Use of Spectroscopic Techniques to Monitor Changes in Food Quality during Application of Natural Preservatives: A Review
Abstract
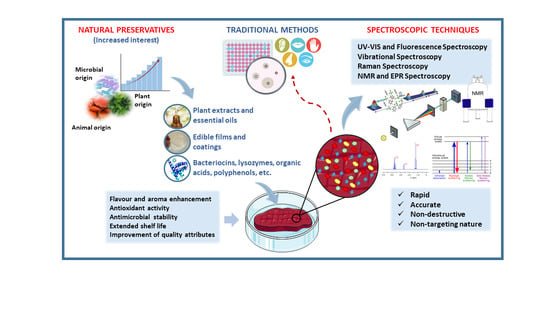
:1. Introduction
2. Natural Food Preservation Technologies
2.1. Essential Oils and Plant Extracts
2.2. Edible Films and Coatings
2.3. Microbial-Derived Compounds
2.4. Other Natural Compounds
3. Traditional Methods Used to Evaluate Quality Changes
3.1. Sensory Analysis
3.2. Microbiological Evaluation
3.3. Physical and Chemical/Biochemical Parameters
4. Spectroscopic Monitoring Techniques
4.1. Overview of Spectroscopic Techniques
4.2. Examples of Applications of Spectroscopic Techniques
4.2.1. UV-Vis and Fluorescence Spectroscopy
4.2.2. Vibrational Spectroscopy
4.2.3. Raman Spectroscopy
4.2.4. NMR and EPR Spectroscopy
5. Final Remarks and Future Trends
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef] [Green Version]
- Hassoun, A.; Emir Çoban, Ö. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends Food Sci. Technol. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent advances in quality retention of non-frozen fish and fishery products: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1747–1759. [Google Scholar] [CrossRef]
- Bondi, M.; Lauková, A.; De Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural preservatives to improve food quality and safety. J. Food Qual. 2017, 2017, 1090932. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Chen, S.; Hao, S.; Yang, X. Effect of low-temperature preservation on quality changes in Pacific white shrimp, Litopenaeus vannamei: A review. J. Sci. Food Agric. 2019, 99, 6121–6128. [Google Scholar] [CrossRef] [PubMed]
- Sampels, S. The effects of processing technologies and preparation on the final quality of fish products. Trends Food Sci. Technol. 2015, 44, 131–146. [Google Scholar] [CrossRef]
- Hassoun, A.; Heia, K.; Lindberg, S.; Nilsen, H. Spectroscopic techniques for monitoring thermal treatments in fish and other seafood: A review of recent developments and applications. Foods 2020, 6, 767. [Google Scholar] [CrossRef]
- Aubourg, S.P. Effect of natural preservatives on chemical changes related to quality and shelf life in processed aquatic foods. In Innovative Technologies in Seafood Processing; Özoğul, Y., Ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 219–241. ISBN 9780815366447. [Google Scholar]
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Inanli, A.G.; Tümerkan, E.T.A.; Abed, N.E.; Regenstein, J.M.; Özogul, F. The impact of chitosan on seafood quality and human health: A review. Trends Food Sci. Technol. 2020, 97, 404–416. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products. Meat Sci. 2020, 108272. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, N.; Rotabakk, B.T.; Lerfall, J. Effect of heat treatment and packaging technology on the microbial load of lightly processed seafood. LWT 2019, 101, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, L.M.; Leyton, A.; Elena, M. Reformulation of preserved fish products. In Trends in Fish Processing Technologies; Daniela, B., Anca, I., Nicolau, P.R., Eds.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 135–160. [Google Scholar]
- Bar, E.M.S.; Hoel, S.; Lerfall, J. New product development. In Trends in Fish Processing Technologies; Borda, D., Nicolau, A.I., Raspor, P., Eds.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 161–170. [Google Scholar]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Potential application of essential oils as natural antioxidants in meat and meat products: A review. Food Rev. Int. 2014, 30, 71–90. [Google Scholar] [CrossRef]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Xavier, K.A.M.; Balange, A.; Layana, P.; Nayak, B.B. Chitosan gel addition in pre-emulsified fish mince—Effect on quality parameters of sausages under refrigerated storage. LWT 2019, 110, 283–291. [Google Scholar] [CrossRef]
- Vieira, B.B.; Mafra, J.F.; da Bispo, A.S.R.; Ferreira, M.A.; de Silva, F.L.; Rodrigues, A.V.N.; Evangelista-Barreto, N.S. Combination of chitosan coating and clove essential oil reduces lipid oxidation and microbial growth in frozen stored tambaqui (Colossoma macropomum) fillets. LWT 2019, 116, 108546. [Google Scholar] [CrossRef]
- Cai, L.; Leng, L.; Cao, A.; Cheng, X.; Li, J. The effect of chitosan-essential oils complex coating on physicochemical, microbiological, and quality change of grass carp (Ctenopharyhgodon idella) fillets. J. Food Saf. 2018, 38, 1–9. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.; Qazi, I.M.; Pavase, T.R.; Lv, L. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Skandamis, P.N. Applications of active packaging for increasing microbial stability in foods: Natural volatile antimicrobial compounds. Curr. Opin. Food Sci. 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Pavli, F.G.; Argyri, A.A.; Chorianopoulos, N.G.; Nychas, G.J.E.; Tassou, C.C. Effect of Lactobacillus plantarum L125 strain with probiotic potential on physicochemical, microbiological and sensorial characteristics of dry-fermented sausages. LWT 2020, 118, 108810. [Google Scholar] [CrossRef]
- Pavli, F.; Argyri, A.A.; Nychas, G.J.E.; Tassou, C.; Chorianopoulos, N. Use of Fourier transform infrared spectroscopy for monitoring the shelf life of ham slices packed with probiotic supplemented edible films after treatment with high pressure processing. Food Res. Int. 2018, 106, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D. Infrared spectroscopy as a versatile analytical tool for the quantitative determination of antioxidants in agricultural products, foods and plants. Antioxidants 2015, 4, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Clemente, I.; Aznar, M.; Salafranca, J.; Nerín, C. Raman spectroscopy, electronic microscopy and SPME-GC-MS to elucidate the mode of action of a new antimicrobial food packaging material. Anal. Bioanal. Chem. 2017, 409, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Moudache, M.; Nerín, C.; Colon, M.; Zaidi, F. Antioxidant effect of an innovative active plastic film containing olive leaves extract on fresh pork meat and its evaluation by Raman spectroscopy. Food Chem. 2017, 229, 98–103. [Google Scholar] [CrossRef]
- Li, P.; Zhou, Q.; Chu, Y.; Lan, W.; Mei, J.; Xie, J. Effects of chitosan and sodium alginate active coatings containing ε-polysine on qualities of cultured pufferfish (Takifugu obscurus) during cold storage. Int. J. Biol. Macromol. 2020, 160, 418–428. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Xie, J. Effect of glazing with different materials on the quality of tuna during frozen storage. Foods 2020, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Karoui, R.; Hassoun, A. Efficiency of rosemary and basil essential oils on the shelf-life extension of Atlantic mackerel (Scomber scombrus) fillets stored at 2 °C. J. AOAC Int. 2017, 100, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Rustad, T. Assessment of lipid oxidation in Atlantic mackerel (Scomber scombrus) subjected to different antioxidant and sous-vide cooking treatments by conventional and fluorescence microscopy methods. Food Control 2019, 104, 1–8. [Google Scholar] [CrossRef]
