Afzal, I. Exogenous application of growth promoting substances improves growth, yield and quality of spring maize (Zea mays L.) hybrids under late sown conditions. Bull. Biol. Allied Sci. Res. 2017, 2017, 9. [Google Scholar] [CrossRef]
- Qi, H. Method for Producing Abscisic Acid by Solid State Fermentation of Fungi 2013. Available online: https://patents.google.com/patent/CN103409474A/en (accessed on 6 June 2023).
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Thomaz-Soccol, V.; Martínez-Burgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-State Fermentation Technology and Innovation for the Production of Agricultural and Animal Feed Bioproducts. Syst. Microbiol. Biomanuf. 2021, 1, 142–165. [Google Scholar] [CrossRef]
- Rodrigues, C.; Vandenberghe, L.P.D.S.; De Oliveira, J.; Soccol, C.R. New Perspectives of Gibberellic Acid Production: A Review. Crit. Rev. Biotechnol. 2012, 32, 263–273. [Google Scholar] [CrossRef]
- Yang, S.; Xie, J.; Hu, N.; Liu, Y.; Zhang, J.; Ye, X.; Liu, Z. Bioconversion of Gibberellin Fermentation Residue into Feed Supplement and Organic Fertilizer Employing Housefly (Musca domestica L.) Assisted by Corynebacterium variabile. PLoS ONE 2015, 10, e0110809. [Google Scholar] [CrossRef] [PubMed]
- Camara, M.C.; Vandenberghe, L.P.S.; Rodrigues, C.; de Oliveira, J.; Faulds, C.; Bertrand, E.; Soccol, C.R. Current Advances in Gibberellic Acid (GA3) Production, Patented Technologies and Potential Applications. Planta 2018, 248, 1049–1062. [Google Scholar] [CrossRef]
- Brückner, B.; Blechschmidt, D. The Gibberellin Fermentation. Crit. Rev. Biotechnol. 1991, 11, 163–192. [Google Scholar] [CrossRef]
- Maria, C.; Machado, M.; Soccol, C.R. Gibberellic Acid Production. In Current Developments in Solid-State Fermentation; Pandey, A., Soccol, C.R., Larroche, C., Eds.; Springer: New York, NY, USA, 2008; pp. 277–301. ISBN 978-0-387-75213-6. [Google Scholar]
- Saeed, S.; Mehmood, T.; Irfan, M. Statistical Optimization of Cultural Parameters for the Optimized Production of Alginic Acid Using Apple (Malus Domestica) Peels through Solid-State Fermentation. Biomass Conv. Bioref. 2023, 13, 1269–1277. [Google Scholar] [CrossRef]
- Dos Santos Silva, M.C.; De Farias Silva, C.E.; dos Santos, L.M.; Medeiros, J.A.; Vieira, R.C.; de Souza Abud, A.K.; Almeida, R.M.R.G.; Tonholo, J. Alginate Lyase Produced by Filamentous Fungus Through Solid State Fermentation Using Sargassum from the Brazilian Coast. Waste Biomass Valor. 2022, 13, 2947–2962. [Google Scholar] [CrossRef]
- Wozniak, E.; Blaszczak, A.; Wiatrak, P.; Canady, M. Biostimulant Mode of Action; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar]
- Rodríguez-Jasso, R.M.; Mussatto, S.I.; Sepúlveda, L.; Agrasar, A.T.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Fungal Fucoidanase Production by Solid-State Fermentation in a Rotating Drum Bioreactor Using Algal Biomass as Substrate. Food Bioprod. Process. 2013, 91, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Bergé, J.P. Marine Biotechnology: An Overview of Leading Field. In Proceedings of the IXth ESMB Meeting, Nantes, France, 12–14 May 2002. [Google Scholar]
- Patel, J.S.; Selvaraj, V.; More, P.; Bahmani, R.; Borza, T.; Prithiviraj, B. A Plant Biostimulant from Ascophyllum Nodosum Potentiates Plant Growth Promotion and Stress Protection Activity of Pseudomonas Protegens CHA0. Plants 2023, 12, 1208. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Yuan, Y.; Jing, C.; Cao, J.; Wang, Y.; Zhang, L.; Zhang, C.; Li, Y. Purification and Characterization of a Fucoidan from the Brown Algae Macrocystis Pyrifera and the Activity of Enhancing Salt-Stress Tolerance of Wheat Seedlings. Int. J. Biol. Macromol. 2021, 180, 547–558. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Tarafdar, J.C. Chapter 15—Biostimulants for Sustainable Crop Production. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 299–313. ISBN 978-0-323-85579-2. [Google Scholar]
- Solid-State Fermentation for Humic Acids Production by a Trichoderma Reesei Strain Using an Oil Palm Empty Fruit Bunch as the Substrate|SpringerLink. Available online: https://link.springer.com/article/10.1007/s12010-013-0668-2 (accessed on 27 May 2023).
- Zhang, Y.; Dou, S.; Hamza, B.; Ye, S.; Zhang, D. Mechanisms of Three Fungal Types on Humic-Like Substances Formation during Solid-State Fermentation of Corn Straw. Int. J. Agric. Biol. 2020, 23, 970–976. [Google Scholar]
- Yang, Y.; Wang, L.; Zhang, Y.; Li, L.; Shi, X.; Liu, X.; Ren, X.; Dou, S. Transformation of Corn Stalk Residue to Humus-like Substances during Solid-State Fermentation. Sustainability 2019, 11, 6771. [Google Scholar] [CrossRef] [Green Version]
- Bettoni, M.M.; Mogor, Á.F.; Pauletti, V.; Goicoechea, N. Growth and Metabolism of Onion Seedlings as Affected by the Application of Humic Substances, Mycorrhizal Inoculation and Elevated CO2. Sci. Hortic. 2014, 180, 227–235. [Google Scholar] [CrossRef]
- Hölker, U.; Höfer, M. Solid Substrate Fermentation of Lignite by the Coal-Solubilizing Mould, Trichoderma Atroviride, in a New Type of Bioreactor. Biotechnol. Lett. 2002, 24, 1643–1645. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and Fulvic Acids as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Deswal, D.; Khasa, Y.P.; Kuhad, R.C. Optimization of Cellulase Production by a Brown Rot Fungus Fomitopsis Sp. RCK2010 under Solid State Fermentation. Bioresour. Technol. 2011, 102, 6065–6072. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, H.K.; Sharma, K.; Gupta, J.K.; Soni, S.K. Production of a Thermostable α-Amylase from Bacillus Sp. PS-7 by Solid State Fermentation and Its Synergistic Use in the Hydrolysis of Malt Starch for Alcohol Production. Process Biochem. 2005, 40, 525–534. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The Effect of a Plant-Derived Biostimulant on Metabolic Profiling and Crop Performance of Lettuce Grown under Saline Conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Asri, N.M.; Muhialdin, B.J.; Zarei, M.; Saari, N. Low Molecular Weight Peptides Generated from Palm Kernel Cake via Solid State Lacto-Fermentation Extend the Shelf Life of Bread. LWT 2020, 134, 110206. [Google Scholar] [CrossRef]
- Li, W.; Wang, T. Effect of Solid-State Fermentation with Bacillus Subtilis Lwo on the Proteolysis and the Antioxidative Properties of Chickpeas. Int. J. Food Microbiol. 2021, 338, 108988. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Hua, Y.; Liu, H.; Dai, X. A New Approach to Recycling Cephalosporin Fermentation Residue into Plant Biostimulants. J. Hazard. Mater. 2021, 413, 125393. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, G.N.; Bindu, P. Optimization of Process Parameters for Siderophore Production Under Solid State Fermentation Using Polystyrene Beads as Inert Support. JSIR 2016, 75, 621–625. [Google Scholar]
- Le, H.; ZongHao, Y.; Can, C.; ChunYan, L.; Juan, L.; ZhongKe, S. Enhancing Iron Uptake and Alleviating Iron Toxicity in Wheat by Plant Growth-Promoting Bacteria: Theories and Practices. Int. J. Agric. Biol. 2020, 23, 190–196. [Google Scholar]
