The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Analysis of Grape Pomace’s Dry Extract
2.3.1. Quantification of Phenolics and Flavonoids
2.3.2. In Vitro Antioxidant Activity
2.3.3. HPLC and ESI–MS
2.4. Formulations
2.4.1. In Vitro Antioxidant Activity of the Formulations
2.4.2. Photoprotection Efficacy and Photostability
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Grape Pomace’s Dry Extract
3.1.1. Quantification of Phenolics and Flavonoids
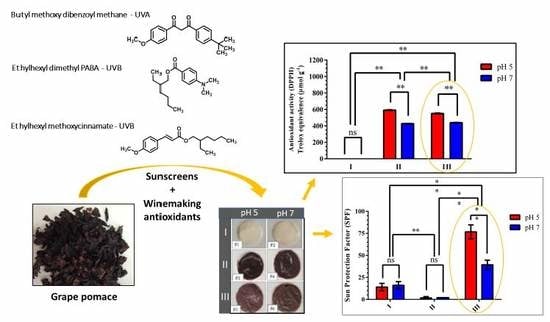
3.1.2. In Vitro Antioxidant Activity
3.1.3. HPLC and ESI-MS/MS
3.2. Formulations
3.2.1. In Vitro Antioxidant Activity of the Formulations
3.2.2. In Vitro Photoprotection Efficacy and Photostability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cavalcante, C.M. An institutional approach to the history of wine in brazil. In Proceedings of the BIO Web of Conferences, Bento Gonçalves, Brazil, 24–28 October 2016; Volume 7, p. 03025. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Ky, I.; Teissedre, P.L. Characterisation of mediterranean grape pomace seed and skin extracts: Polyphenolic content and antioxidant activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef] [PubMed]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical solvent extraction of anthocyanins from dried red grape pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, K.; Hosseinian, F.; Rod, M.R. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Texeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Cosmeceuticals: Undefined, unclassified, and unregulated. Clin. Dermatol. 2009, 27, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Close, D.C.; McArthur, C. Rethinking the role of many plant phenolics–protection from photodamage not herbivores? Oikos 2002, 99, 166–172. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Peres, D.D.; Graziola, F.; Chacra, N.A.B.; Araújo, G.L.B.; Florido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.; Rodrigues, L.M.; et al. Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef]
- da Silva, R.A.D. Farmacopeia dos Estados Unidos do Brasil, 2nd ed.; Siqueira: São Paulo, Brazil, 1959; p. 448. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Pozzi, A.C.S. Desenvolvimento de Métodos Espectrofotométricos de Análise de Flavonóides do Maracujá (Passiflora alata e Passiflota edulis). Master’s Thesis, Universidade de São Paulo, São Paulo, Brasil, 2007. [Google Scholar] [CrossRef]
- Noriega, P.; Mafud, D.F.; Souza, B.; Soares-Scott, M.; Rivelli, D.P.; Barros, S.B.M.; Bacchi, E.M. Applying design of experiments (DOE) to flavonoid extraction from Passiflora alata and P. edulis. Rev. Bras. Farmacogn. 2012, 22, 1119–1129. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rubilar, M.; Pinelo, M.; Shene, C.; Sineiro, J.; Nuñez, M.J. Separation and HPLC-MS identification of phenolic antioxidants from agricultural residues: Almond hulls and grape pomace. J. Agric. Food Chem. 2007, 55, 10101–10109. [Google Scholar] [CrossRef] [PubMed]
- Japan Ministry of Health and Welfare. Pharmaceutical and Medical Safety Bureau Notification, No. 990/2000; Partial Amendments to the Enforcement Regulations of the Pharmaceutical Affairs Law Pertaining to the Relaxation of Regulations for Cosmetics; Japan Ministry of Health and Welfare: Tokyo, Japan, 2000.