- Xie, W.; Huang, Y.; Xiang, Y.; Xiong, S.; Manyande, A.; Du, H. Insights into the binding mechanism of polyphenols and fish myofibrillar proteins explored using multi-spectroscopic methods. Food Bioprocess Technol. 2020, 13, 797–806. [Google Scholar] [CrossRef]
- Lopes, T.S.; Fontoura, P.S.; Oliveira, A.; Rizzo, F.A.; Silveira, S.; Streck, A.F. Use of plant extracts and essential oils in the control of bovine mastitis. Res. Vet. Sci. 2020, 131, 186–193. [Google Scholar] [CrossRef]
- Nhu, T.Q.; Bich Hang, B.T.; Vinikas, A.; Bach, L.T.; Buu Hue, B.T.; Thanh Huong, D.T.; Quetin-Leclercq, J.; Scippo, M.L.; Phuong, N.T.; Kestemont, P. Screening of immuno-modulatory potential of different herbal plant extracts using striped catfish (Pangasianodon hypophthalmus) leukocyte-based in vitro tests. Fish Shellfish Immunol. 2019, 93, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Harhaun, R.; Kunik, O.; Saribekova, D.; Lazzara, G. Biologically active properties of plant extracts in cosmetic emulsions. Microchem. J. 2020, 154, 104543. [Google Scholar] [CrossRef] [Green Version]
- Dima, C.; Dima, S. Essential oils in foods: Extraction, stabilization, and toxicity. Curr. Opin. Food Sci. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E. Production of essential oils. In Handbook of Essential Oils: Science, Technology and Applications; Can Başer, K.H., Buchbauer, G., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 83–120. ISBN 9781466590472. [Google Scholar]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments—A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- Tlili, N.; Sarikurkcu, C. Bioactive compounds profile, enzyme inhibitory and antioxidant activities of water extracts from five selected medicinal plants. Ind. Crops Prod. 2020, 151, 112448. [Google Scholar] [CrossRef]
- Ben Jemaa, M.; Falleh, H.; Neves, M.A.; Isoda, H.; Nakajima, M.; Ksouri, R. Quality preservation of deliberately contaminated milk using thyme free and nanoemulsified essential oils. Food Chem. 2017, 217, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Falleh, H.; Ben Jemaa, M.; Djebali, K.; Abid, S.; Saada, M.; Ksouri, R. Application of the mixture design for optimum antimicrobial activity: Combined treatment of Syzygium aromaticum, Cinnamomum zeylanicum, Myrtus communis, and Lavandula stoechas essential oils against Escherichia coli. J. Food Process. Preserv. 2019, 43, 1–11. [Google Scholar] [CrossRef]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Dwivedy, A.K.; Dubey, N.K. Trachyspermum ammi L. essential oil as plant based preservative in food system. Ind. Crops Prod. 2015, 69, 104–109. [Google Scholar] [CrossRef]
- Dwivedy, A.K.; Prakash, B.; Chanotiya, C.S.; Bisht, D.; Dubey, N.K. Chemically characterized Mentha cardiaca L. essential oil as plant based preservative in view of efficacy against biodeteriorating fungi of dry fruits, aflatoxin secretion, lipid peroxidation and safety profile assessment. Food Chem. Toxicol. 2017, 106, 175–184. [Google Scholar] [CrossRef]
- Ozogul, Y.; Yuvka, İ.; Ucar, Y.; Durmus, M.; Kösker, A.R.; Öz, M.; Ozogul, F. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT Food Sci. Technol. 2017, 75, 677–684. [Google Scholar] [CrossRef]
- Alparslan, Y.; Yapici, H.H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT Food Sci. Technol. 2016, 72, 457–466. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Naghibi, S.S.; Mohammadi, S.; Khalili Sadaghiani, S. Properties of Bunium Persicum essential oil and its application in Iranian white cheese against Listeria Monocytogenes and Escherichia Coli O157:H7. J. Food Saf. 2016, 36, 563–570. [Google Scholar] [CrossRef]
- De Carvalho, R.J.; de Souza, G.T.; Honório, V.G.; de Sousa, J.P.; da Conceição, M.L.; Maganani, M.; de Souza, E.L. Comparative inhibitory effects of Thymus vulgaris L. essential oil against Staphylococcus aureus, Listeria monocytogenes and mesophilic starter co-culture in cheese-mimicking models. Food Microbiol. 2015, 52, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Es’haghi Gorji, M.; Noori, N.; Nabizadeh Nodehi, R.; Jahed Khaniki, G.; Rastkari, N.; Alimohammadi, M. The evaluation of Zataria multiflora Boiss: Essential oil effect on biogenic amines formation and microbiological profile in Gouda cheese. Lett. Appl. Microbiol. 2014, 59, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.P.; Zhang, Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef]
- De Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef]
- Jayawardana, B.C.; Warnasooriya, V.B.; Thotawattage, G.H.; Dharmasena, V.A.K.I.; Liyanage, R. Black and green tea (Camellia sinensis L.) extracts as natural antioxidants in uncured pork sausages. J. Food Process. Preserv. 2019, 43, 1–8. [Google Scholar] [CrossRef]
- Ju, J.; Xu, X.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Inhibitory effects of cinnamon and clove essential oils on mold growth on baked foods. Food Chem. 2018, 240, 850–855. [Google Scholar] [CrossRef]
- Koné, A.P.; Desjardins, Y.; Gosselin, A.; Cinq-Mars, D.; Guay, F.; Saucier, L. Plant extracts and essential oil product as feed additives to control rabbit meat microbial quality. Meat Sci. 2019, 150, 111–121. [Google Scholar] [CrossRef]
- Đurović, S.; Vujanović, M.; Radojković, M.; Filipović, J.; Filipović, V.; Gašić, U.; Tešić, Ž.; Mašković, P.; Zeković, Z. The functional food production: Application of stinging nettle leaves and its extracts in the baking of a bread. Food Chem. 2020, 312, 126091. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Pasqualone, A.; Silletti, R.; Cosmai, L.; Summo, C.; Paradiso, V.M.; Caponio, F. Use of olive leaf extract to reduce lipid oxidation of baked snacks. Food Res. Int. 2018, 108, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef] [Green Version]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Essential oils and their principal constituents as antimicrobial agents for synthetic packaging films. J. Food Sci. 2011, 76, 164–177. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid -based natural edible films in food packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on edible films based on fruits and vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [Green Version]
- Boarca, B.; Lungu, I.; Holban, A.M. Bioactive packaging for modern beverage industry. In Trends in Beverage Packaging; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 51–71. ISBN 9780128166833. [Google Scholar]