- Marschner, H.; Römheld, V.; Kissel, M. Different Strategies in Higher Plants in Mobilization and Uptake of Iron. J. Plant Nutr. 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Stanley-Raja, V.; Senthil-Nathan, S.; Chanthini, K.M.P.; Sivanesh, H.; Ramasubramanian, R.; Karthi, S.; Shyam-Sundar, N.; Vasantha-Srinivasan, P.; Kalaivani, K. Biological Activity of Chitosan Inducing Resistance Efficiency of Rice (Oryza sativa L.) after Treatment with Fungal Based Chitosan. Sci. Rep. 2021, 11, 20488. [Google Scholar] [CrossRef] [PubMed]
- Nwe, N.; Chandrkrachang, S.; Stevens, W.F.; Maw, T.; Tan, T.K.; Khor, E.; Wong, S.M. Production of Fungal Chitosan by Solid State and Submerged Fermentation. Carbohydr. Polym. 2002, 49, 235–237. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Lau, S.-E.; Teo, W.F.A.; Teoh, E.Y.; Tan, B.C. Microbiome Engineering and Plant Biostimulants for Sustainable Crop Improvement and Mitigation of Biotic and Abiotic Stresses. Discov. Food 2022, 2, 9. [Google Scholar] [CrossRef]
- Ben Mrid, R.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary Metabolites as Biostimulant and Bioprotectant Agents: A Review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Caccavo, V.; Forlano, P.; Mang, S.M.; Fanti, P.; Nuzzaci, M.; Battaglia, D.; Trotta, V. Effects of Trichoderma Harzianum Strain T22 on the Arthropod Community Associated with Tomato Plants and on the Crop Performance in an Experimental Field. Insects 2022, 13, 418. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Rasera, R.; Causin, R. Trichoderma Harzianum Seed Treatment Controls Fusarium Verticillioides Colonization and Fumonisin Contamination in Maize under Field Conditions. Crop Prot. 2014, 65, 51–56. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing Biostimulants from Agro-Food and Industrial By-Products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [Green Version]
- Francesca, S.; Arena, C.; Mele, B.H.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The Use of a Plant-Based Biostimulant Improves Plant Performances and Fruit Quality in Tomato Plants Grown at Elevated Temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.; Kumar, G.; Hussain, S. Utilization of Seaweed-Based Biostimulants in Improving Plant and Soil Health: Current Updates and Future Prospective. Int. J. Environ. Sci. Technol. 2022, 19, 12839–12852. [Google Scholar] [CrossRef]
- da Silva, L.C.A.; Honorato, T.L.; Cavalcante, R.S.; Franco, T.T.; Rodrigues, S. Effect of PH and Temperature on Enzyme Activity of Chitosanase Produced Under Solid Stated Fermentation by Trichoderma spp. Indian J. Microbiol. 2012, 52, 60–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, N.A.; Saleem, M.H.; Shafiq, S.; Naz, H.; Farid-ul-Haq, M.; Ali, B.; Shafiq, F.; Iqbal, M.; Jaremko, M.; Qureshi, K.A. Phytoextracts as Crop Biostimulants and Natural Protective Agents—A Critical Review. Sustainability 2022, 14, 14498. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Lopez, A.; Martos, V.; Reyes, A.; Maksimovic, I.; Eichler-Löbermann, B.; Malusà, E. Unexploited Potential of Some Biotechnological Techniques for Biofertilizer Production and Formulation. Appl. Microbiol. Biotechnol. 2015, 99, 4983–4996. [Google Scholar] [CrossRef]
- Chen, H. Modern Solid State Fermentation; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent Advances in Solid-State Fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Szabo, O.E.; Csiszar, E.; Koczka, B.; Kiss, K. Ultrasonically Assisted Single Stage and Multiple Extraction of Enzymes Produced by Aspergillus Oryzae on a Lignocellulosic Substrate with Solid-State Fermentation. Biomass Bioenergy 2015, 75, 161–169. [Google Scholar] [CrossRef]
- Yadav, J.S.; Tripathi, J.P. Optimization of Cultivation and Nutrition Conditions and Substrate Pretreatment for Solid-Substrate Fermentation of Wheat Straw ByCoriolus Versicolor. Folia Microbiol. 1991, 36, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Kunamneni, A.; Permaul, K.; Singh, S. Amylase Production in Solid State Fermentation by the Thermophilic Fungus Thermomyces Lanuginosus. J. Biosci. Bioeng. 2005, 100, 168–171. [Google Scholar] [CrossRef]
- Elibol, M.; Moreira, A.R. Optimizing Some Factors Affecting Alkaline Protease Production by a Marine Bacterium Teredinobacter Turnirae under Solid Substrate Fermentation. Process Biochem. 2005, 40, 1951–1956. [Google Scholar] [CrossRef]
- Alias, C.; Bulgari, D.; Gobbi, E. It Works! Organic-Waste-Assisted Trichoderma Spp. Solid-State Fermentation on Agricultural Digestate. Microorganisms 2022, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Raghavarao, K.S.M.S.; Ranganathan, T.V.; Karanth, N.G. Some Engineering Aspects of Solid-State Fermentation. Biochem. Eng. J. 2003, 13, 127–135. [Google Scholar] [CrossRef]
- General Considerations about Solid-State Fermentation Processes|SpringerLink. Available online: https://link.springer.com/chapter/10.1007/978-0-387-75213-6_2 (accessed on 23 July 2023).
- Kamilova, F.; Okon, Y.; de Weert, S.; Hora, K. Commercialization of Microbes: Manufacturing, Inoculation, Best Practice for Objective Field Testing, and Registration. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 319–327. ISBN 978-3-319-08575-3. [Google Scholar]
- Kiruba, N.J.M.; Saeid, A. An Insight into Microbial Inoculants for Bioconversion of Waste Biomass into Sustainable “Bio-Organic” Fertilizers: A Bibliometric Analysis and Systematic Literature Review. Int. J. Mol. Sci. 2022, 23, 13049. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in Solid-State Fermentation for Bioconversion of Agricultural Wastes to Value-Added Products: Opportunities and Challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef]
- Weber, F.J.; Tramper, J.; Rinzema, A. A Simplified Material and Energy Balance Approach for Process Development and Scale-up of Coniothyrium Minitans Conidia Production by Solid-State Cultivation in a Packed-Bed Reactor. Biotechnol. Bioeng. 1999, 65, 447–458. [Google Scholar] [CrossRef]
- de Oliveira, J.; Rodrigues, C.; Vandenberghe, L.P.S.; Câmara, M.C.; Libardi, N.; Soccol, C.R. Gibberellic Acid Production by Different Fermentation Systems Using Citric Pulp as Substrate/Support. BioMed Res. Int. 2017, 2017, e5191046. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, D.; Alias, C.; Peron, G.; Ribaudo, G.; Gianoncelli, A.; Savino, S.; Boureghda, H.; Bouznad, Z.; Monti, E.; Gobbi, E. Solid-State Fermentation of Trichoderma Spp.: A New Way to Valorize the Agricultural Digestate and Produce Value-Added Bioproducts. J. Agric. Food Chem. 2023, 71, 3994–4004. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent Developments and Key Barriers to Advanced Biofuels: A Short Review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef]
- Nitayapat, N.; Prakarnsombut, N.; Lee, S.J.; Boonsupthip, W. Bioconversion of Tangerine Residues by Solid-State Fermentation with Lentinus Polychrous and Drying the Final Products. LWT Food Sci. Technol. 2015, 63, 773–779. [Google Scholar] [CrossRef]