- Cosmetics Europe. Guidelines. International sun Protection Factor (SPF) Test Method; COLIPA, CTFA SA, JCIA, CTFA: Auderghem, Belgium, 2006; p. 46. [Google Scholar]
- Chisvert, A.; Salvador, A. UV filters in sunscreens and other cosmetics. Regulatory aspects and analytical methods. In Analysis of Cosmetic Products; Chisvert, A., Salvador, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 83–120. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Peres, D.D.; Hubner, A.; Oliveira, C.A.; Almeida, T.S.; Kaneko, T.M.; Consiglieri, V.O.; Pinto, C.A.S.O.; Velasco, M.V.R.; Baby, A.R. Hydrolyzed collagen interferes with in vitro photoprotective effectiveness of sunscreens. Braz. J. Pharm. Sci. 2017, 53, 1–7. [Google Scholar] [CrossRef]
- Baby, A.R.; Haroutiounian-Filho, C.A.; Sarruf, F.D.; Tavante-Júnior, C.R.; Pinto, C.A.S.O.; Zague, V.; Arêas, E.P.G.; Kaneko, T.M.; Velasco, M.V.R. Stability and in vitro penetration study of rutin incorporated in a cosmetic emulsion through an alternative model biomembrane. Rev. Bras. Ciênc. Farm. 2008, 44, 233–248. [Google Scholar] [CrossRef]
- Velasco, M.V.; Balogh, T.S.; Pedriali, C.A.; Sarruf, F.D.; Pinto, C.A.S.O.; Kaneko, T.M.; Rolim, A.R. Associação da rutina com p-metoxicinamato de octila e benzofenona-3: Avaliação in vitro da eficácia fotoprotetora por espectrofotometria de refletância. Lat. Am. J. Pharm. 2008, 27, 23–27. [Google Scholar]
- Springsteen, A.; Yurek, R.; Frazier, M.; Carr, K.F. In vitro measurement of sun protection factor of sunscreens by diffuse transmittance. Anal. Chim. Acta 1999, 380, 155–164. [Google Scholar] [CrossRef]
- Diffey, B.L.; Tanner, P.R.; Matts, P.J.; Nash, J.F. In vitro assessment of the broad-spectrum ultraviolet protection of sunscreen products. J. Am. Acad. Dermatol. 2000, 43, 1024–1035. [Google Scholar] [CrossRef] [Green Version]
- Cosmetics Europe. In Vitro Method for the Determination of the UVA Protection Factor and “Critical Wavelength” Values of Sunscreen Products; COLIPA: Auderghem, Belgium, 2011; pp. 1–29. [Google Scholar]
- Food and Drug Administration, Department of Health and Human Services. 21 CFR Parts 201 and 310. Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed. Regist. 2011, 76, 35620–35665. [Google Scholar]
- Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Silva, G.L.; Rodrigues, E.; Kuskoski, E.M.; Fett, R. Solvent Influence on total polyphenol content, anthocyanins, and antioxidant activity of grape (Vitis vinifera) bagasse extracts from Tannat and Ancelota-different varieties of Vitis vinifera varieties. Ciênc. Tecnol. Aliment. 2008, 28, 238–244. [Google Scholar] [CrossRef]
- Rimac-Brnčić, S.; Sabolović, M.B.; Žlabur, J.S.; Jelovečki, M. Colour stability and antioxidant activity of some berry extracts. Croat. J. Food Technol. Biotechnol. Nutr. 2015, 10, 115–119. [Google Scholar]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Choquenet, B.; Couteau, C.; Paparis, E.; Coiffard, L.J.M. Quercetin and Rutin as Potential Sunscreen Agents: Determination of Efficacy by an in Vitro Method. J. Nat. Prod. 2008, 71, 1117–1118. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Jiphimai, P.; Prutanopajai, P.; Chanathong, B.; Sapwarobol, S.; Ariyapitipan, T. Evaluation of α-glucosidase, α-amylase and protein glycation inhibitory activities of edible plants. Int. J. Food Sci. Nutr. 2010, 61, 295–305. [Google Scholar] [CrossRef]