- Umaraw, P.; Verma, A.K. Comprehensive review on application of edible film on meat and meat products: An eco-friendly approach. Crit. Rev. Food Sci. Nutr. 2017, 57, 1270–1279. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Leceta, I.; Molinaro, S.; Guerrero, P.; Kerry, J.P.; De la Caba, K. Quality attributes of map packaged ready-to-eat baby carrots by using chitosan-based coatings. Postharvest Biol. Technol. 2015, 100, 142–150. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Khomeiri, M.; Ziaiifar, A.M. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol. Technol. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; de Ramos, A.L.S.; de Gomide, L.A.M.; Ramos, E.M. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef]
- Farajzadeh, F.; Motamedzadegan, A.; Shahidi, S.A.; Hamzeh, S. The effect of chitosan-gelatin coating on the quality of shrimp (Litopenaeus vannamei) under refrigerated condition. Food Control 2016, 67, 163–170. [Google Scholar] [CrossRef]
- Frazão, G.G.S.; Blank, A.F.; de Aquino Santana, L.C.L. Optimisation of edible chitosan coatings formulations incorporating Myrcia ovata Cambessedes essential oil with antimicrobial potential against foodborne bacteria and natural microflora of mangaba fruits. LWT Food Sci. Technol. 2017, 79, 1–10. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. LWT Food Sci. Technol. 2011, 44, 2324–2327. [Google Scholar] [CrossRef]
- Oh, Y.A.; Oh, Y.J.; Song, A.Y.; Won, J.S.; Song, K.B.; Min, S.C. Comparison of effectiveness of edible coatings using emulsions containing lemongrass oil of different size droplets on grape berry safety and preservation. LWT 2017, 75, 742–750. [Google Scholar] [CrossRef]
- Lekjing, S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Wang, L.; Hu, Y.; Chen, J.; Liu, D.; Ye, X. Efficacy of chitosan-gallic acid coating on shelf life extension of refrigerated Pacific mackerel fillets. Food Bioprocess Technol. 2016, 9, 675–685. [Google Scholar] [CrossRef]
- García, M.A.; Ferrero, C.; Bértola, N.; Martino, M.; Zaritzky, N. Edible coatings from cellulose derivatives to reduce oil uptake in fried products. Innov. Food Sci. Emerg. Technol. 2002, 3, 391–397. [Google Scholar] [CrossRef]
- Suppakul, P.; Jutakorn, K.; Bangchokedee, Y. Efficacy of cellulose-based coating on enhancing the shelf life of fresh eggs. J. Food Eng. 2010, 98, 207–213. [Google Scholar] [CrossRef]
- Arnon, H.; Granit, R.; Porat, R.; Poverenov, E. Development of polysaccharides-based edible coatings for citrus fruits: A layer-by-layer approach. Food Chem. 2015, 166, 465–472. [Google Scholar] [CrossRef]
- Genevois, C.E.; De Escalada Pla, M.F.; Flores, S.K. Application of edible coatings to improve global quality of fortified pumpkin. Innov. Food Sci. Emerg. Technol. 2016, 33, 506–514. [Google Scholar] [CrossRef]
- Viña, S.Z.; Mugridge, A.; García, M.A.; Ferreyra, R.M.; Martino, M.N.; Chaves, A.R.; Zaritzky, N.E. Effects of polyvinylchloride films and edible starch coatings on quality aspects of refrigerated Brussels sprouts. Food Chem. 2007, 103, 701–709. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Ranjitha, K.; Sudhakar Rao, D.V.; Shivashankara, K.S.; Oberoi, H.S.; Roy, T.K.; Bharathamma, H. Shelf-life extension and quality retention in fresh-cut carrots coated with pectin. Innov. Food Sci. Emerg. Technol. 2017, 42, 91–100. [Google Scholar] [CrossRef]
- Kang, H.J.; Jo, C.; Kwon, J.H.; Kim, J.H.; Chung, H.J.; Byun, M.W. Effect of a pectin-based edible coating containing green tea powder on the quality of irradiated pork patty. Food Control 2007, 18, 430–435. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Wang, L.F.; Rhim, J.W. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The effect of edible coatings on the nutritional quality of ‘Bravo de Esmolfe’ fresh-cut apple through shelf-life. LWT Food Sci. Technol. 2017, 75, 210–219. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The effect of alginate-based edible coatings enriched with essential oils constituents on Arbutus unedo L. fresh fruit storage. Postharvest Biol. Technol. 2015, 100, 226–233. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Effect of sodium alginate and carboxymethyl cellulose edible coating with epigallocatechin gallate on quality and shelf life of fresh pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar] [CrossRef]
- Wu, S.; Chen, J. Using pullulan-based edible coatings to extend shelf-life of fresh-cut “Fuji” apples. Int. J. Biol. Macromol. 2013, 55, 254–257. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T.V.R. Xanthan gum based edible coating enriched with cinnamic acid prevents browning and extends the shelf-life of fresh-cut pears. LWT Food Sci. Technol. 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Feng, X.; Ng, V.K.; Mikš-Krajnik, M.; Yang, H. Effects of fish gelatin and tea polyphenol coating on the spoilage and degradation of myofibril in fish fillet during cold storage. Food Bioprocess Technol. 2017, 10, 89–102. [Google Scholar] [CrossRef]
- Feng, X.; Bansal, N.; Yang, H. Fish gelatin combined with chitosan coating inhibits myofibril degradation of golden pomfret (Trachinotus blochii) fillet during cold storage. Food Chem. 2016, 200, 283–292. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Effect of fish gelatin coating enriched with oregano essential oil on the quality of refrigerated rainbow trout fillet. J. Aquat. Food Prod. Technol. 2016, 25, 835–842. [Google Scholar] [CrossRef]
- Azarifar, M.; Ghanbarzadeh, B.; Sowti khiabani, M.; Akhondzadeh Basti, A.; Abdulkhani, A. The effects of gelatin-CMC films incorporated with chitin nanofiber and Trachyspermum ammi essential oil on the shelf life characteristics of refrigerated raw beef. Int. J. Food Microbiol. 2020. [Google Scholar] [CrossRef]
- Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Effects of edible coatings based on ultrasound-treated whey proteins in quality attributes of frozen Atlantic salmon (Salmo salar). Innov. Food Sci. Emerg. Technol. 2012, 14, 92–98. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Carrión-Granda, X.; Maté, J.I. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 2014, 36, 69–75. [Google Scholar] [CrossRef]
- Shokri, S.; Ehsani, A. Efficacy of whey protein coating incorporated with lactoperoxidase and α-tocopherol in shelf life extension of Pike-Perch fillets during refrigeration. LWT Food Sci. Technol. 2017, 85, 225–231. [Google Scholar] [CrossRef]