- Vassileva, M.; Malusá, E.; Eichler-Löbermann, B.; Vassilev, N. Aspegillus Terreus: From Soil to Industry and Back. Microorganisms 2020, 8, 1655. [Google Scholar] [CrossRef]
- Dos Santos, V.Z.; Vieira, K.R.; Nass, P.P.; Zepka, L.Q.; Jacob-Lopes, E. Application of Microalgae Consortia/Cocultures in Wastewater Treatment. In Recent Advances in Microbial Degradation; Ahamed, M.I., Prasad, R., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2021; pp. 131–154. ISBN 9789811605185. [Google Scholar]
- Tong, C.Y.; Honda, K.; Derek, C.J.C. A Review on Microalgal-Bacterial Co-Culture: The Multifaceted Role of Beneficial Bacteria towards Enhancement of Microalgal Metabolite Production. Environ. Res. 2023, 228, 115872. [Google Scholar] [CrossRef]
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and Assessment of Cell Viability in Formulation of Non-Sporulating Bacterial Inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef] [Green Version]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 2012, e491206. [Google Scholar] [CrossRef] [PubMed]
- Fuess, L.T.; Lens, P.N.L.; Garcia, M.L.; Zaiat, M. Exploring Potentials for Bioresource and Bioenergy Recovery from Vinasse, the “New” Protagonist in Brazilian Sugarcane Biorefineries. Biomass 2022, 2, 374–411. [Google Scholar] [CrossRef]
- Vassileva, M.; Malusà, E.; Sas-Paszt, L.; Trzcinski, P.; Galvez, A.; Flor-Peregrin, E.; Shilev, S.; Canfora, L.; Mocali, S.; Vassilev, N. Fermentation Strategies to Improve Soil Bio-Inoculant Production and Quality. Microorganisms 2021, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Shetty, K. Cranberry Processing Waste for Solid State Fungal Inoculant Production. Process Biochem. 1998, 33, 323–329. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New Developments in Solid State Fermentation: I-Bioprocesses and Products. Process Biochem. 2000, 35, 1153–1169. [Google Scholar] [CrossRef]
- Malik, T.; Rawat, S. Biotechnological Interventions for Production of Flavour and Fragrance Compounds. In Sustainable Bioeconomy: Pathways to Sustainable Development Goals; Venkatramanan, V., Shah, S., Prasad, R., Eds.; Springer: Singapore, 2021; pp. 131–170. ISBN 9789811573217. [Google Scholar]
- Hassan, G.; Shabbir, M.A.; Ahmad, F.; Pasha, I.; Aslam, N.; Ahmad, T.; Rehman, A.; Manzoor, M.F.; Inam-Ur-Raheem, M.; Aadil, R.M. Cereal Processing Waste, an Environmental Impact and Value Addition Perspectives: A Comprehensive Treatise. Food Chem. 2021, 363, 130352. [Google Scholar] [CrossRef] [PubMed]
- Finkler, A.T.J.; Biz, A.; Pitol, L.O.; Medina, B.S.; Luithardt, H.; Luz, L.F.d.L.; Krieger, N.; Mitchell, D.A. Intermittent Agitation Contributes to Uniformity across the Bed during Pectinase Production by Aspergillus Niger Grown in Solid-State Fermentation in a Pilot-Scale Packed-Bed Bioreactor. Biochem. Eng. J. 2017, 121, 1–12. [Google Scholar] [CrossRef]
- Berovic, M. Cultivation of Medicinal Mushroom Biomass by Solid-State Bioprocessing in Bioreactors. In Solid State Fermentation: Research and Industrial Applications; Steudler, S., Werner, A., Cheng, J.J., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–25. ISBN 978-3-030-23675-5. [Google Scholar]
- Khairy, M.F.A.; Mohamed, A.A.I.; Khlil, M.M.N. Development of bioreactor to enrich the protein of agricultural residues. Misr J. Agric. Eng. 2015, 32, 1625–1640. [Google Scholar] [CrossRef]
- Werle, L.B. Obtention Gibberellic Acid by Solid State Ferment. Employing Brew. Residue Crude Rice Brand Substrates. Master’s Thesis, Federal University of Santa Maria, Santa Maria, Brazil, 2017. [Google Scholar]
- Monrroy, M.; García, J.R. Gibberellic Acid Production from Corn Cob Residues via Fermentation with Aspergillus niger. J. Chem. 2022, 2022, e1112941. [Google Scholar] [CrossRef]
- Jain, B.M.; Badve, M.P. A Novel Process for Synthesis of Soybean Protein Hydrolysates and Study of Its Effectiveness as a Biostimulant and Emulsifier. Chem. Eng. Process. Process Intensif. 2022, 174, 108880. [Google Scholar] [CrossRef]
- Wei, X.; Sui, Z.; Guo, M.; Chen, S.; Zhang, Z.; Geng, J.; Xiao, J.; Huang, D. The Potential of Degrading Natural Chitinous Wastes to Oligosaccharides by Chitinolytic Enzymes from Two Talaromyces Sp. Isolated from Rotten Insects (Hermetia Illucens) under Solid State Fermentation. Braz. J. Microbiol. 2023, 54, 223–238. [Google Scholar] [CrossRef]
- Volpi, M.P.C.; Corzo, I.J.M.; Bastos, R.G.; Santana, M.H.A. Production of Humic Acids by Solid-State Fermentation of Trichoderma Reesei in Raw Oil Palm Empty Fruit Bunch Fibers. 3 Biotech 2019, 9, 393. [Google Scholar] [CrossRef]
- Ghanavati, H.; Ramezanipour, N.; Jouzani, G.S.; Kowsari, M.; Valijanian, E.; Nikrad, M.; Mostajeran, F.; Tahmasbi, M. Submerged Fermentation as a Suitable Solution to Produce Humic and Fulvic Acids from Sugarcane Bagasse. Sci. Iran. 2022, 29, 3554–3569. [Google Scholar] [CrossRef]
- Amadou, I.; Le, G.-W.; Shi, Y.-H.; Gbadamosi, O.S.; Kamara, M.T.; Jin, S. Optimized Lactobacillus Plantarum Lp6 Solid-State Fermentation and Proteolytic Hydrolysis Improve Some Nutritional Attributes of Soybean Protein Meal. J. Food Biochem. 2011, 35, 1686–1694. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as Biostimulant: Exploiting the Multilevel Properties of a Plant Beneficial Fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Dikilitas, M.; Tuna, A.L. Alleviation of Salt Stress-Induced Adverse Effects on Maize Plants by Exogenous Application of Indoleacetic Acid (IAA) and Inorganic Nutrients—A Field Trial. Aust. J. Crop Sci. 2013, 7, 249–254. [Google Scholar]
- Richards, D.E.; King, K.E.; Ait-ali, T.; Harberd, N.P. How gibberellin regulates plant growth and development: A Molecular Genetic Analysis of Gibberellin Signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 67–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brian, P.W. Effects of Gibberellins on Plant Growth and Development. Biol. Rev. 1959, 34, 37–77. [Google Scholar] [CrossRef]
- Soper, F.M.; Paungfoo-Lonhienne, C.; Brackin, R.; Rentsch, D.; Schmidt, S.; Robinson, N. Arabidopsis and Lobelia Anceps Access Small Peptides as a Nitrogen Source for Growth. Funct. Plant Biol. 2011, 38, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Song, S.; Zhou, W.; Dossou, S.S.K.; Zhou, R.; Zhang, Y.; Li, D.; You, J.; Wang, L. Integrating Transcriptome and Phytohormones Analysis Provided Insights into Plant Height Development in Sesame. Plant Physiol. Biochem. 2023, 198, 107695. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xie, Y.; Yu, S.; Yang, J.; Chen, S.; Yuan, X.; Guo, T.; Wang, H.; Liu, Y.; Chen, C.; et al. The DnaJ Domain-Containing Heat-Shock Protein NAL11 Determines Plant Architecture by Mediating Gibberellin Homeostasis in Rice (Oryza sativa). New Phytol. 2023, 237, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Tsavkelova, E.A.; Cherdyntseva, T.A.; Klimova, S.Y.; Shestakov, A.I.; Botina, S.G.; Netrusov, A.I. Orchid-Associated Bacteria Produce Indole-3-Acetic Acid, Promote Seed Germination, and Increase Their Microbial Yield in Response to Exogenous Auxin. Arch. Microbiol. 2007, 188, 655–664. [Google Scholar] [CrossRef]