- David, A.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Mierziak, J.; Kostyn, K.; Kulma, A.; Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Abe, L.T.; Mota, R.V.; Lajolo, F.M.; Genovese, M.I. Compostos fenólicos e capacidade antioxidante de cultivares de uvas Vitis labrusca L. e Vitis vinifera L. Ciênc. Tecnol. Aliment. 2007, 27, 394–400. [Google Scholar] [CrossRef]
- Salazar, S.A.; Juárez, L.A.M.; Valdez, H.S.; López, F.M.; Meza, N.G. Influence of the solvent system on the composition of phenolic substances and antioxidant capacity of extracts of grape (Vitis vinifera L.) marc. Aust. J. Grape Wine Res. 2014, 20, 208–213. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- Flamini, R. Recent applications of mass spectrometry in the study of grape and wine polyphenols. ISRN Spectrosc. 2013, 2013, 1–46. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Zhang, Z.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Liquid chromatographic/electrospray ionization mass spectrometric studies of proanthocyanidins in foods. J. Mass Spectrom. 2003, 38, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Deinzer, M.L. Tandem mass spectrometry for sequencing proanthocyanidins. Anal. Chem. 2007, 79, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Capistrano, R.; Dhooghe, L.; Foubert, K.; Lemière, F.; Maregesi, S.; Baldé, A.; Apers, S.; Pieters, L. Herbal medicines and infectious diseases: Characterization by LC-SPE-NMR of some medicinal plant extracts used against malaria. Planta Med. 2011, 77, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.G.; Duan, T.T.; He, B.; Tang, D.; Jia, X.B.; Wang, R.S.; Zhu, J.X.; Xu, Y.H.; Zhu, Q.; Feng, L. Macrophage biospecific extraction and HPLC–ESI-MS n analysis for screening immunological active components in Smilacis Glabrae Rhizoma. J. Pharm. Biomed. Anal. 2013, 77, 44–48. [Google Scholar] [CrossRef]
- Traldi, P.; Flamini, R. Mass Spectrometry in Grape and Wine Chemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 163–221. [Google Scholar]
- Soto, M.L.; Parada, M.; Falqué, E.; Domínguez, H. Personal-Care Products Formulated with Natural Antioxidant Extracts. Cosmetics 2018, 5, 13. [Google Scholar] [CrossRef]
- Bleasel, M.D.; Aldous, S. In vitro evaluation of sun protection factors of sunscreen agents using a novel UV spectrophotometric technique. Int. J. Cosmet. Sci. 2008, 30, 259–270. [Google Scholar] [CrossRef]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J. Am. Acad. Dermatol. 2017, 76, S100–S109. [Google Scholar] [CrossRef] [Green Version]
- Lourith, N.; Kanlayavattanakul, M.; Chingunpitak, J. Development of sunscreen products containing passion fruit seed extract. Braz. J. Pharm. Sci. 2017, 53, 1–8. Available online: http://www.scielo.br/pdf/bjps/v53n1/2175-9790-bjps-53-01-e16116.pdf (accessed on 21 October 2019). [CrossRef]
- Fujikake, K.; Tago, S.; Plasson, R.; Nakazawa, R.; Okano, K.; Maezawa, D.; Mukawa, T.; Kuroda, A.; Asakura, K. Problems of in vitro SPF Measurements Brought about by Viscous Fingering Generated during Sunscreen Applications. Skin Pharmacol. Physiol. 2014, 27, 254–262. [Google Scholar] [CrossRef]
- Babaloo, F.; Jamei, R. Anthocyanin pigment stability of Cornus mas–Macrocarpa under treatment with pH and some organic acids. Food Sci. Nutr. 2018, 6, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Agrapidis-Paloympis, L.E.; Nash, R.A.; Shaath, N.A. The effect of solvents on the ultraviolet absorbance of sunscreens. J. Soc. Cosmet. Chem. 1987, 38, 209–221. [Google Scholar]