- Oregel-Zamudio, E.; Angoa-Pérez, M.V.; Oyoque-Salcedo, G.; Aguilar-González, C.N.; Mena-Violante, H.G. Effect of candelilla wax edible coatings combined with biocontrol bacteria on strawberry quality during the shelf-life. Sci. Hortic. 2017, 214, 273–279. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Rojas-Molina, R.; Aguilera-Carbó, A.F.; Saenz-Galindo, A.; de La Garza, H.; Jasso-Cantú, D.; Aguilar, C.N. Edible film based on candelilla wax to improve the shelf life and quality of avocado. Food Res. Int. 2009, 42, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Chiumarelli, M.; Hubinger, M.D. Stability, solubility, mechanical and barrier properties of cassava starch—Carnauba wax edible coatings to preserve fresh-cut apples. Food Hydrocoll. 2012, 28, 59–67. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Nanjappa, C.; Ashok, N.; Ravi, N.; Roopa, N.; Raju, P.S. Shellac and Aloe vera gel based surface coating for shelf life extension of tomatoes. J. Food Sci. Technol. 2013, 52, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, V.; Khan, M.S.; Jamal, Q.M.S.; Alzohairy, M.A.; Al Karaawi, M.A.; Siddiqui, M.U. Antimicrobial potential of bacteriocins: In therapy, agriculture and food preservation. Int. J. Antimicrob. Agents 2017, 49, 1–11. [Google Scholar] [CrossRef]
- Balciunas, E.M.; Castillo Martinez, F.A.; Todorov, S.D.; de Franco, B.D.G.M.; Converti, A.; de Oliveira, R.P.S. Novel biotechnological applications of bacteriocins: A review. Food Control 2013, 32, 134–142. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; de Jesus, M.B.; Kapila, Y.L. Bacterial anti-microbial peptides and nano-sized drug delivery systems: The state of the art toward improved bacteriocins. J. Control. Release 2020, 321, 100–118. [Google Scholar] [CrossRef]
- Bagde, P.; Nadanathangam, V. Mechanical, antibacterial and biodegradable properties of starch film containing bacteriocin immobilized crystalline nanocellulose. Carbohydr. Polym. 2019, 222, 115021. [Google Scholar] [CrossRef]
- Iseppi, R.; Camellini, S.; Sabia, C.; Messi, P. Combined antimicrobial use of essential oils and bacteriocin bacLP17 as seafood biopreservative to control Listeria monocytogenes both in planktonic and in sessile forms. Res. Microbiol. 2020. [Google Scholar] [CrossRef]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocin-antimicrobial synergy: A medical and food perspective. Front. Microbiol. 2017, 8, 1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, D.; Ross, R.P.; Hill, C. Developing bacteriocins of lactic acid bacteria into next generation biopreservatives. Curr. Opin. Food Sci. 2018, 20, 1–6. [Google Scholar] [CrossRef]
- Ananou, S.; Zentar, H.; Martínez-Bueno, M.; Gálvez, A.; Maqueda, M.; Valdivia, E. The impact of enterocin AS-48 on the shelf-life and safety of sardines (Sardina pilchardus) under different storage conditions. Food Microbiol. 2014, 44, 185–195. [Google Scholar] [CrossRef]
- Sofra, C.; Tsironi, T.; Taoukis, P.S. Modeling the effect of pre-treatment with nisin enriched osmotic solution on the shelf life of chilled vacuum packed tuna. J. Food Eng. 2018, 216, 125–131. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Bhandari, B.; Xu, J.; Yang, C. Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control 2020, 107, 106771. [Google Scholar] [CrossRef]
- Koné, A.P.; Zea, J.M.V.; Gagné, D.; Cinq-Mars, D.; Guay, F.; Saucier, L. Application of Carnobacterium maltaromaticum as a feed additive for weaned rabbits to improve meat microbial quality and safety. Meat Sci. 2018, 135, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Zohri, M.; Shafiee Alavidjeh, M.; Mirdamadi, S.S.; Behmadi, H.; Hossaini Nasr, S.M.; Eshghi Gonbaki, S.; Shafiee Ardestani, M.; Jabbari Arabzadeh, A. Nisin-loaded chitosan/alginate nanoparticles: A hopeful hybrid biopreservative. J. Food Saf. 2013, 33, 40–49. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, P.R. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Nigam, A.; Gupta, D.; Sharma, A. Treatment of infectious disease: Beyond antibiotics. Microbiol. Res. 2014, 169, 643–651. [Google Scholar] [CrossRef]
- Crist, C.A.; Williams, J.B.; Schilling, M.W.; Hood, A.F.; Smith, B.S.; Campano, S.G. Impact of sodium lactate and vinegar derivatives on the quality of fresh Italian pork sausage links. Meat Sci. 2014, 96, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.; Whyte, P.; Brunton, N.; Lyng, J.; Fagan, J.; Bolton, D.J. The effect of organic acid and sodium chloride dips on the shelf-life of refrigerated Irish brown crab (Cancer pagurus) meat. LWT 2018, 98, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Hong, H.; Jia, S.; Liu, Y.; Luo, Y. Effects of phytic acid and lysozyme on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets stored at 4 °C. Food Microbiol. 2020, 86, 103313. [Google Scholar] [CrossRef]
- Pattarasiriroj, K.; Kaewprachu, P.; Rawdkuen, S. Properties of rice flour-gelatine-nanoclay film with catechin-lysozyme and its use for pork belly wrapping. Food Hydrocoll. 2020, 107, 105951. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Rawdkuen, S. Quality attributes of minced pork wrapped with catechin-lysozyme incorporated gelatin film. Food Packag. Shelf Life 2015, 3, 88–96. [Google Scholar] [CrossRef]
- Shokri, S.; Ehsani, A.; Jasour, M.S. Efficacy of lactoperoxidase system-whey protein coating on shelf-life extension of rainbow trout fillets during cold storage (4 °C). Food Bioprocess Technol. 2015, 8, 54–62. [Google Scholar] [CrossRef]
- Çoban, M.Z.; Çoban, Ö.E.; Fadiloğlu, E.E.; Çoban, M.Z. Microbiological and physicochemical quality of carp sausage enriched with propolis natural extract during chilled storage microbiological and physicochemical quality of carp sausage. J. Aquat. Food Prod. Technol. 2019, 28, 960–966. [Google Scholar] [CrossRef]
- Khodanazary, A. Quality characteristics of refrigerated mackerel Scomberomorus commerson using gelatin-polycaprolactone composite film incorporated with lysozyme and pomegranate peel extract. Int. J. Food Prop. 2019, 22, 2057–2071. [Google Scholar] [CrossRef] [Green Version]
- Arulkumar, A.; Swain, B.; Paramasivam, S. Shelf life extension of sardines (Sardinella albella) using betel leaf (Piper betle) incorporated ice. Food Bioprocess Technol. 2020, 13, 1255–1260. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Q.; Ma, T.; Bao, K.; Yu, X.; Duan, Z.; Shen, X. Effect of EGCG-gelatin biofilm on the quality and microbial composition of tilapia fillets during chilled storage. Food Chem. 2020, 305, 125454. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Pan, D.D.; Cao, J.X.; Shao, X.F.; Chen, Y.J.; Sun, Y.Y.; Ou, C.R. Effect of black pepper essential oil on the quality of fresh pork during storage. Meat Sci. 2016, 117, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lahmar, A.; Morcuende, D.; Andrade, M.J.; Chekir-Ghedira, L.; Estévez, M. Prolonging shelf life of lamb cutlets packed under high-oxygen modified atmosphere by spraying essential oils from North-African plants. Meat Sci. 2018, 139, 56–64. [Google Scholar] [CrossRef]