- Egamberdieva, D. Alleviation of Salt Stress by Plant Growth Regulators and IAA Producing Bacteria in Wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Florido Bacallao, M.; Bao Fundora, L. Tolerancia a Estrés Por Déficit Hídrico En Tomate (Solanum lycopersicum L.). Cultiv. Trop. 2014, 35, 70–88. [Google Scholar]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Shen, Y.; Jin, S.; Huang, S.; Cheng, X.; Wang, Z.; Li, P.; Zhao, J.; Bao, M.; Ning, G. Overexpression of Rosa Rugosa Anthocyanidin Reductase Enhances Tobacco Tolerance to Abiotic Stress through Increased ROS Scavenging and Modulation of ABA Signaling. Plant Sci. 2016, 245, 35–49. [Google Scholar] [CrossRef]
- Yazdani, M.; Croen, M.G.; Fish, T.L.; Thannhauser, T.W.; Ahner, B.A. Overexpression of Native ORANGE (OR) and OR Mutant Protein in Chlamydomonas Reinhardtii Enhances Carotenoid and ABA Accumulation and Increases Resistance to Abiotic Stress. Metab. Eng. 2021, 68, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, M.; Hu, J.; Zhang, X.; Wang, K.; Ashraf, M. Modulation Role of Abscisic Acid (ABA) on Growth, Water Relations and Glycinebetaine Metabolism in Two Maize (Zea mays L.) Cultivars under Drought Stress. Int. J. Mol. Sci. 2012, 13, 3189–3202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Patel, J.S. Seaweed Extract: Biostimulator of Plant Defense and Plant Productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Berthon, J.-Y.; Michel, T.; Wauquier, A.; Joly, P.; Gerbore, J.; Filaire, E. Seaweed and microalgae as major actors of blue biotechnology to achieve plant stimulation and pest and pathogen biocontrol—A review of the latest advances and future prospects. J. Agric. Sci. 2021, 159, 523–534. [Google Scholar] [CrossRef]
- Kapur, B.; Sarıdaş, M.A.; Çeliktopuz, E.; Kafkas, E.; Kargı, S.P. Health and Taste Related Compounds in Strawberries under Various Irrigation Regimes and Bio-Stimulant Application. Food Chem. 2018, 263, 67–73. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Corio-Costet, M.-F.; Stadnik, M.J.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. An Ulva Armoricana Extract Protects Plants against Three Powdery Mildew Pathogens. Eur. J. Plant. Pathol. 2011, 131, 393–401. [Google Scholar] [CrossRef]
- Khaled, H.; Fawy, H.A. Effect of Different Levels of Humic Acids on the Nutrient Content, Plant Growth, and Soil Properties under Conditions of Salinity. Soil Water Res. 2011, 6, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Fagbenro, J.A.; Agboola, A.A. Effect of Different Levels of Humic Acid on the Growth and Nutrient Uptake of Teak Seedlings. J. Plant Nutr. 1993, 16, 1465–1483. [Google Scholar] [CrossRef]
- Khan, R.U.; Khan, M.Z.; Khan, A.; Saba, S.; Hussain, F.; Jan, I.U. Effect of Humic Acid on Growth and Crop Nutrient Status of Wheat on Two Different Soils. J. Plant Nutr. 2018, 41, 453–460. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative Effects of Humic and Fulvic Acids as Biostimulants on Growth, Antioxidant Activity and Nutrient Content of Yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Maach, M.; Boudouasar, K.; Akodad, M.; Skalli, A.; Moumen, A.; Baghour, M. Application of Biostimulants Improves Yield and Fruit Quality in Tomato. Int. J. Veg. Sci. 2021, 27, 288–293. [Google Scholar] [CrossRef]
- Chanthini, K.M.-P.; Senthil-Nathan, S.; Pavithra, G.-S.; Asahel, A.-S.; Malarvizhi, P.; Murugan, P.; Deva-Andrews, A.; Sivanesh, H.; Stanley-Raja, V.; Ramasubramanian, R.; et al. The Macroalgal Biostimulant Improves the Functional Quality of Tomato Fruits Produced from Plants Grown under Salt Stress. Agriculture 2023, 13, 6. [Google Scholar] [CrossRef]
- Graziani, G.; Ritieni, A.; Cirillo, A.; Cice, D.; Di Vaio, C. Effects of Biostimulants on Annurca Fruit Quality and Potential Nutraceutical Compounds at Harvest and during Storage. Plants 2020, 9, 775. [Google Scholar] [CrossRef]
- Zwack, P.J.; Rashotte, A.M. Cytokinin Inhibition of Leaf Senescence. Plant Signal. Behav. 2013, 8, e24737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Jones, M.L.; Banowetz, G.M.; Clark, D.G. Overproduction of Cytokinins in Petunia Flowers Transformed with PSAG12-IPT Delays Corolla Senescence and Decreases Sensitivity to Ethylene. Plant Physiol. 2003, 132, 2174–2183. [Google Scholar] [CrossRef] [Green Version]
- Lara, M.E.B.; Garcia, M.-C.G.; Fatima, T.; Ehneß, R.; Lee, T.K.; Proels, R.; Tanner, W.; Roitsch, T. Extracellular Invertase Is an Essential Component of Cytokinin-Mediated Delay of Senescence. Plant Cell 2004, 16, 1276–1287. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A Novel Type of Seaweed Extract as a Natural Alternative to the Use of Iron Chelates in Strawberry Production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Enhancing Soil Health and Productivity of Lycopersicon esculentum Mill. Using Sargassum Johnstonii Setchell & Gardner as a Soil Conditioner and Fertilizer. J. Appl. Phycol. 2013, 25, 1225–1235. [Google Scholar] [CrossRef]
- Meng, C.; Gu, X.; Liang, H.; Wu, M.; Wu, Q.; Yang, L.; Li, Y.; Shen, P. Optimized Preparation and High-Efficient Application of Seaweed Fertilizer on Peanut. J. Agric. Food Res. 2022, 7, 100275. [Google Scholar] [CrossRef]
- Matthews, S.; Ali, A.; Siddiqui, Y.; Supramaniam, C.V. Plant Bio-Stimulant: Prospective, Safe and Natural Resources. J. Soil Sci. Plant Nutr. 2022, 22, 2570–2586. [Google Scholar] [CrossRef]
- Zeljković, S.; Parađiković, N.; Maksimović, I.; Teklić, T.; Kojić, M.T. Growth and Nutrient Status of French Marigold (Tagetes patula L.) under Biostimulant Application. N. Z. J. Crop Hortic. Sci. 2022, 1–11. [Google Scholar] [CrossRef]
- Basavaraja, P.K.; Yogendra, N.D.; Zodape, S.T.; Prakash, R.; Ghosh, A. Effect of Seaweed Sap as Foliar Spray on Growth and Yield of Hybrid Maize. J. Plant Nutr. 2018, 41, 1851–1861. [Google Scholar] [CrossRef]
- Pal, A.; Dwivedi, S.K.; Maurya, P.K.; Kanwar, P. Effect of Seaweed Saps on Growth, Yield, Nutrient Uptake and Economic Improvement of Maize (Sweet Corn). J. Appl. Nat. Sci. 2015, 7, 970–975. [Google Scholar] [CrossRef] [Green Version]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of Seaweed Extracts from Laminaria and Ascophyllum nodosum Spp. as Biostimulants in Zea mays L. Using a Combination of Chemical, Biochemical and Morphological Approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Antioxidant Activity of a Proanthocyanidin-Rich Extract from Grape Seed in Whey Protein Isolate Stabilized Algae Oil-in-Water Emulsions. J. Agric. Food Chem. 2004, 52, 5272–5276. [Google Scholar] [CrossRef] [PubMed]
- Efecto Del Extracto de Células de Alga Verde Como Aerosol Foliar Sobre El Crecimiento Vegetativo, El Rendimiento y La Calidad de Las Bayas de Vides Superiores. Available online: https://www.researchgate.net/publication/237566381_Effect_of_Green_Alga_Cells_Extract_as_Foliar_Spray_on_Vegetative_Growth_Yield_and_Berries_Quality_of_Superior_Grapevines (accessed on 3 June 2023).
