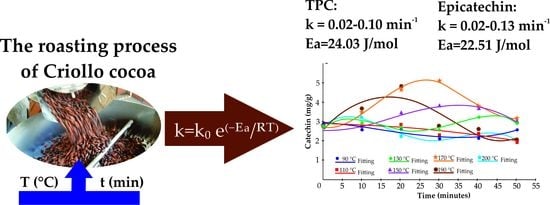
The Kinetics of Total Phenolic Content and Monomeric Flavan-3-ols during the Roasting Process of Criollo Cocoa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals and Standards
2.3. Roasting Process
2.4. Chemical Analysis
2.4.1. Methanolic Extraction of Phenolic Compounds and Monomeric Flavan-3-ols
2.4.2. Total Phenolic Content
2.4.3. Quantification of Epicatechin and Catechin of the Methanolic Extract
2.4.4. TPC and Monomeric Flavan-3-ol Degradation
2.5. Experimental Desing for Kinetics of TPC and Monomeric Flavan-3-ols
2.6. Statistical Analysis
3. Results
3.1. Effect of Roasting on Monomeric Flavan-3-ols and TPC
3.2. Roasting Kinetics of Monomeric Flavan-3-ols and TPC
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Żyżelewicz, D.; Budryn, G.; Oracz, J.; Antolak, H.; Kręgiel, D.; Kaczmarska, M. The effect on bioactive components and characteristics of chocolate by functionalization with raw cocoa beans. Food Res. Int. 2018, 113, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of aromatic compounds precursors during fermentation of Criollo and Forastero cocoa. Heliyon 2019, 5, e01157. [Google Scholar] [CrossRef] [Green Version]
- Ascrizzi, R.; Flamini, G.; Tessieri, C.; Pistelli, L. From the raw seed to chocolate: Volatile profile of Blanco de Criollo in different phases of the processing chain. Microchem. J. 2017, 133, 474–479. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Krysiak, W.; Oracz, J.; Sosnowska, D.; Budryn, G.; Nebesny, E. The influence of the roasting process conditions on the polyphenol content in cocoa beans, nibs and chocolates. Food Res. Int. 2016, 89, 918–929. [Google Scholar] [CrossRef]
- Stanley, T.H.; Van Buiten, C.B.; Baker, S.A.; Elias, R.J.; Anantheswaran, R.C.; Lambert, J.D. Impact of roasting on the flavan-3-ol composition, sensory-related chemistry, and in vitro pancreatic lipase inhibitory activity of cocoa beans. Food Chem. 2018, 255, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Nebesny, E. Influence of roasting conditions on the biogenic amine content in cocoa beans of different Theobroma cacao cultivars. Food Res. Int. 2014, 55, 1–10. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, Z.J.; Liao, W.; Li, J.; Gruget, P.; Kitts, D.D.; Lu, X. Determination of antioxidant capacity and phenolic content of chocolate by attenuated total reflectance-Fourier transformed-infrared spectroscopy. Food Chem. 2016, 202, 254–261. [Google Scholar] [CrossRef]
- Sacchetti, G.; Ioannone, F.; De Gregorio, M.; Di Mattia, C.; Serafini, M.; Mastrocola, D. Non enzymatic browning during cocoa roasting as affected by processing time and temperature. J. Food Eng. 2016, 169, 44–52. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Santos, H.M.; Coutinho, J.P.; Lôbo, I.P.; da Silva Junior, A.L.S.; Santos, A.G.; de Jesus, R.M. Optimization of chromatographic separation and classification of artisanal and fine chocolate based on its bioactive compound content through multivariate statistical techniques. Microchem. J. 2020, 152, 104342. [Google Scholar] [CrossRef]