- Chiralt, A.; Atares, L. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Fadıloğlu, E.E.; Çoban, Ö.E. Effects of chitosan edible coatings enriched with sumac on the quality and the shelf life of rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) fillets. J. Food Saf. 2018, 38, e12545. [Google Scholar] [CrossRef]
- Cilli, L.P.; Contini, L.R.F.; Sinnecker, P.; Lopes, P.S.; Andreo, M.A.; Neiva, C.R.P.; Nascimento, M.S.; Yoshida, C.M.P.; Venturini, A.C. Effects of grape pomace flour on quality parameters of salmon burger. J. Food Process. Preserv. 2020, 44, 1–11. [Google Scholar] [CrossRef]
- He, Q.; Gong, B.; He, J.; Xiao, K. A novel superchilling storage-ice glazing (SS-IG) approach using anti-oxidative and antimicrobial essential oil (EO) for freshness-keeping of sea bass (Dicentrarchus labrax). Aquaculture 2019, 500, 243–249. [Google Scholar] [CrossRef]
- Spence, C. Multisensory flavour perception. In Flavour: From Food to Perception; Guichard, E., Salles, C., Morzel, M., Le Bon, A., Eds.; John Wiley & Sons Inc.: Chichester, UK, 2017; pp. 373–394. ISBN 9781118929384. [Google Scholar]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef] [PubMed]
- Chuesiang, P.; Sanguandeekul, R.; Siripatrawan, U. Phase inversion temperature-fabricated cinnamon oil nanoemulsion as a natural preservative for prolonging shelf-life of chilled Asian seabass (Lates calcarifer) fillets. LWT Food Sci. Technol. 2020, 125, 109122. [Google Scholar] [CrossRef]
- He, Q.; Xiao, K. The effects of tangerine peel (Citri reticulatae pericarpium) essential oils as glazing layer on freshness preservation of bream (Megalobrama amblycephala) during superchilling storage. Food Control 2016, 69, 339–345. [Google Scholar] [CrossRef]
- Ozogul, F.; Oztekin, R.; Kulawik, P. Biogenic amine formation and microbiological quality of anchovy (Engraulis encrasicolus) treated with lavender and lemon balm ethanol extracts. J. Food Sci. 2017, 82, 1278–1284. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Jia, S.; Zhang, L.; Luo, Y. The effect of essential oils on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Int. J. Food Microbiol. 2018, 266, 52–59. [Google Scholar] [CrossRef]
- Özyurt, G.; Kuley, E.; Balikçi, E. Effect of the Icing with rosemary extract on the oxidative stability and biogenic amine formation in sardine (Sardinella aurita) During chilled storage. Food Bioprocess Technol. 2012, 5, 2777–2786. [Google Scholar] [CrossRef]
- Bensid, A.; Ucar, Y.; Bendeddouche, B.; Özogul, F. Effect of the icing with thyme, oregano and clove extracts on quality parameters of gutted and beheaded anchovy (Engraulis encrasicholus) during chilled storage. Food Chem. 2014, 145, 681–686. [Google Scholar] [CrossRef]
- Yavuzer, E.; Özogul, F.; Özogul, Y. Impact of icing with potato, sweet potato, sugar beet, and red beet peel extract on the sensory, chemical, and microbiological changes of rainbow trout (Oncorhynchus mykiss) fillets stored at (3 ± 1 °C). Aquac. Int. 2020, 28, 187–197. [Google Scholar] [CrossRef]
- Li, Y.; Zhuang, S.; Liu, Y.; Zhang, L.; Liu, X.; Cheng, H.; Liu, J. E ff ect of grape seed extract on quality and microbiota community of container-cultured snakehead (Channa argus) fillets during chilled storage. J. Food Microbiol. 2020, 91, 103492. [Google Scholar] [CrossRef]
- Ozogul, I.; Polat, A.; Ozogul, Y.; Boga, E.K.; Ozogul, F.; Ayas, D. Effects of laurel and myrtle extracts on the sensory, chemical and microbiological properties of vacuum-packed and refrigerated European eel (Anguilla anguilla) fillets. Int. J. Food Sci. Technol. 2014, 49, 847–853. [Google Scholar] [CrossRef]
- Lahreche, T.; Uçar, Y.; Kosker, A.R.; Hamdi, T.; Ozogul, F. Combined impacts of oregano extract and vacuum packaging on the quality changes of frigate tuna muscles stored at 3 ± 1 °C. Vet. World 2019, 12, 155–164. [Google Scholar] [CrossRef]
- Fang, S.; Zhou, Q.; Hu, Y.; Liu, F.; Mei, J.; Xie, J. Antimicrobial carvacrol incorporated in flaxseed gum-sodium alginate active films to improve the quality attributes of Chinese sea bass (Lateolabrax maculatus) during cold storage. Molecules 2019, 24, 3292. [Google Scholar] [CrossRef] [Green Version]
- Grande-tovar, C.D.; Chaves-lopez, C.; Serio, A.; Rossi, C. Chitosan coatings enriched with essential oils: Effects on fungi involved in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Junca, M.A.V.; Valencia, C.; López, E.F.; Delgado-Ospina, J.; Zapata, P.A.; Solano, M.; Grande Tovar, C.D. Chitosan beads incorporated with essential oil of Thymus capitatus: Stability studies on red Tilapia fillets. Biomolecules 2019, 9, 458. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, S.; Noori, A.; Khanzadi, S.; Fazlara, A.; Najafzadehvarzi, H. Effect of lactic acid and ajwain (Carum copticum) on the biogenic amines and quality of refrigerated common carp (Cyprinus carpio). LWT Food Sci. Technol. 2018, 97, 434–439. [Google Scholar] [CrossRef]
- Hosseini, S.V.; Hamzeh, A.; Moslemi, M.; Lashkan, A.B.; Iglesias, A.; Feás, X. Effect of Delayed icing on biogenic amines formation and bacterial contribution of iced common carp (Cyprinus carpio). Molecules 2013, 18, 15464–15473. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Cao, A.; Li, T.; Wu, X.; Xu, Y.; Li, J. Effect of the fumigating with essential oils on the microbiological characteristics and quality changes of refrigerated turbot (Scophthalmus maximus) Fillets. Food Bioprocess Technol. 2015, 8, 844–853. [Google Scholar] [CrossRef]
- Gao, M.; Feng, L.; Jiang, T.; Zhu, J.; Fu, L.; Yuan, D.; Li, J. The use of rosemary extract in combination with nisin to extend the shelf life of pompano (Trachinotus ovatus) fillet during chilled storage. Food Control 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Cao, Q.; Du, H.; Huang, Y.; Hu, Y.; You, J.; Liu, R.; Xiong, S.; Manyande, A. The inhibitory effect of chlorogenic acid on lipid oxidation of grass carp (Ctenopharyngodon idellus) during chilled storage. Food Bioprocess Technol. 2019, 12, 2050–2061. [Google Scholar] [CrossRef]
- Nie, X.; Wang, L.; Wang, Q.; Lei, J.; Hong, W.; Huang, B.; Zhang, C. Effect of a sodium alginate coating infused with tea polyphenols on the quality of fresh Japanese sea bass (Lateolabrax japonicas) Fillets. J. Food Sci. 2018, 83, 1695–1700. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, G.; Yang, Q.; Fan, X.; Lyu, S. Effects of chitosan-based coatings on storage quality of Chinese shrimp. Food Sci. Nutr. 2019, 7, 4085–4094. [Google Scholar] [CrossRef]
- Fan, W.; Sun, J.; Chen, Y.; Qiu, J.; Zhang, Y.; Chi, Y. Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chem. 2009, 115, 66–70. [Google Scholar] [CrossRef]