- Martincigh, B.S.; Ollengo, M.A. The Photostabilizing Effect of grape seed extract on three common sunscreen absorbers. Photochem. Photobiol. 2016, 92, 870–884. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Guia Para Avaliação de Segurança de Produtos Cosméticos, 2nd ed.; Anvisa: Brasília, Brasil, 2012; pp. 34–37. [Google Scholar]
- Balogh, T.S.; Velasco, M.V.R.; Pedriali, C.A.; Kaneko, T.M.; Baby, A.R. Ultraviolet radiation protection: Current available resources in photoprotection. An. Bras. Dermatol. 2011, 86, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Legouin, B.; Lohézic-Le Dévéhat, F.; Ferron, S.; Rouaud, I.; Le Pogam, P.; Cornevin, L.; Bertrand, M.; Boustie, J. Specialized metabolites of the lichen Vulpicida pinastri act as photoprotective agents. Molecules 2017, 22, 1162. [Google Scholar] [CrossRef]
- Apel, C.; Tang, J.; Ebinghaus, R. Environmental occurrence and distribution of organic UV stabilizers and UV filters in the sediment of Chinese Bohai and Yellow Seas. Environ. Pollut. 2018, 235, 85–94. [Google Scholar] [CrossRef]
- Hübner, A.A. Caracterização Fitoquímica e Eficácia Fotoprotetora Clínica de Formulações Cosméticas Contendo Extrato do Bagaço de uva Cabernet Sauvignon. Master’s Thesis, Universidade de São Paulo, São Paulo, Brasil, 2017. [Google Scholar] [CrossRef]
- Peyrefitte, G.; Martini, C.M.; Chivot, M. Cosmetologia/ Biologia Geral/ Biologia da Pele Estética Cosmética, 1st ed.; Andrei: São Paulo, Brasil, 1998; pp. 329–424. [Google Scholar]
- Dutra, E.A.; Oliveira, D.A.G.C.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev. Bras. Ciênc. Farm. 2004, 40, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Ruvolo, J.E.; Kollias, N.; Cole, C. New noninvasive approach assessing in vivo sun protection factor (SPF) using diffuse reflectance spectroscopy (DRS) and in vitro transmission. Photodermatol. Photoimmunol. Photomed. 2014, 30, 202–211. [Google Scholar] [CrossRef] [PubMed]




| Ingredients (INCI a) | Concentration (% w/w) | |||||
|---|---|---|---|---|---|---|
| Type I | Type II | Type III | ||||
| F1 – pH 5 | F2 – pH 7 | F3 – pH 5 | F4 – pH 7 | F5 – pH 5 | F6 – pH 7 | |
| Oil phase | ||||||
| Ethylhexyl methoxycinnamate | 10.0 | 10.0 | - | - | 10.0 | 10.0 |
| Ethylhexyl dimethyl PABA | 10.0 | 10.0 | - | - | 10.0 | 10.0 |
| Butyl methoxy dibenzoyl methane | 5.0 | 5.0 | - | - | 5.0 | 5.0 |
| Mixture of phenoxyethanol and paraben esters * | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Aqueous phase | ||||||
| Ammonium acryloyldimethyltaurate vinylpyrrolidone | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Grape pomace extract of V. vinifera L. | - | - | 10.0 | 10.0 | 10.0 | 10.0 |
| Purified water | 72.9 | 72.9 | 87.9 | 87.9 | 62.9 | 62.9 |
| Peak | MW (Da) | RT (min) | [M+H]+ (m/z) | MS/MS (m/z) | Major Fragment Ion (m/z) | Formula | Peak Identity | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 578 | 29.9 | 579.26 | 561.34, 453.31, 427.23, 409.24, 291.10, 247.05 | [M+H−H2O]+, [M+H−HRFC]+, [M+H−RDA]+, [M+H−RDA−H2O]+, [M+H−QM]+ | C30H26O12 | B-type procyanidin dimers | [40,41,42] |
| 2 | 578 | 31.6 | 579.26 | 543.33, 409.25, 291.10, 247.05, 200.88 | ||||
| 3 | 578 | 33.7 | 579.25 | 561.19, 409.17, 291.11, 246,98 | ||||
| 4 | 578 | 37.9 | 579.28 | 561.35, 453.29, 427.22, 409.23, 291.14 | ||||
| 5 | 578 | 41.5 | 579.30 | 561.33, 453.31, 409.23, 291.11, 247.02, 164.98 | ||||