- Arioli, T.; Mattner, S.W.; Hepworth, G.; McClintock, D.; McClinock, R. Effect of Seaweed Extract Application on Wine Grape Yield in Australia. J. Appl. Phycol. 2021, 33, 1883–1891. [Google Scholar] [CrossRef]
- Jayaraman, J.J.J.; Ali, N. Use of Seaweed Extracts for Disease Management of Vegetable Crops. In Sustainable Crop Disease Management Using Natural Products; CABI: Wallingford, UK, 2015; pp. 160–183. [Google Scholar] [CrossRef]
- Murtic, S.; Oljaca, R.; Murtic, M.S.; Vranac, A.; Akagic, A.; Civic, H. Cherry Tomato Productivity as Influenced by Liquid Organic Fertilizer under Different Growth Conditions. J. Cent. Eur. Agric. 2018, 19, 503–516. [Google Scholar] [CrossRef]
- Demir, N.; Dural, B.; Yildirim, K. Effect of Seaweed Suspensions on Seed Germination of Tomato, Pepper and Aubergine. J. Biol. Sci. 2006, 6, 1130–1133. [Google Scholar]
- Yusuf, R.; Kristiansen, P.; Warwick, N. Effect of Two Seaweed Products and Equivalent Mineral Treatments on Lettuce (Lactuca sativa L.) Growth. J. Agron. 2019, 18, 100–106. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Nardelli, A.E.; Chiozzini, V.G.; Braga, E.S.; Chow, F. Integrated Multi-Trophic Farming System between the Green Seaweed Ulva Lactuca, Mussel, and Fish: A Production and Bioremediation Solution. J. Appl. Phycol. 2019, 31, 847–856. [Google Scholar] [CrossRef]
- Righini, H.; Roberti, R.; Baraldi, E. Use of Algae in Strawberry Management. J. Appl. Phycol. 2018, 30, 3551–3564. [Google Scholar] [CrossRef]
- Abbas, M.; Anwar, J.; Zafar-ul-Hye, M.; Iqbal Khan, R.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of Seaweed Extract on Productivity and Quality Attributes of Four Onion Cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Almaroai, Y.A.; Eissa, M.A. Role of Marine Algae Extracts in Water Stress Resistance of Onion Under Semiarid Conditions. J. Soil Sci. Plant Nutr. 2020, 20, 1092–1101. [Google Scholar] [CrossRef]
- Prajapati, A.; Jain, S.; Chongtham, S.; Maheshwari, M.; Patel, C.; Patel, R.; Patel, C.; Singh, N.; Prajapati, A. Evaluation of Seaweed Extract on Growth and Yield of Potato. Environ. Ecol. 2016, 34, 605–608. [Google Scholar]
- Dziugieł, T.; Wadas, W. Possibility of Increasing Early Crop Potato Yield with Foliar Application of Seaweed Extracts and Humic Acids. J. Cent. Eur. Agric. 2020, 21, 300–310. [Google Scholar] [CrossRef]
- Manpuhro, N.; Dawson, J. Influence of Indole Acetic Acid (IAA) and Boron on Growth and Yield of Maize (Zea mays. L). Int. J. Plant Soil Sci. 2023, 35, 33–41. [Google Scholar] [CrossRef]
- Hagaggi, N.S.A.; Mohamed, A.A.A. Enhancement of Zea mays (L.) Growth Performance Using Indole Acetic Acid Producing Endophyte Mixta Theicola Isolated from Solenostemma Argel (Hayne). S. Afr. J. Bot. 2020, 134, 64–71. [Google Scholar] [CrossRef]
- Marag, P.S.; Suman, A. Growth Stage and Tissue Specific Colonization of Endophytic Bacteria Having Plant Growth Promoting Traits in Hybrid and Composite Maize (Zea mays L.). Microbiol. Res. 2018, 214, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-Based Biostimulants Optimize N Use Efficiency and Stimulate Growth of Leafy Vegetables in Greenhouse Intensive Cropping Systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef] [Green Version]
- Kondhare, K.R.; Patil, A.B.; Giri, A.P. Auxin: An Emerging Regulator of Tuber and Storage Root Development. Plant Sci. 2021, 306, 110854. [Google Scholar] [CrossRef]
- Romanov, G.A.; Aksenova, N.P.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kossmann, J.; Willmitzer, L. Effect of Indole-3-Acetic Acid and Kinetin on Tuberisation Parameters of Different Cultivars and Transgenic Lines of Potato in Vitro. Plant Growth Regul. 2000, 32, 245–251. [Google Scholar] [CrossRef]
- Ekin, Z. Integrated Use of Humic Acid and Plant Growth Promoting Rhizobacteria to Ensure Higher Potato Productivity in Sustainable Agriculture. Sustainability 2019, 11, 3417. [Google Scholar] [CrossRef] [Green Version]
- Hye, M.; Haque, M.; Karim, M. Influence of Growth Regulators and Their Time of Application on Yield of Onion. Pak. J. Biol. Sci. 2002, 5, 1021–1023. [Google Scholar] [CrossRef] [Green Version]
- Bista, D.; Sapkota, D.; Paudel, H.; Adhikari, G. Effect of Foliar Application of Growth Regulators on Growth and Yield of Onion (Allium cepa). Int. J. Hortic. Sci. Technol. 2022, 9, 247–254. [Google Scholar] [CrossRef]
- Gupta, S.; Stirk, W.A.; Plačková, L.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Interactive Effects of Plant Growth-Promoting Rhizobacteria and a Seaweed Extract on the Growth and Physiology of Allium cepa L. (Onion). J. Plant Physiol. 2021, 262, 153437. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement Using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus Licheniformis QA1 and Enterobacter Asburiae QF11 Isolated from Chenopodium Quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef]
- Azarakhsh, M.R.; Bagherieh-Najjar, M.B.; Sadeghipour, H.R.; Raeisi, S. Improved Grain Yield by Phytohormones-Driven Suppression of Pod Abscission and Revitalization of Source-Sink Relationships in Soybean. Int. J. Plant Prod. 2022, 16, 467–481. [Google Scholar] [CrossRef]
- Hanaa, H.; Safaa, A. Foliar Application Foliar Application of IAA at Different Growth Stages and Their Influenced on Growth and Productivity of Bread Wheat (Triticum aestivum l.). J. Phys. Conf. Ser. 2019, 1294, 092029. [Google Scholar] [CrossRef]
- Çakmakçı, R.; Erat, M.; Erdoğan, Ü.; Dönmez, M.F. The Influence of Plant Growth–Promoting Rhizobacteria on Growth and Enzyme Activities in Wheat and Spinach Plants. J. Plant Nutr. Soil Sci. 2007, 170, 288–295. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-Inoculation of Glomus Intraradices and Trichoderma Atroviride Acts as a Biostimulant to Promote Growth, Yield and Nutrient Uptake of Vegetable Crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma Virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth through an Auxin-Dependent Mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, S.; Dubey, A. Effects of Biozyme (Ascophyllum nodosum) Biostimulant on Growth and Development of Soybean [Glycine max (L.) Merill]. Commun. Soil Sci. Plant Anal. 2015, 46, 845–858. [Google Scholar] [CrossRef]
- Marathe, R.; Phatake, Y.; Shaikh, A.; Shinde, B.; Gajbhiye, M. Effect of IAA Produced by Pseudomonas Aeruginosa 6a (Bc4) on Seed Germination and Plant Growth of Glycin Max. J. Exp. Biol. Agric. Sci. 2017, 5, 351–358. [Google Scholar] [CrossRef]
- Susilowati, D.N.; Riyanti, E.I.; Setyowati, M.; Mulya, K. Indole-3-Acetic Acid Producing Bacteria and Its Application on the Growth of Rice. AIP Conf. Proc. 2018, 2002, 020016. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing Auxin Accumulation in Maize Root Tips Improves Root Growth and Dwarfs Plant Height. Plant Biotechnol. J. 2018, 16, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Husen, A.; Iqbal, M.; Aref, I.M. Plant Growth and Foliar Characteristics of Faba Bean (Vicia faba L.) as Affected by Indole-Acetic Acid under Water-Sufficient and Water-Deficient Conditions. J. Environ. Biol. 2017, 38, 179–186. [Google Scholar] [CrossRef]
- Hamidon, A.; Shah, R.M.; Razali, R.M.; Lob, S. Effect of different types and concentration of rooting hormones on momordica cochinensis (gac fruit) root vine cuttings. Malays. Appl. Biol. 2020, 49, 127–132. [Google Scholar] [CrossRef]