- Dong, Z.; Luo, C.; Guo, Y.; Ahmed, I.; Pavase, T.R.; Lv, L.; Li, Z.; Lin, H. Characterization of new active packaging based on PP/LDPE composite films containing attapulgite loaded with Allium sativum essence oil and its application for large yellow croaker (Pseudosciaena crocea) fillets. Food Packag. Shelf Life 2019, 20, 100320. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Jia, S.; Luo, Y. Antimicrobial effects of cinnamon bark oil on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Food Control 2017, 82, 316–324. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Sun, D.-W.; Cheng, J.-H.; Gao, W.-H. Recent advances for rapid identification of chemical information of muscle foods by hyperspectral imaging analysis. Food Eng. Rev. 2016, 8, 336–350. [Google Scholar] [CrossRef]
- He, H.-J.; Sun, D.-W. Microbial evaluation of raw and processed food products by visible/infrared, Raman and fluorescence spectroscopy. Trends Food Sci. Technol. 2015, 46, 199–210. [Google Scholar] [CrossRef]
- Nawrocka, A.; Lamorska, J. Determination of food quality by using spectroscopic methods. In Advances in Agrophysical Research; Grundas, S., Stępniewski, A., Eds.; IntechOpen: Rijeka, Croatia, 2013; pp. 347–367. [Google Scholar]
- Sikorska, E.; Khmelinskii, I.; Sikorski, M. Fluorescence spectroscopy and imaging instruments for food quality evaluation. Eval. Technol. Food Qual. 2019, 491–533. [Google Scholar] [CrossRef]
- Albani, J.R. Principles and Applications of Fluorescence Spectroscopy; Blackwell Publishing: Oxford, UK, 2007; ISBN 9780470692059. [Google Scholar]
- Su, W.H.; Sun, D.W. Mid-infrared (MIR) spectroscopy for quality analysis of liquid foods. Food Eng. Rev. 2019, 11, 142–158. [Google Scholar] [CrossRef]
- Sun, D.-W. Infrared Spectroscopy for Food Quality Analysis and Control; Academic Press: New York, NY, USA, 2009; ISBN 9780123741363. [Google Scholar]
- Bellamy, L. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall Ltd.: London, UK, 1975; ISBN 978-94-011-6017-9. [Google Scholar]
- Su, W.-H.; Sun, D.-W. Fourier transform infrared and Raman and hyperspectral imaging techniques for quality determinations of powdery foods: A review. Compr. Rev. Food Sci. Food Saf. 2017, 17. [Google Scholar] [CrossRef]
- Pavli, F.; Argyri, A.A.; Skandamis, P.; Nychas, G.J.; Tassou, C.; Chorianopoulos, N. Antimicrobial activity of oregano essential oil incorporated in sodium alginate edible films: Control of Listeria monocytogenes and spoilage in ham slices treated with high pressure processing. Materials 2019, 12, 3726. [Google Scholar] [CrossRef] [Green Version]
- Ammor, M.S.; Argyri, A.; Nychas, G.J.E. Rapid monitoring of the spoilage of minced beef stored under conventionally and active packaging conditions using Fourier transform infrared spectroscopy in tandem with chemometrics. Meat Sci. 2009, 81, 507–514. [Google Scholar] [CrossRef]
- Zhang, Z. Raman spectroscopic sensing in food safety and quality analysis. In Sensing Techniques for Food Safety and Quality Control; Lu, X., Ed.; RSC: London, UK, 2017; pp. 2–16. [Google Scholar]
- Sueters-Di Meo, J.; Liv, N.; Hoogenboom, J.P. Using Advanced Correlative Microscopy to Study Complex Biological Samples. Biomol. Anal. 2016. ISBN 9780470027318. [Google Scholar] [CrossRef]
- Power, A.C.; Chapman, J.; Chandra, S.; Cozzolino, D. Ultraviolet-visible spectroscopy for food quality analysis. In Evaluation Technologies for Food Quality; Zhong, J., Wang, X., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 91–104. [Google Scholar]
- Albani, J.R. AFluorescence spectroscopy in food analysis. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley and Sons: New York, NY, USA, 2012; p. 32. [Google Scholar]
- Zhang, L.; Li, Q.; Hong, H.; Luo, Y. Prevention of protein oxidation and enhancement of gel properties of silver carp (Hypophthalmichthys molitrix) surimi by addition of protein hydrolysates derived from surimi processing by-products. Food Chem. 2020, 316, 126343. [Google Scholar] [CrossRef]
- Spyros, A.; Dais, P. NMR Spectroscopy in Food Analysis; RSC: London, UK, 2012; ISBN 9781849731751. [Google Scholar]
- Ezeanaka, M.C.; Nsor-Atindana, J.; Zhang, M. Online low-field nuclear magnetic resonance (LF-NMR) and magnetic resonance imaging (MRI) for food quality optimization in food processing. Food Bioprocess Technol. 2019, 12, 1435–1451. [Google Scholar] [CrossRef]
- Belton, P.S. New methods for monitoring changes in proteins. Food Rev. Int. 1993, 9, 551–573. [Google Scholar] [CrossRef]
- Amigo, J.M.; Babamoradi, H.; Elcoroaristizabal, S. Hyperspectral image analysis. A tutorial. Anal. Chim. Acta 2015, 896, 34–51. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, R.P. Evaluation of antioxidant activity in foods with special reference to TEAC method. Am. J. Food Technol. 2013, 8, 83–101. [Google Scholar]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Brodowska, M.; Guzek, D.; Godziszewska, J.; Górska-Horczyczak, E.; Pogorzelska, E.; Sakowska, A.; Wojtasik-Kalinowska, I.; Gantner, M.; Wierzbicka, A. Cherry (Prunus cerasus cv Montmorency) extract with standardised antioxidant potential as preservative for refrigerated storage of ground pork. Int. J. Food Sci. Technol. 2017, 52, 2555–2563. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Oxidative stability of cooked pork patties incorporated with Clitoria ternatea extract (Blue Pea Flower Petal) during refrigerated storage. J. Food Process. Preserv. 2017, 41, e12751. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef]
- Al-Juhaimi, F.Y.; Almusallam, I.A.; Mohamed Ahmed, I.A.; Ghafoor, K.; Babiker, E.E. Potential of Acacia nilotica fruit flesh extract as an anti-oxidative and anti-microbial agent in beef burger. J. Food Process. Preserv. 2020, 44. [Google Scholar] [CrossRef]
- Grotta, L.; Castellani, F.; Palazzo, F.; Naceur Haouet, M.; Martino, G. Treatment optimisation and sample preparation for the evaluation of lipid oxidation in various meats through TBARs assays before analysis. Food Anal. Methods 2017, 10, 1870–1880. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Viji, P.; Binsi, P.K.; Visnuvinayagam, S.; Mohan, C.O.; Venkateshwarlu, G.; Srinivasa Gopal, T.K. Lipid Oxidation and Biochemical Quality of Indian Mackerel during Frozen Storage: Effect of Previous Treatment with Plant Extracts. J. Food Biochem. 2017, 41, e12308. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Lei, Y.; Shen, H.; Hong, H.; Yu, X.; Zhu, B.; Luo, Y. Effect of glazing and rosemary (Rosmarinus officinalis) extract on preservation of mud shrimp (Solenocera melantho) during frozen storage. Food Chem. 2019, 272, 604–612. [Google Scholar] [CrossRef]
- Tan, M.; Li, P.; Yu, W.; Wang, J.; Xie, J. Effects of glazing with preservatives on the quality changes of squid during frozen storage. Appl. Sci. 2019, 9, 3847. [Google Scholar] [CrossRef] [Green Version]
- Tao, F.; Peng, Y. A method for nondestructive prediction of pork meat quality and safety attributes by hyperspectral imaging technique. J. Food Eng. 2014, 126, 98–106. [Google Scholar] [CrossRef]