- Steinberg, F.M.; Bearden, M.M.; Keen, C.L. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet. Assoc. 2003, 103, 215–223. [Google Scholar] [CrossRef]
- Do Carmo Brito, B.D.N.; Campos Chisté, R.; Da Silva Pena, R.; Abreu Gloria, M.B.; Santos Lopes, A. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Alean, J.; Chejne, F.; Rojano, B. Degradation of polyphenols during the cocoa drying process. J. Food Eng. 2016, 189, 99–105. [Google Scholar] [CrossRef]
- Wang, Y.; Feltham, B.A.; Suh, M.; Jones, P.J.H. Cocoa flavanols and blood pressure reduction: Is there enough evidence to support a health claim in the United States? Trends Food Sci. Technol. 2019, 83, 203–210. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castle, S.M.; Siswanto, P.J.; Cifuentes-Gómez, T.; Spencer, J.P.E. Assessment of flavanol stereoisomers and caffeine and theobromine content in commercial chocolates. Food Chem. 2016, 208, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Fayeulle, N.; Vallverdu-Queralt, A.; Meudec, E.; Hue, C.; Boulanger, R.; Cheynier, V.; Sommerer, N. Characterization of new flavan-3-ol derivatives in fermented cocoa beans. Food Chem. 2018, 259, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ioannone, F.; Di Mattia, C.D.; De Gregorio, M.; Sergi, M.; Serafini, M.; Sacchetti, G. Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem. 2015, 174, 256–262. [Google Scholar] [CrossRef]
- Krysiak, W. Influence of roasting conditions on coloration of roasted cocoa beans. J. Food Eng. 2006, 77, 449–453. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Monagas, M.; Khan, N.; Izquierdo-Pulido, M.; Urpi-Sarda, M.; Permanyer, J.; Lamuela-Raventós, R.M. Flavanol and Flavonol Contents of Cocoa Powder Products: Influence of the Manufacturing Process. J. Agric. Food Chem. 2008, 56, 3111–3117. [Google Scholar] [CrossRef]
- Martín, M.A.; Ramos, S. Cocoa polyphenols in oxidative stress: Potential health implications. J. Funct. Foods 2016, 27, 570–588. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.Á.; Ramos, S. Health beneficial effects of cocoa phenolic compounds: A mini-review. Curr. Opin. Food Sci. 2017, 14, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Della Pelle, F.; Blandón-Naranjo, L.; Alzate, M.; Del Carlo, M.; Compagnone, D. Cocoa powder and catechins as natural mediators to modify carbon-black based screen-printed electrodes. Application to free and total glutathione detection in blood. Talanta 2020, 207, 120349. [Google Scholar] [CrossRef] [PubMed]