- Sabir, A. Improvement of Grafting Efficiency in Hard Grafting Grape Berlandieri Hybrid Rootstocks by Plant Growth-Promoting Rhizobacteria (PGPR). Sci. Hortic. 2013, 164, 24–29. [Google Scholar] [CrossRef]
- Shahzad, K.; Siddiqi, E.H.; Ahmad, S.; Zeb, U.; Muhammad, I.; Khan, H.; Zhao, G.-F.; Li, Z.-H. Exogenous Application of Indole-3-Acetic Acid to Ameliorate Salt Induced Harmful Effects on Four Eggplants (Solanum melongena L.) Varieties. Sci. Hortic. 2022, 292, 110662. [Google Scholar] [CrossRef]
- Lur, H.-S.; Setter, T.L. Endorsperm Development of Maize Defective Kernel (Dek) Mutants. Auxin and Cytokinin Levels. Ann. Bot. 1993, 72, 1–6. [Google Scholar] [CrossRef]
- Rady, M.M.; Talaat, N.B.; Abdelhamid, M.T.; Shawky, B.T.; Desoky, E.-S.M. Maize (Zea mays L.) Grains Extract Mitigates the Deleterious Effects of Salt Stress on Common Bean (Phaseolus vulgaris L.) Growth and Physiology. J. Hortic. Sci. Biotechnol. 2019, 94, 777–789. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, D.; Zhang, G.; Gao, S.; Liu, L.; Xu, F.; Che, R.; Wang, Y.; Tong, H.; Chu, C. Big Grain3, Encoding a Purine Permease, Regulates Grain Size via Modulating Cytokinin Transport in Rice. J. Integr. Plant Biol. 2019, 61, 581–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, W.; Xiao, Y.; Niu, M.; Meng, W.; Li, L.; Zhang, X.; Liu, D.; Zhang, G.; Qian, Y.; Sun, Z.; et al. ARGONAUTE2 Enhances Grain Length and Salt Tolerance by Activating BIG GRAIN3 to Modulate Cytokinin Distribution in Rice. Plant Cell 2020, 32, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, Y.; Shi, Y.; Cui, Z.; Luo, Y.; Zheng, M.; Chen, J.; Li, Y.; Yin, Y.; Wang, Z. Exogenous Cytokinins Increase Grain Yield of Winter Wheat Cultivars by Improving Stay-Green Characteristics under Heat Stress. PLoS ONE 2016, 11, e0155437. [Google Scholar] [CrossRef] [Green Version]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M.F.; Erinle, K.O.; Iqbal, R.; Ahmad, S. Effect of Rhizobacteria and Cytokinins Application on Wheat Growth and Yield under Normal vs Drought Conditions. Commun. Soil Sci. Plant Anal. 2019, 50, 2521–2533. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, Y.; Liu, W. High Nitrogen Application Rate and Planting Density Reduce Wheat Grain Yield by Reducing Filling Rate of Inferior Grain in Middle Spikelets. Crop J. 2021, 9, 412–426. [Google Scholar] [CrossRef]
- Nagel, L.; Brewster, R.; Riedell, W.E.; Reese, R.N. Cytokinin Regulation of Flower and Pod Set in Soybeans (Glycine max (L.) Merr.). Ann. Bot. 2001, 88, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Kron, A.P.; Souza, G.M.; Ribeiro, R.V. Water Deficiency at Different Developmental Stages of Glycine max Can Improve Drought Tolerance. Bragantia 2008, 67, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Mady, M.A. Effect of foliar application with salicylic acid and vitamin e on growth and productivity of tomato (Lycopersicon esculentum, Mill.) Plant. J. Plant Prod. 2009, 34, 6715–6726. [Google Scholar] [CrossRef]
- Caldiz, D.O. Seed Potato (Solanum tuberosum L.) Yield and Tuber Number Increase after Foliar Applications of Cytokinins and Gibberellic Acid under Field and Glasshouse Conditions. Plant Growth Regul 1996, 20, 185–188. [Google Scholar] [CrossRef]
- Pavlista, A.D. Growth Regulators Increased Yield of Atlantic Potato. Am. J. Potato Res 2011, 88, 479–484. [Google Scholar] [CrossRef]
- Carvajal-Millán, E.; Carvallo, T.; Orozco, J.A.; Martínez, M.A.; Tapia, I.; Guerrero, V.M.; Rascón-Chu, A.; Llamas, J.; Gardea, A.A. Polyphenol Oxidase Activity, Color Changes, and Dehydration in Table Grape Rachis during Development and Storage As Affected by N-(2-Chloro-4-Pyridyl)-N-Phenylurea. J. Agric. Food Chem. 2001, 49, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Peppi, M.C.; Fidelibus, M.W. Effects of Forchlorfenuron and Abscisic Acid on the Quality of ‘Flame Seedless’ Grapes. HortScience 2008, 43, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Guan, S.C.; Wen, C.; Li, P.; Gao, Z.; Chen, X. Auxin and Cytokinin Coordinate the Dormancy and Outgrowth of Axillary Bud in Strawberry Runner. BMC Plant Biol. 2019, 19, 528. [Google Scholar] [CrossRef]
- Dale, A.; Elfving, D.C.; Chandler, C.K. Benzyladenine and Gibberellic Acid Increase Runner Production in Dayneutral Strawberries. HortScience 1996, 31, 1190–1194. [Google Scholar] [CrossRef] [Green Version]
- Costa, G.; Corelli-Grappadelli, L.; Bucchi, F. Studies on apple fruit abscission and growth as affected by cytokinins. Acta Hortic. 2001, 243–252. [Google Scholar] [CrossRef]
- Kumari, S.; Bakshi, P.; Sharma, A.; Wali, V.; Jasrotia, A.; Kour, S. Use of Plant Growth Regulators for Improving Fruit Production in Sub Tropical Crops. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, C.; Martiz, J.; Saa, S.; Cautín, R. Increase in Final Fruit Size of Tangor (Citrus reticulata × C. sinensis) Cv W. Murcott by Application of Benzyladenine to Flowers. Sci. Hortic. 2017, 223, 38–43. [Google Scholar] [CrossRef]
- Yousif, K.H. Application Method of Potassium Humate on Growth and Yield of Green Onion (Allium cepa L.). Sci. J. Univ. Zakho 2014, 2, 323–328. [Google Scholar] [CrossRef]
- Forotaghe, Z.A.; Souri, M.K.; Jahromi, M.G.; Torkashvand, A.M. Influence of Humic Acid Application on Onion Growth Characteristics under Water Deficit Conditions. J. Plant Nutr. 2022, 45, 1030–1040. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Ashraf, M.; Sonmez, O. Exogenous Application of Humic Acid Mitigates Salinity Stress in Maize (Zea mays L.) Plants by Improving Some Key Physico-Biochemical Attributes. Cereal Res. Commun. 2018, 46, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Shafi, M.I.; Adnan, M.; Fahad, S.; Wahid, F.; Khan, A.; Yue, Z.; Danish, S.; Zafar-ul-Hye, M.; Brtnicky, M.; Datta, R. Application of Single Superphosphate with Humic Acid Improves the Growth, Yield and Phosphorus Uptake of Wheat (Triticum aestivum L.) in Calcareous Soil. Agronomy 2020, 10, 1224. [Google Scholar] [CrossRef]
- Lamlom, S.F.; Irshad, A.; Mosa, W.F.A. The Biological and Biochemical Composition of Wheat (Triticum aestivum) as Affected by the Bio and Organic Fertilizers. BMC Plant Biol. 2023, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Mindari, W.; Sasongko, P.E.; Kusuma, Z.; Syekhfani; Aini, N. Efficiency of Various Sources and Doses of Humic Acid on Physical and Chemical Properties of Saline Soil and Growth and Yield of Rice. AIP Conf. Proc. 2018, 2019, 030001. [Google Scholar] [CrossRef]
- Khedr, R.A.; Sorour, S.G.R.; Aboukhadrah, S.H.; El Shafey, N.M.; Elsalam, H.E.A.; El-Sharnouby, M.E.; El-Tahan, A.M. Alleviation of Salinity Stress Effects on Agro-Physiological Traits of Wheat by Auxin, Glycine Betaine, and Soil Additives. Saudi J. Biol. Sci. 2022, 29, 534–540. [Google Scholar] [CrossRef]
- Suh, H.Y.; Yoo, K.S.; Suh, S.G. Effect of Foliar Application of Fulvic Acid on Plant Growth and Fruit Quality of Tomato (Lycopersicon esculentum L.). Hortic. Environ. Biotechnol. 2014, 55, 455–461. [Google Scholar] [CrossRef]
- Yildirim, E. Foliar and Soil Fertilization of Humic Acid Affect Productivity and Quality of Tomato. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2007, 57, 182–186. [Google Scholar] [CrossRef]
- Hemida, K.A.; Eloufey, A.Z.A.; El-Yazal, M.A.S.; Rady, M.M. Integrated Effect of Potassium Humate and α-Tocopherol Applications on Soil Characteristics and Performance of Phaseolus vulgaris Plants Grown on a Saline Soil. Arch. Agron. Soil Sci. 2017, 63, 1556–1571. [Google Scholar] [CrossRef]