| 6 | 866 | 867.38 | 697.34, 579.33, 409.25, 289.15 | [M+H−RDA−H2O]+, [M+H−QMC]+, [M+H−QM−RDA−H2O]+, [M+H−QMCD]+ | C45H38O18 | Trimer procyanidins | ||
| 7 | 450 | 55.8 | 451 | 415.19 305.06 | [M+H−2H2O]+ [M+H−Rham]+ | C21H22O11 | Di-hydroxyquercetin-O-rhamnose | [43,44] |
| 8 | 478 | 55.9 | 479.20 | 303.10 | [M+H−Gluc]+ | C21H18O13 | Quercetin-O-glucuronide | [40,45] |
| 9 | 610 | 59.3 | 611.28 | 465.25 303.10 | [M+H−Rham]+ [M+H-Rham−Glc]+ | C27H30O16 | Rutin | [45] |
| 10 | 464 | 59.6 | 465.21 | 303.07 | [M+H−Glc]+ | C21H18O13 | Quercetin-3-O-glucoside | [40,45] |
| 11 | 560 | 59.7 | 561.14 | 399.21 | [M+H−Glc]+ | C26H25O14 | Malvidin-3-O-glucoside pyruvate | [45] |
| 12 | 462 | 61.2 | 463.25 | 287.09 | [M+H−Gluc]+ | C21H18O12 | Kaempferol-3-O-glucuronide | [40,45] |
| 13 | 302 | 63.4 | 303.15 | 303.08, 257.03 | [M+H-H2O−CO]+ | C15H10O7 | Quercetin | [40,45] |
| 14 | 478 | 64.4 | 479.22 | 317.15 | [M+H−Glc]+ | C22H23O12 | Petunidin-3-O-glucoside | [43,44] |
| 15 | 530 | 64.6 | 531.18 | 369.15 | [M+H−Glc]+ | C25H23O13 | Peonidin-3-O-glucoside pyruvate | [45] |
| 16 | 492 | 65.3 | 493.22 | 331.16 | [M+H−Glc]+ | C23H25O12 | Malvidin-3-O-glucoside | [45] |
| 17 | 678 | 67 | 679.33 | 661.69 585.31 | [M+H−C6H6O]+ M+H−H2O]+ | C25H23O13 | Delphinidin-3-O-(6”-O-p-coumaryl) glucoside pyruvate | [45] |
| 18 | 534 | 76.1 | 535.27 | 517.42 331.18 | [M+H−2H2O]+ [M+H-acetylGlc]+ | C23H25O12 | Malvidin-3-(6”-O-acetylglucoside) | [45] |
| 19 | 654 | 81.1 | 655.32 | 636.75 331.17 | [M+H−H2O]+ M+H−caffeoylGlc]+ | C33H27O16 | Malvidin-3-(6”-O-caffeoylglucoside) | [45] |
| Formulations | pH | SPF * | Critical λ ** | |||||
|---|---|---|---|---|---|---|---|---|
| T 0 | T 1 | T 2 | T 0 | T 1 | T 2 | |||
| I | F1 | 5.0 | 14.00 ± 1.70 H I | 7.67 ± 1.53 J K L M N | 6.67 ± 1.53 L M N O | 381.67± 0.60 A B | 380.33 ± 0.58 A B | 380.00 ± 1.00 A B |
| F2 | 7.6 | 16.00 ± 1.70 G H I | 7.67 ± 0.58 J K L M N | 6.00 ± 0.00 L M NO | 381.33± 0.60 A B | 379.67 ± 0.58 A B | 379.33 ± 0.58 A B | |
| II | F3 | 5.2 | 1.67 ± 0.58 O | 1.67 ± 0.58 O | 1.67 ± 0.58 O | 360.33± 1.50 D | 364.67 ± 2.08 C D | 366.00 ± 1.73 C D |
| F4 | 7.0 | 2.00 ± 0.00 O | 2.00 ± 0.00 O | 2.00 ± 0.0 O | 365.33± 2.10 C D | 368.67 ± 2.08 C | 369.00 ± 2.65 C | |
| III | F5 | 5.4 | 76.67 ± 3.21 B | 26.33 ± 1.53 E | 17.33 ± 0.58 F G H | 380.00± 0.00 A B | 377.67 ± 0.58 A B | 376.67 ± 0.58 A B |
| F6 | 7.2 | 39.33 ± 2.08 D | 16.67 ± 1.15 G H | 12.33 ± 0.58 H I J | 380.33± 0.60 AB | 377.67 ± 0.58 AB | 376.00 ± 1.00 B | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubner, A.; Sobreira, F.; Vetore Neto, A.; Pinto, C.A.S.d.O.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System. Antioxidants 2019, 8, 530. https://doi.org/10.3390/antiox8110530
Hubner A, Sobreira F, Vetore Neto A, Pinto CASdO, Dario MF, Díaz IEC, Lourenço FR, Rosado C, Baby AR, Bacchi EM. The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System. Antioxidants. 2019; 8(11):530. https://doi.org/10.3390/antiox8110530
Chicago/Turabian StyleHubner, Alexandra, Flávia Sobreira, Alberto Vetore Neto, Claudinéia Aparecida Sales de Oliveira Pinto, Michelli Ferrera Dario, Ingrit Elida Collantes Díaz, Felipe Rebello Lourenço, Catarina Rosado, André Rolim Baby, and Elfriede Marianne Bacchi. 2019. "The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System" Antioxidants 8, no. 11: 530. https://doi.org/10.3390/antiox8110530