- Hii, C.L.; Menon, A.S.; Chiang, C.L.; Sharif, S. Kinetics of hot air roasting of cocoa nibs and product quality. J. Food Process Eng. 2017, 40, e12467. [Google Scholar] [CrossRef]
- Afoakwa, E. Roasting effects on phenolic content and free radical scavening actiivities of pulp preconditioned and fermented (Theobroma cacao) beans. Afr. J. Food Agric. Nutr. Dev. 2015, 15, 9635–9650. [Google Scholar]
- Van Durme, J.; Ingels, I.; De Winne, A. Inline roasting hyphenated with gas chromatography–mass spectrometry as an innovative approach for assessment of cocoa fermentation quality and aroma formation potential. Food Chem. 2016, 205, 66–72. [Google Scholar] [CrossRef]
- Hu, S.; Kim, B.-Y.; Baik, M.-Y. Physicochemical properties and antioxidant capacity of raw, roasted and puffed cacao beans. Food Chem. 2016, 194, 1089–1094. [Google Scholar] [CrossRef]
- Teh, Q.T.M.; Tan, G.L.Y.; Loo, S.M.; Azhar, F.Z.; Menon, A.S.; Hii, C.L. The Drying Kinetics and Polyphenol Degradation of Cocoa Beans: Cocoa Drying and Polyphenol Degradation. J. Food Process Eng. 2016, 39, 484–491. [Google Scholar] [CrossRef]
- Hii, C.L.; Law, C.L.; Cloke, M.; Suzannah, S. Thin layer drying kinetics of cocoa and dried product quality. Biosyst. Eng. 2009, 102, 153–161. [Google Scholar] [CrossRef]
- Kyi, T.M.; Daud, W.R.W.; Mohammad, A.B.; Wahid Samsudin, M.; Kadhum, A.A.H.; Talib, M.Z.M. The kinetics of polyphenol degradation during the drying of Malaysian cocoa beans. Int. J. Food Sci. Technol. 2005, 40, 323–331. [Google Scholar] [CrossRef]
- Summa, C.; Raposo, F.C.; McCourt, J.; Scalzo, R.L.; Wagner, K.-H.; Elmadfa, I.; Anklam, E. Effect of roasting on the radical scavenging activity of cocoa beans. Eur. Food Res. Technol. 2006, 222, 368–375. [Google Scholar] [CrossRef]
- Jonfia-Essien, W.A.; West, G.; Alderson, P.G.; Tucker, G. Phenolic content and antioxidant capacity of hybrid variety cocoa beans. Food Chem. 2008, 108, 1155–1159. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela-Raventos, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteau reagent. In Methods in Enzymology; Academic Press: Cambridge, UK, 1999; pp. 152–178. [Google Scholar]
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of Fermentation, Drying, Roasting, and Dutch Processing on Epicatechin and Catechin Content of Cacao Beans and Cocoa Ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S.; Tijskens, L.M.M. Kinetic modelling. In Food Process Modelling; Woodhead Publishing Limited: Cambridge, UK, 2001; pp. 35–59. ISBN 978-1-85573-565-1. [Google Scholar]
- Rabeler, F.; Feyissa, A.H. Kinetic Modeling of Texture and Color Changes During Thermal Treatment of Chicken Breast Meat. Food Bioprocess Technol. 2018, 11, 1495–1504. [Google Scholar] [CrossRef] [Green Version]