- Kandil, A.A.; Sharief, A.E.; Fathalla, F.H. Onion yield as affected by foliar application with amino and humic acids under nitrogen fertilizer levels. Crop Prod. 2013, 2, 62–72. [Google Scholar]
- Sruthi, B.E.S. Influence of organic manures on yield, quality and economics of aggregatum onion (Allium cepa. L. var. aggregatum). J. Pharmacogn. Phytochem. 2019, 8, 1768–1770. [Google Scholar]
- Omar, M.; Ramadan, A. Response of Carrot (Daucus carota L.) to Foliar Application of Potassium Fertilizers and Some Soil Amendments under Clay Soil Conditions. J. Soil Sci. Agric. Eng. 2018, 9, 197–202. [Google Scholar] [CrossRef]
- Raheem, S.M.; Al-Jaf, H.I.; Tofiq, G.K. Influence of Foliar and Soil Application of Humic Acid on Growth and Yield of Lettuce. Euphrates J. Agric. Sci. 2018, 10, 199–204. [Google Scholar]
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Chapter 23—Solid-State Fermentation Strategy for Microbial Metabolites Production: An Overview. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–354. ISBN 978-0-444-63504-4. [Google Scholar]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae Biostimulants: A Critical Look at Microalgal Biostimulants for Sustainable Agricultural Practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-Treated Seedlings under Sustainable Agriculture: A Global Perspective Facing Climate Change. Agronomy 2021, 11, 14. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
| Natural Product | Type of NB | Molecules Present | Action Mode | Biostimulant Effect | SSF-Relevant Origin | Refs. |
|---|---|---|---|---|---|---|
| Hormone-Containing Products (HCP) | Auxins | 3-indoleacetic Acid (IAA) | Promotes cell elongation | Stimulates cell elongation and rooting | Produced by SSF | [22,23] |
| Indole Propionic Acid (AIP) | Promotes vegetative growth and cell division | Stimulates growth, flowering, and rooting in plants | Not produced by SSF | [24,25] | ||
| Cytokinins | Zeatin | Stimulates cell division and vegetative growth | Promotes growth and development of plants | Not produced by SSF | [26,27,28] | |
| Kinetin | Stimulates cell division and vegetative growth | Improves the quality of the crops, increasing the size and weight of the fruits | Produced by SSF and vermicompost | [29,30,31] | ||
| Abscisic Acid (ABA) | ABA | Regulates stress responses and plant development | Improves stress tolerance and fruit ripening | Produced by SSF | [32,33] | |
| Gibberellins | Gibberellin A3 (GA3) | Stimulates growth and vigor in plants | Inducts germination and flowering | Produced by SSF | [34,35,36] | |
| Gibberellin A4 (GA4) | Promotes plant growth and development | Stimulates germination, development of lateral shoots, and flowering | Produced by SSF | [37,38] | ||
| Seaweed Extract (AM) | Alginic Acids | Improves nutrient absorption and stimulates enzyme activity | Increases growth and resistance to abiotic stress | Produced by SSF | [39,40,41] | |
| AM | Fucoidan | Improves the defense mechanisms of plants | Increases resistance to abiotic stress | Produced by SSF | [42,43,44,45] | |
| Oligosaccharides | Stimulates physiological responses in plants | Improves immune response and growth | Produced by SSF | [46,47,48,49] | ||
| Humic Substances | Humic and Fulvic Acids (AHF) | Humic Acids | Improves soil structure and nutrient availability | Stimulates root growth and nutrient absorption | Produced by SSF | [50,51,52,53] |
| Humic Acids | Stimulates plant growth and development | Improves nutrient uptake and stress resistance. | Produced by SSF | [54,55] | ||
| Amino-Acid-Containing Products (AACP) | Amino Acids | L-proline | Regulates plant stress and development | Enhances stress tolerance and resistance | Produced by SSF | [56,57,58] |
| Peptides | Low Molecular Weight Peptides | Stimulates plant growth and development | Improves plant nutrition and growth | Produced by SSF | [59,60,61] | |
| Other NBs | Siderophores | Siderophores | Binds to Fe and is solubilized | Improves absorption and mobilization of Fe | Produced by SSF | [62,63,64] |
| Chitosan Fungal | Chitosan Fungal | Promotes plant growth, cell division, increases enzyme activity, and improves nutrient transport | Presents biostimulant activity in seed germination | Produced by SSF | [65,66] |
| Substrate | Characteristics and Advantages of Substrate | Microorganism Selection | Production Mode | Bioreactor Type | Refs. |
|---|---|---|---|---|---|
| Crop Residues | Abundant local availability, nutrient source, and microorganism support | Bacteria, Fungi | Batch, Continuous, Fed-Batch | Fixed-Bed, Packed-Bed | [89,90,91] |
| Agroindustrial Waste | Waste valorization and reduced environmental impact | Filamentous Fungi | Batch, Continuous | Fluidized-Bed, Packed-Bed | [47,92] |
| Food Residues | Rich in nutrients and organic matter, avoids food waste | Bacteria, Filamentous Fungi | Batch, Fed-Batch | Fixed-Bed, Packed-Bed | [37,93] |
| Plant Residues | High content of bioactive compounds and phytohormones | Bacteria, Filamentous Fungi | Batch, Continuous | Fluidized-Bed, Packed-Bed | [94,95] |
| Algal Biomass | Rich in bioactive compounds and auxins | Microalgae | Batch, Fed-Batch | Bubble-Column | [96,97] |
| Wood Residues | Sustainable source with lignocellulosic content | Filamentous Fungi | Fed-Batch, Continuous | Fluidized-Bed, Packed-Bed | [98,99] |
| Residual Sludge | Reduces waste volume and provides rich source of nutrients | Bacteria, Filamentous Fungi | Batch, Continuous | Plug- Flow, Packed-Bed | [100,101] |
| Fishery Waste | Utilization of waste from the fishing industry | Filamentous Fungi | Batch, Continuous | Packed-Bed | [102,103] |
| Brewery Waste | Valorization of waste from brewing processes | Filamentous Fungi | Continuous | Packed-Bed | [104,105] |
| Citrus Waste | Abundant source of bioactive compounds and antioxidants | Filamentous Fungi | Batch, Fed-Batch | Fixed-Bed, Packed-Bed | [33,106] |
| Coffee Residues | Rich in bioactive compounds and promotes soil health | Filamentous Fungi | Batch, Continuous | Packed-Bed | [107] |
| Rice Husk | Rich in organic matter and bioactive substances | Filamentous Fungi | Fed-Batch, Continuous | Packed-Bed | [108] |
| NB | Substrate | Microorganism | Pretreatment | Optimal SSF Conditions | Effect of NBs on Crop | Refs. | |||
|---|---|---|---|---|---|---|---|---|---|
| Trituration | pH | Sterilization | Moisture % | Temperature °C | |||||
| IAA | Pruning Waste + Grass | Trichoderma harzianum | 1 cm | 6.8 | 2 times | 74 | 25 | [15] | |
| IAA | Yuca Bagasse Soy Bran Wheat Bran Sorghum Dried Distiller’s Grains Corn Dried Distiller’s Grains | Aspergillus flavipes Aspergillus ustus Bacillus subtilis Bacillus megaterium Bacillus amyloliquefaciens Trichoderma atroviride Trichoderma koningii Trichoderma harzianum | 0.5, 1.0 y > 1.0 mm | 50 | Room Temperature | Clon IPB2 Eucalyptus grandis and Eucalyptus urophylla Increasing Rooting | [14,18] | ||
| Kinetin | Cow Dung + Leaf Litter | Selenomonas ruminantium | 2–5 mm | 6.9 | 70–75 | 25 ± 3 | [29] | ||
| ABA | Millet Rice | Botrytis cinerea | Millet and Rice | 1 time | 26.5–25.5 | [32] | |||
| GA3 | Rice Bran | Gibberella fujikuroi | 50 °C | 65.95% | 28 ± 2 | [109] | |||