- Weng, S.; Zhu, W.; Zhang, X.; Yuan, H.; Zheng, L.; Zhao, J.; Huang, L.; Han, P. Recent advances in Raman technology with applications in agriculture, food and biosystems: A review. Artif. Intell. Agric. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Badii, F.; Howell, N.K. Effect of antioxidants, citrate, and cryoprotectants on protein denaturation and texture of frozen cod (Gadus morhua). J. Agric. Food Chem. 2002, 50, 2053–2061. [Google Scholar] [CrossRef]
- Zhang, H.; He, P.; Li, X.; Kang, H. Antioxidant effect of essential oils on RTC pork chops and its evaluation by Raman spectroscopy. J. Food Process. Preserv. 2018, 42, 1–7. [Google Scholar] [CrossRef]
- Howes, B.D.; Milazzo, L.; Droghetti, E.; Nocentini, M.; Smulevich, G. Addition of sodium ascorbate to extend the shelf-life of tuna meat fish: A risk or a benefit for consumers? J. Inorg. Biochem. 2019, 200, 110813. [Google Scholar] [CrossRef]
- Drouza, C.; Smaragda, S.; Keramidas, A.D. EPR methods applied on food analysis. In EPR Methods Applied on Food Analysis; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef] [Green Version]
- Hatzakis, E. Nuclear magnetic resonance (NMR) spectroscopy in food science: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 189–220. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Shen, Y.; Nisar, M.F.; Qi, W.; Chen, C.; Chen, J. The antifungal potential of carvacrol against Penicillium digitatum through 1H-NMR based metabolomics approach. Appl. Sci. 2019, 9, 2240. [Google Scholar] [CrossRef] [Green Version]
- Policegoudra, R.S.; Divakar, S.; Aradhya, S.M. Identification of difurocumenonol, a new antimicrobial compound from mango ginger (Curcuma amada Roxb.) rhizome. J. Appl. Microbiol. 2007, 102, 1594–1602. [Google Scholar] [CrossRef]
- Del Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Machi, L.; Santacruz, H.; Villegas-Ochoa, M.A.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Controlled release of antifungal volatiles of thyme essential oil from β-cyclodextrin capsules. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 431–441. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, P.; Fang, S.; Mei, J.; Xie, J. Preservative effects of gelatin active coating containing eugenol and higher CO2 concentration. Molecules 2020, 25, 871. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Shen, Y.; Li, N.; Mei, J.; Xie, J.; Kraśniewska, K. Application of gelatin incorporated with red pitaya peel methanol extract as edible coating for quality enhancement of crayfish (Procambarus clarkii) during refrigerated storage. J. Food Qual. 2019, 2019, 1715946. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Peng, Y.; Mei, J.; Xie, J. Effects of microencapsulated eugenol emulsions on microbiological, chemical and organoleptic qualities of farmed Japanese sea bass (Lateolabrax japonicus) during cold storage. LWT 2020, 118, 108831. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, P.; Fang, S.; Liu, W.; Mei, J.; Xie, J. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass. Coatings 2019, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Plasma enhanced-nutmeg essential oil solid liposome treatment on the gelling and storage properties of pork meat batters. J. Food Eng. 2020, 266, 109696. [Google Scholar] [CrossRef]
- Barba, F.J.; Roohinejad, S.; Ishikawa, K.; Leong, S.Y.; El-Din, A.; Bekhit, A.; Saraiva, J.A.; Lebovka, N. Electron spin resonance as a tool to monitor the influence of novel processing technologies on food properties. Trends Food Sci. Technol. 2020, 100, 77–87. [Google Scholar] [CrossRef]
- Duda, M.; Cygan, K.; Wisniewska-Becker, A. Effects of curcumin on lipid membranes: An EPR spin-label study. Cell Biochem. Biophys. 2020, 78, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Arivizhivendhan, K.V.; Mahesh, M.; Boopathy, R.; Swarnalatha, S.; Regina Mary, R.; Sekaran, G. Antioxidant and antimicrobial activity of bioactive prodigiosin produces from Serratia marcescens using agricultural waste as a substrate. J. Food Sci. Technol. 2018, 55, 2661–2670. [Google Scholar] [CrossRef]
- Sadžak, A.; Mravljak, J.; Maltar-Strmečki, N.; Arsov, Z.; Baranović, G.; Erceg, I.; Kriechbaum, M.; Strasser, V.; Přibyl, J.; Šegota, S. The structural integrity of the model lipid membrane during induced lipid peroxidation: The role of flavonols in the inhibition of lipid peroxidation. Antioxidants 2020, 9, 430. [Google Scholar] [CrossRef]
- Paari, A.; Kanmani, P.; Satishkumar, R.; Yuvaraj, N.; Pattukumar, V.; Agrawal, M.; Arul, V. The combined effect of irradiation and antioxidant packaging on shelf life extension of goat fish (Parupeneus indicus): Microbial, chemical and EPR spectral assessment. J. Food Process. Preserv. 2012, 36, 152–160. [Google Scholar] [CrossRef]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Mechanisms of radical formation in beef and chicken meat during high pressure processing evaluated by electron spin resonance detection and the addition of antioxidants. Food Chem. 2014, 150, 422–428. [Google Scholar] [CrossRef]
- Bao, Y.; Zhu, Y.; Ren, X.; Zhang, Y.; Peng, Z.; Zhou, G. Formation and inhibition of lipid alkyl radicals in roasted meat. Foods 2020, 9, 572. [Google Scholar] [CrossRef]
- Bolumar, T.; LaPeña, D.; Skibsted, L.H.; Orlien, V. Rosemary and oxygen scavenger in active packaging for prevention of high-pressure induced lipid oxidation in pork patties. Food Packag. Shelf Life 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Nissen, L.R.; Månsson, L.; Bertelsen, G.; Huynh-Ba, T.; Skibsted, L.H. Protection of dehydrated chicken meat by natural antioxidants as evaluated by electron spin resonance spectrometry. J. Agric. Food Chem. 2000, 48, 5548–5556. [Google Scholar] [CrossRef]
- Pénicaud, C.; Peyron, S.; Gontard, N.; Guillard, V. Oxygen quantification methods and application to the determination of oxygen diffusion and solubility coefficients in food. Food Rev. Int. 2012, 28, 113–145. [Google Scholar] [CrossRef]
- Yu, L.; Cheng, Z. Application of electron spin resonance (ESR) spectrometry in nutraceutical and food research. Mol. Nutr. Food Res. 2008, 52, 62–78. [Google Scholar] [CrossRef] [PubMed]




| Plant | Product | Properties | Ref. |
|---|---|---|---|
| Essential Oils | |||
| Thyme (Thymus capitatus) | Semi-skimmed ultra-high-temperature (UHT) Milk | Antimicrobial activity against Staphylococcus aureus, Bacillus licheniformis, and Enterococcus hirae Improve oxidative and fermentative stability | [51] |
| Clove (Syzygium aromaticum) Cinnamon (Cinnamomum zeylanicum), Myrtle (Myrtus communis, and Lavender (Lavandula stoechas) | UHT Milk | Antimicrobial activity against Escherichia coli | [52] |
| Trachyspermum ammi fruit | Wheat and chickpea | High antifungal activity against Aspergillus flavus and anti-aflatoxigenic activity against aflatoxin B1 | [53] |