- García-Alamilla, P.; Lagunes-Gálvez, L.M.; Barajas-Fernández, J.; García-Alamilla, R. Physicochemical Changes of Cocoa Beans during Roasting Process. J. Food Qual. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Hurst, W.J.; Krake, S.H.; Bergmeier, S.C.; Payne, M.J.; Miller, K.B.; Stuart, D.A. Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem. Cent. J. 2011, 5, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghdadi, Y.M.; Hii, C.L. Mass transfer kinetics and effective diffusivities during cocoa roasting. J. Eng. Sci. Technol. 2017, 12, 127–137. [Google Scholar]
- Menon, A.S.; Hii, C.L.; Law, C.L.; Shariff, S.; Djaeni, M. Effects of drying on the production of polyphenol-rich cocoa beans. Dry. Technol. 2017, 35, 1799–1806. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K.; You, X. Isocratic elution system for the determination of catechins, caffeine and gallic acid in green tea using HPLC. Food Chem. 2000, 68, 115–121. [Google Scholar] [CrossRef]
- Martins, L.M.; Sant’Ana, A.S.; Iamanaka, B.T.; Berto, M.I.; Pitt, J.I.; Taniwaki, M.H. Kinetics of aflatoxin degradation during peanut roasting. Food Res. Int. 2017, 97, 178–183. [Google Scholar] [CrossRef]
- Molaveisi, M.; Beigbabaei, A.; Akbari, E.; Noghabi, M.S.; Mohamadi, M. Kinetics of temperature effect on antioxidant activity, phenolic compounds and color of Iranian jujube honey. Heliyon 2019, 5, e01129. [Google Scholar] [CrossRef] [Green Version]
- Kothe, L.; Zimmermann, B.F.; Galensa, R. Temperature influences epimerization and composition of flavanol monomers, dimers and trimers during cocoa bean roasting. Food Chem. 2013, 141, 3656–3663. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Quelal-Vásconez, M.A.; Lerma-García, M.J.; Pérez-Esteve, É.; Arnau-Bonachera, A.; Barat, J.M.; Talens, P. Changes in methylxanthines and flavanols during cocoa powder processing and their quantification by near-infrared spectroscopy. LWT 2020, 117, 108598. [Google Scholar] [CrossRef]
- Kim, H.; Keeney, P.G. (-)-Epicatechin Content in Fermented and Unfermented Cocoa Beans. J. Food Sci. 1984, 49, 1090–1092. [Google Scholar] [CrossRef]
- Mazor Jolić, S.; Radojčić Redovniković, I.; Marković, K.; Ivanec Šipušić, Đ.; Delonga, K. Changes of phenolic compounds and antioxidant capacity in cocoa beans processing: Changes of phenolic in cocoa beans processing. Int. J. Food Sci. Technol. 2011, 46, 1793–1800. [Google Scholar] [CrossRef]
- Peláez, P.; Bardón, I.; Camasca, P. Methylxanthine and catechin content of fresh and fermented cocoa beans, dried cocoa beans, and cocoa liquor. Sci. Agropecu. 2016, 7, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Prakash, M.; Basavaraj, B.V.; Chidambara Murthy, K.N. Biological functions of epicatechin: Plant cell to human cell health. J. Funct. Foods 2019, 52, 14–24. [Google Scholar] [CrossRef]