| GA3 | Corn Cob Residues | Aspergillus niger | 5.1 | 24% | [110] | ||||
| GA3 | Citric Pulp | Fusarium moniliforme LPB03 + Gibberella fujikuroi | 5.5–5.8 | 75 | 29 | [91] | |||
| Alginic Acids | Apple Peels | Azotobacter vinelandii, NRRL-14641 | 0.1 mm | 7 | 60 °C | 70 | 37.5 | [39] | |
| Alginic Acids | Sargassum Macroalgae | Cunninghamella echinulate Aspergillus niger Penicillium oxalicum | 7–8.5 | 1 time 121 °C | 65–75 | 28–30 | [40] | ||
| Fucoida | Seaweed Fucus Vesiculosus | Aspergillus niger Mucor sp | 80 | 30 | [42] | ||||
| Oligosaccharides | Soybean Meal | - | Room Temperature | Effect on Germination | [111] | ||||
| Chitin Oligosaccharides | Powder of Molting of Mealworms | Talaromyces allahabadensis Hi-4 Talaromyces funiculosus | 6 | 40 | [112] | ||||
| Humic Acid | Oil Palm Empty Fruit Bunch | Trichoderma reesei | 6 | 64–72 | 30 | [50,113] | |||
| Fulvic Acid | Sugarcane Bagasse | Trichoderma Sp. | 70 | 20 | [114] | ||||
| L-proline | Wheat Straw Ice Straw Wheat Bran Corn Cob Corn Stover | Fomitopsis sp. | Small Pieces | 5.5 | 25–30 | [56] | |||
| Low Molecular Weight Peptides | Chickpeas | Bacillus subtilis | [60] | ||||||
| Siderophores | Soybean Protein Meal | Lactobacillus plantarum | 37 | [115] | |||||
| Chitosan Fungal | Sweet Potato | Gongronella butleri USDB 0201 | 28 | [66] | |||||
| Crop | NB Type | Effect | Scale | Refs. |
|---|---|---|---|---|
| Arabidopsis thaliana | Low Molecular Weight Peptides | Increase in plant biomass | Laboratory | [120] |
| Sesame | GA3 | Improvement of plant architecture | Laboratory | [121] |
| Rice | GA3 | Improvement of plant architecture | Laboratory | [122] |
| Tomato Pepper Seed Arabidopsis Orchid | IAA | Promotion of seed germination and seedling emergence | Greenhouse Laboratory | [17,123,124] |
| Crop | NB Type | Effect | Scale | Refs. |
|---|---|---|---|---|
| Orange Tobacco Corn | ABA | Abiotic stress tolerance | Laboratory | [127,128,129] |
| Strawberry Bean Vine Cucumber | Seaweed Polysaccharides | Resistance to diseases and pests | Field | [130,131,132,133] |
| Crop | NB Type | Effect | Scale | Refs. |
|---|---|---|---|---|
| Gerbera Tectona Grandis Peas Yarrow | Humic Acid | Increased nutrient concentration | Greenhouse | [134,135,136,137] |
| Tomato Apple | Amino Acids | Improved organoleptic quality | Greenhouse | [138,139,140] |
| Soy Petunia Flowers Lettuce | Cytokinins | Delayed tissue senescence | Greenhouse | [141,142,143] |
| Crop | NB Type | Effect | Scale | Refs. |
|---|---|---|---|---|
| Tomato Strawberries Peanut | Alginic Acids | Improvement of nutrient availability in the soil | Greenhouse | [144,145,146] |
| French Marigold | Oligosaccharides | Reduced nutrient losses | Greenhouse | [147,148] |
| Crop | NB Type | Effect of Productivity on Crops | Scale | Refs. |
|---|---|---|---|---|
| Corn | Seaweed Extract | Increases grain yield, crop residue, and improves nutritional quality | Field | [149,150,151] |
| Grapes | Seaweed Extract | Increases grape production, improves stress resistance, and increases polyphenol content | Greenhouse | [152,153,154] |
| Tomato | Seaweed Extract | Increases fruit yield and quality | Greenhouse | [155,156,157] |
| Lettuce | Seaweed Extract | Higher yield increase and increases shoot growth | Greenhouse | [158,159,160] |
| Strawberries | Seaweed Extract | Improves fruit quality and flavor, higher yield | Greenhouse | [132,161] |
| Onion | Seaweed Extract | Increases bulb diameter and weight | Field | [162,163] |
| Potato | Seaweed Extract | Increases tuber yield and quality | Field | [164,165] |
| Corn | IAA | Stimulates vegetative growth and increases grain production | Greenhouse | [166,167,168] |
| Lettuce | IAA | Increases biomass | Greenhouse | [169] |
| Potato | IAA | Promotes tuber growth and improves yield | Greenhouse | [170,171,172] |
| Onion | IAA | Increases bulb size and enhances production | Greenhouse Laboratory | [173,174,175] |
| Quinoa | IAA | Boosts grain yield and improves quality | Field | [176,177] |
| Wheat | IAA | Stimulates plant growth and increases yield | Field | [178,179] |
| Tomato | IAA | Improves rooting, increases fruit production, and enhances antioxidant content | Greenhouse | [180,181] |
| Soybean | IAA | Improves root development and increases production | Greenhouse | [182,183] |
| Rice | IAA | Promotes rooting and improves yield | Field | [184,185] |
| Broad Beans | IAA | Stimulates vegetative growth and increases production | Greenhouse | [183,186] |
| Grapes | IAA | Enhances root formation and increases yield | Greenhouse | [187,188,189] |
| Corn | Cytokinins | Stimulates cell division and increases yield | Greenhouse | [190,191] |
| Rice | Cytokinins | Promotes grain growth and improves yield | Greenhouse | [192,193] |
| Wheat | Cytokinins | Increases the number of grains per spike and improves production | Field | [194,195,196] |
| Soybean | Cytokinins | Improves vegetative growth and increases production | Greenhouse | [197,198] |
| Tomato | Cytokinins | Stimulates flower formation and increases yield | Greenhouse | [28,199] |
| Potato | Cytokinins | Promotes tuber development and improves yield | Field | [200,201] |
| Grapes | Cytokinins | Enhances cluster size and quality | Greenhouse | [202,203] |
| Strawberry | Cytokinins | Increases stolon formation and improves production | Greenhouse | [204,205] |
| Strawberry | Cytokinins | Stimulates bud break and improves yield | Greenhouse | [206] |
| Citrus | Cytokinins | Increases fruit size and improves production | Greenhouse | [207,208] |
| Onion | Humic Acids | Enhances bulb yield, improves quality and disease resistance | Greenhouse | [209,210] |
| Corn | Humic Acids | Improves nutrient absorption and increases yield | Greenhouse | [28,211] |
| Wheat | Humic Acids | Increases grain size and weight | Greenhouse | [212,213] |
| Rice | Humic Acids | Boosts the number of spikes and improves production | Greenhouse | [214,215] |
| Tomato | Humic Acids | Enhances fruit quality and increases yield | Greenhouse | [216,217] |
| Beans | Humic Acids | Improves vegetative growth and increases production | Field | [218] |
| Onion | Humic Acids | Increases bulb size and quality | Greenhouse | [219,220] |
| Carrot | Humic Acids | Promotes root development and improves production | Greenhouse | [221] |
| Lettuce | Humic Acids | Stimulates leaf growth and increases yield | Greenhouse | [222] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solano Porras, R.C.; Artola, A.; Barrena, R.; Ghoreishi, G.; Ballardo Matos, C.; Sánchez, A. Breaking New Ground: Exploring the Promising Role of Solid-State Fermentation in Harnessing Natural Biostimulants for Sustainable Agriculture. Processes 2023, 11, 2300. https://doi.org/10.3390/pr11082300
Solano Porras RC, Artola A, Barrena R, Ghoreishi G, Ballardo Matos C, Sánchez A. Breaking New Ground: Exploring the Promising Role of Solid-State Fermentation in Harnessing Natural Biostimulants for Sustainable Agriculture. Processes. 2023; 11(8):2300. https://doi.org/10.3390/pr11082300
Chicago/Turabian StyleSolano Porras, Roberto Carlos, Adriana Artola, Raquel Barrena, Golafarin Ghoreishi, Cindy Ballardo Matos, and Antoni Sánchez. 2023. "Breaking New Ground: Exploring the Promising Role of Solid-State Fermentation in Harnessing Natural Biostimulants for Sustainable Agriculture" Processes 11, no. 8: 2300. https://doi.org/10.3390/pr11082300