- Miller, K.B.; Hurst, W.J.; Flannigan, N.; Ou, B.; Lee, C.Y.; Smith, N.; Stuart, D.A. Survey of Commercially Available Chocolate- and Cocoa-Containing Products in the United States. 2. Comparison of Flavan-3-ol Content with Nonfat Cocoa Solids, Total Polyphenols, and Percent Cacao. J. Agric. Food Chem. 2009, 57, 9169–9180. [Google Scholar] [CrossRef]
- Djikeng, F.T.; Teyomnou, W.T.; Tenyang, N.; Tiencheu, B.; Morfor, A.T.; Touko, B.A.H.; Houketchang, S.N.; Boungo, G.T.; Karuna, M.S.L.; Ngoufack, F.Z.; et al. Effect of traditional and oven roasting on the physicochemical properties of fermented cocoa beans. Heliyon 2018, 4, e00533. [Google Scholar] [CrossRef] [Green Version]
- Suazo, Y.; Davidov-Pardo, G.; Arozarena, I. Effect of Fermentation and Roasting on the Phenolic Concentration and Antioxidant Activity of Cocoa from Nicaragua: Effect of Process on Cocoa from Nicaragua. J. Food Qual. 2014, 37, 50–56. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [Green Version]
- Lončarić, A.; Pablo Lamas, J.; Guerra, E.; Kopjar, M.; Lores, M. Thermal stability of catechin and epicatechin upon disaccharides addition. Int. J. Food Sci. Technol. 2018, 53, 1195–1202. [Google Scholar] [CrossRef]
- Bernatova, I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Changes in the flavan-3-ols, anthocyanins, and flavanols composition of cocoa beans of different Theobroma cacao L. groups affected by roasting conditions. Eur. Food Res. Technol. 2015, 241, 663–681. [Google Scholar] [CrossRef] [Green Version]
- Taoukis, P.S.; Labuza, T.P.; Saguy, I.S. Kinetics of Food Deterioration and Shelf-Life Prediction. In Handbook of Food Engineering Practice; CRC Press: New York, NY, USA, 1997; pp. 366–408. [Google Scholar]
- Henríquez, C.; Córdova, A.; Almonacid, S.; Saavedra, J. Kinetic modeling of phenolic compound degradation during drum-drying of apple peel by-products. J. Food Eng. 2014, 143, 146–153. [Google Scholar] [CrossRef]
- Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Effect of thermal treatment on phenolic compounds from plum (prunus domestica) extracts—A kinetic study. J. Food Eng. 2016, 171, 200–207. [Google Scholar] [CrossRef]
- Garvín, A.; Ibarz, R.; Ibarz, A. Kinetic and thermodynamic compensation. A current and practical review for foods. Food Res. Int. 2017, 96, 132–153. [Google Scholar] [CrossRef]
- Olivares-Tenorio, M.-L.; Verkerk, R.; van Boekel, M.A.J.S.; Dekker, M. Thermal stability of phytochemicals, HMF and antioxidant activity in cape gooseberry (Physalis peruviana L.). J. Funct. Foods 2017, 32, 46–57. [Google Scholar] [CrossRef]


| T (°C) | Time (min) | Concentration 1 | Epi/Cat Ratio 1 | Degradation 1 (%) | ||||
|---|---|---|---|---|---|---|---|---|
| TPC (mg GAE/gdf) | Epicatechin (mg/gdf) | Catechin (mg/gdf) | TPC | Epicatechin | Catechin 2 | |||
| Control | 0 | 110.98 ± 1.43a | 30.29 ± 1.09a | 2.71 ± 0.13a | 11.20 ± 0.40a | |||
| 90 | 10 | 54.60 ± 10.86b | 14.97 ± 1.45b | 2.48 ± 0.14ab | 6.08 ± 0.96b | 50.84 ± 9.47a | 50.41 ± 6.61a | 8.31 ± 5.43a |
| 20 | 72.70 ± 7.44b | 17.78 ± 3.13b | 2.48 ± 0.05ab | 7.20 ± 1.37b | 34.44 ± 7.46a | 41.25 ± 10.23a | 8.31 ± 6.24a | |
| 30 | 45.10 ± 7.61b | 11.78 ± 1.22b | 2.20 ± 0.11b | 5.35 ± 0.32b | 59.41 ± 6.47a | 61.07 ± 4.13a | 18.69 ± 3.99a | |
| 40 | 62.10 ± 19.80b | 12.92 ± 4.89b | 2.22 ± 0.28b | 5.93 ± 2.62b | 43.90 ± 18.7a | 57.0 ± 17.7a | 18.25 ± 6.22a | |
| 50 | 51.58 ± 7.44b | 13.75 ± 2.92b | 2.48 ± 0.04ab | 5.55 ± 1.24b | 53.46 ± 7.29a | 54.78 ± 8.05a | 8.08 ± 5.82a | |
| 110 | 10 | 68.54 ± 4.05c | 27.24 ± 0.53c | 2.60 ± 0.07cd | 10.50 ± 0.36c | 38.21 ± 4.44c | 10.02 ± 2.08d | 2.22 ± 1.45d |
| 20 | 60.84 ± 5.59cd | 15.36 ± 0.47d | 2.67 ± 0.21c | 5.78 ± 0.50d | 45.14 ± 5.72bc | 49.20 ± 3.07c | 7.78 ± 2.00cd | |
| 30 | 52.25 ± 3.86d | 11.47 ± 0.46e | 2.28 ± 0.08de | 5.05 ± 0.28de | 52.91 ± 3.59b | 62.10 ± 1.50b | 15.79 ± 4.65bc | |
| 40 | 48.14 ± 6.12d | 8.28 ± 1.36e | 2.33 ± 0.18cde | 3.54 ± 0.31f | 56.58 ± 5.95b | 72.53 ± 5.49b | 13.59 ± 8.53bcd | |
| 50 | 58.92 ± 6.57cd | 8.22 ± 2.37e | 2.06 ± 0.12e | 3.96 ± 0.97ef | 46.88 ± 6.30bc | 72.77 ± 8.31b | 24.00 ± 3.58b | |
| 130 | 10 | 98.85 ± 7.79e | 28.74 ± 0.94f | 3.01 ± 0.03f | 9.55 ± 0.39g | 10.98 ± 6.04f | 8.05 ± 3.01e | 11.46 ± 4.71e |
| 20 | 52.24 ± 3.68f | 11.21 ± 1.24g | 2.69 ± 0.47f | 4.20 ± 0.26h | 52.93 ± 3.24e | 62.87 ± 5.25d | 14.22 ± 6.08e | |
| 30 | 35.34 ± 2.19g | 9.47 ± 1.01gh | 2.62 ± 0.23f | 3.66 ± 0.67hi | 68.14 ± 2.34d | 68.77 ± 2.57cd | 5.30 ± 4.23e | |
| 40 | 44.35 ± 7.73fg | 10.46 ± 1.46g | 3.30 ± 0.36f | 3.23 ± 0.81hi | 60.01 ± 7.15de | 65.40 ± 5.08d | 18.62 ± 7.50e | |
| 50 | 38.43 ± 3.98fg | 6.98 ± 1.01h | 2.95 ± 0.22f | 2.36 ± 0.20i | 65.40 ± 3.16de | 76.99 ± 2.78c | 9.08 ± 7.50e | |
| 150 | 10 | 46.71 ± 7.84h | 11.25 ± 2.11i | 2.62 ± 0.24g | 4.26 ± 0.41j | 57.85 ± 7.61h | 62.70 ± 8.19h | 8.66 ± 0.15f |
| 20 | 48.78 ± 8.12h | 8.70 ± 0.70 i j | 3.49 ± 0.68g | 2.59 ± 0.73k | 56.07 ± 7.02h | 71.29 ± 1.29gh | 32.90 ± 25.60f | |
| 30 | 31.32 ± 2.25i | 6.32 ± 0.53jk | 3.79 ± 0.75g | 1.70 ± 0.25kl | 71.80 ± 1.72g | 79.14 ± 1.51fg | 39.60 ± 21.10f | |
| 40 | 31.27 ± 2.17i | 5.72 ± 0.12k | 3.64 ± 0.10g | 1.57 ± 0.07kl | 71.81 ± 2.18g | 81.11 ± 0.84fg | 34.61 ± 8.41f | |
| 50 | 28.22 ± 1.32i | 4.27 ± 0.68k | 3.01 ± 0.35g | 1.41 ± 0.08l | 74.57 ± 1.37g | 85.95 ± 1.81f | 11.05 ± 8.82f | |
| 170 | 10 | 42.10 ± 7.56j | 12.33 ± 2.33l | 3.48 ± 0.98hij | 0.08 ± 0.02no | 62.12 ± 6.39j | 59.44 ± 6.36k | 11.58 ± 0.01h |
| 20 | 33.43 ± 7.28jk | 6.75 ± 0.77m | 4.66 ± 0.31hi | 0.10 ± 0.02n | 69.84 ± 6.81ij | 77.75 ± 1.80j | 72.34 ± 12.07gh | |
| 30 | 31.50 ± 6.43jk | 5.41 ± 0.69mn | 5.15 ± 1.11h | 0.17 ± 0.05m | 71.66 ± 5.50ij | 82.11 ± 2.36ij | 84.90 ± 37.90g | |
| 40 | 31.33 ± 7.40jk | 4.16 ± 0.59mn | 3.85 ± 0.66hij | 0.12 ± 0.02mn | 71.82 ± 6.36ij | 86.26 ± 1.77ij | 43.00 ± 30.30gh | |
| 50 | 19.54 ± 1.05k | 3.12 ± 0.18n | 3.19 ± 0.42ij | 0.11 ± 0.01mn | 82.40 ± 0.89i | 89.70 ± 0.77i | 22.19 ± 14.41h | |
| 190 | 10 | 41.13 ± 8.69l | 11.61 ± 4.88o | 3.67 ± 0.91l | 3.35 ± 1.62p | 62.95 ± 7.70l | 61.93 ± 15.42m | 42.3 ± 29.40ij |
| 20 | 38.17 ± 6.69l | 5.70 ± 0.50p | 4.87 ± 0.28 k | 1.18 ± 0.17q | 65.63 ± 5.75l | 81.20 ± 0.99l | 80.50 ± 17.40i | |
| 30 | 21.35 ± 4.45m | 3.11 ± 0.60pq | 2.77 ± 0.25lm | 1.11 ± 0.12q | 80.79 ± 3.80k | 89.70 ± 2.32kl | 7.32 ± 6.32j | |
| 40 | 19.81 ± 2.11m | 2.43 ± 0.13pq | 2.67 ± 0.27lm | 0.92 ± 0.10q | 82.17 ± 1.72k | 91.97 ± 0.23kl | 9.97 ± 3.21j | |
| 50 | 12.31 ± 1.36m | 0.00 ± 0.00q | 2.05 ± 0.07m | 0.00 ± 0.00q | 88.91 ± 1.27k | 100.00 ± 0.00k | 23.96 ± 6.02j | |
| 200 | 10 | 33.23 ± 2.44n | 8.10 ± 0.88r | 3.20 ± 0.68n | 2.64 ± 0.83r | 70.04 ± 2.60o | 73.21 ± 3.58p | 21.89 ± 14.12k |
| 20 | 17.33 ± 3.26o | 2.42 ± 0.21s | 2.30 ± 0.30n | 1.06 ± 0.05s | 84.38 ± 2.98n | 92.01 ± 0.39o | 15.17 ± 7.86k | |
| 30 | 13.76 ± 0.53op | 2.32 ± 0.06s | 2.26 ± 0.07n | 1.03 ± 0.03s | 87.60 ± 0.64mn | 92.34 ± 0.10o | 16.56 ± 1.54k | |
| 40 | 14.41 ± 1.86o | 2.53 ± 0.45s | 2.35 ± 0.60n | 1.09 ± 0.09st | 87.00 ± 1.85mn | 91.61 ± 1.81o | 22.29 ± 10.34k | |
| 50 | 8.56 ± 0.10p | 0.00 ± 0.00t | 2.13 ± 0.21n | 0.00 ± 0.00t | 92.29 ± 0.06m | 100.00 ± 0.00n | 21.37 ± 5.74k | |
| Roasting Temperature (°C) | TPC | Epicatechin | ||||
|---|---|---|---|---|---|---|
| (min−1) | RMSE | (min−1) | RMSE | |||
| 90 | 0.02 ± 0.01 | 0.52 | 18.50 | 0.02 ± 0.01 | 0.65 | 4.57 |
| 110 | 0.02 ± 0.01 | 0.70 | 13.97 | 0.03 ± 0.01 | 0.95 | 2.49 |
| 130 | 0.03 ± 0.02 | 0.87 | 13.08 | 0.03 ± 0.02 | 0.86 | 4.31 |
| 150 | 0.04 ± 0.03 | 0.83 | 14.59 | 0.06 ± 0.03 | 0.91 | 3.27 |
| 170 | 0.07 ± 0.03 | 0.96 | 2.24 | 0.07 ± 0.03 | 0.96 | 2.24 |
| 190 | 0.06 ± 0.03 | 0.92 | 11.35 | 0.09 ± 0.02 | 0.99 | 1.05 |
| 200 | 0.10 ± 0.05 | 0.96 | 9.23 | 0.13 ± 0.04 | 0.99 | 1.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Romero, E.; Chavez-Quintana, S.G.; Siche, R.; Castro-Alayo, E.M.; Cardenas-Toro, F.P. The Kinetics of Total Phenolic Content and Monomeric Flavan-3-ols during the Roasting Process of Criollo Cocoa. Antioxidants 2020, 9, 146. https://doi.org/10.3390/antiox9020146
Fernández-Romero E, Chavez-Quintana SG, Siche R, Castro-Alayo EM, Cardenas-Toro FP. The Kinetics of Total Phenolic Content and Monomeric Flavan-3-ols during the Roasting Process of Criollo Cocoa. Antioxidants. 2020; 9(2):146. https://doi.org/10.3390/antiox9020146
Chicago/Turabian StyleFernández-Romero, Editha, Segundo G. Chavez-Quintana, Raúl Siche, Efraín M. Castro-Alayo, and Fiorella P. Cardenas-Toro. 2020. "The Kinetics of Total Phenolic Content and Monomeric Flavan-3-ols during the Roasting Process of Criollo Cocoa" Antioxidants 9, no. 2: 146. https://doi.org/10.3390/antiox9020146