| Mentha cardiac L. | Dry fruits | High antifungal activity against Aspergillus flavus and anti-aflatoxigenic activity against aflatoxin B1 | [54] |
| Rosemary (Rosmarinus officinalis) and basil (Ocimum basilicum L.) | Atlantic Mackerel (Scomber scombrus) fillets | Delay of the development of lipid oxidation and the formation of TVB-N. Extension of shelf-life of products of 2–5 days compared to the control samples | [40] |
| Rosemary (Rosmarinus officinalis), thyme (Thymus vulgaris), laurel (Laurus nobilis), and sage (Salvia officinalis) | Rainbow trout (Oncorhynchus mykiss) | Antimicrobial and antioxidant properties Enhancement of the organoleptic quality of fish | [55] |
| Orange (Citrus sinensis (L.) Osbeck) | Pink shrimp (Parapenaeus longirostris) | Antioxidant activity and antimicrobial activity (total viable counts, psychrotrophic bacteria, and Enterobacteriaceae family). Shelf-life extension of nearly 10 days | [56] |
| Bunium persicum | Iranian cheese | Antioxidant activity, antibacterial properties against Salmonella typhimurium, Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes | [57] |
| Thyme (Thymus vulgaris) | Semi-solid coalho cheese | Antimicrobial activity against Staphylococcus aureus and Listeria monocytogenes | [58] |
| Zataria multiflora Boiss. | Gouda cheese | Decrease in the biogenic amines content (Tyr and His) and antimicrobial activity, especially against yeasts | [59] |
| Oregano | Fresh lettuce | Antimicrobial activity against Listeria monocytogenes, Salmonella typhimurium and Escherichia coli | [60] |
| Plant Extracts | |||
| Guarana (Paullinia cupana) and pitanga (Eugenia uniflora L.) | Lamb burgers | Reduction of the lipid and protein oxidation | [61] |
| Black and green tea (Camellia sinensis L.) | Uncured pork sausages | Antioxidant activity on par with the effect of BHT without any adverse effects on sensory attributes | [62] |
| Cinnamon and clove | Baked foods | Growth inhibition of molds (Aspergillus spp., Penicilium spp.) | [63] |
| Onion and cranberry | Rabbit meat | Improve the microbial control against Pseudomonas and Enterobacteriaceae. Higher phenolic content | [64] |
| Stinging nettle leaves | Bread | Improve the antioxidant activity (DPPH), quality, and sensory profile | [65] |
| Olive (Olea europaea L.) leaves | Baked snacks | Reduction of oxidative degradation. Improvement of sensory data, antioxidant activity, and level of volatile compounds | [66] |
| Coating Material | Product | Properties | Ref. |
|---|---|---|---|
| Polysaccharides | |||
| Chitosan-based | Baby carrots | Delay of the microbial spoilage. Improvement of quality attributes: Product color and texture | [77] |
| Chitosan-green tea extract | Walnut kernel | Inhibition of lipid oxidation and fungal growth and optimization of sensory properties | [78] |
| Chitosan-gelatin | Beef | Improvement of color preservation and reduced weight loss and lipid oxidation | [79] |
| Chitosan-gelatin | White shrimp (Litopenaeus vannamei) | Shelf-life extension by decreasing the total and psychrotrophic bacteria. Decrease of lipid oxidation and improvement of texture and color | [80] |
| Chitosan-cassava starch-Myrcia ovata Cambessedes EO | Mangaba fruits | Growth inhibition of Bacillus cereus, Bacillus subtilis, and Serratia marcescens | [81] |
| Chitosan-pomegranate peel extract | White shrimp (Litopenaeus vannamei) | Retarding melanosis and color changes, enhancing texture and sensory scores during iced storage | [82] |
| Chitosan-whey protein | Ricotta cheese | Extension of shelf-life: Retard development of undesirable acidity and microbial spoilage without modifying sensory characteristics | [83] |
| Chitosan-lemongrass oil | Grape berry | Initial inhibition of Salmonella typhimurium and other microorganisms. Retention of color, total soluble solid content, and improvement of the antioxidant activity during storage | [84] |
| Chitosan clove oil | Cooked pork sausages | Inhibition of microbial growth, late lipid oxidation, and extension of the shelf-life in refrigerated storage | [85] |
| Chitosan-gallic acid | Pacific mackerel fillets | Synergistic effect during chilled storage: Inhibition of microbial growth, BA formation, lipid oxidation, and nucleotide and protein breakdown, without losing optimal sensory characteristics | [86] |
| Cellulose derivatives | Fried potatoes | Oil uptake reduction without causing differences in texture | [87] |
| Cellulose derivatives | Fresh eggs | Lower weight loss and improvement of albumen quality, changing from grade AA to A, and remaining that way during the storage period | [88] |
| Carboxymethyl cellulose (CMC)-chitosan | Citrus fruits | Improvement of fruit qualities: Firmness, weight loss, fruit gloss, fruit ripening progression, sensory evaluation, ethanol concentration, and disease incidence | [89] |
| Tapioca starch-based | Fortified pumpkin | Color, antimicrobial activity, and ascorbic acid retention were improved. | [90] |
| Starch-based | Brussels sprouts | Shelf-life extension by optimizing weight loss, surface color and texture | [91] |
| Starch-gelatin | Red Crimson grapes | Increase of biofilm mechanical strength, solubility in water, water vapor permeability, and thickness | [92] |
| Pectin-based | Fresh-cut carrots | Lower accumulation of phenolic acids (responsible for white blush) and flavonoids (responsible for astringency and bitterness) during refrigerated storage | [93] |
| Pectin-green tea powder | Pork patty | Decrease of lipid oxidation, increase of radical scavenging effects, and reduction of total aerobic bacteria | [94] |
| Pectin-oregano EO-resveratrol | Fresh pork loin | Decrease of pH, color change, lipid and protein oxidation, and inhibition of microbial growth with high oxygen modified atmosphere packaging | [95] |
| Agar-alginate-chitosan-AgNP-Grapefruit seed PE | Potatoes | UV-screening effect, antimicrobial activity against Listeria monocytogenes, and Escherichia coli. Prevention of greening during storage and formation of condensed water on the packaged film surface | [96] |
| Sodium alginate with anti-browning agents | Fresh-cut apple | Improvement of the shelf-life by reducing microbial growth and reducing the browning index | [97] |
| Sodium alginate-EO | Arbutus unedo L. Fresh fruit | Maintenance of postharvest quality (sensory and nutritional) attributes through storage. Reduction of microbial spoilage | [98] |
| Sodium alginate-CMC- epigallocatechin gallate | Fresh pork | Significant inhibitory effect on microbial growth, lipid oxidation, and improvement of sensory scores | [99] |
| K-carrageenan | Fortified pumpkin | Color, antimicrobial activity, and ascorbic acid retention were improved. | [90] |
| Pullulan | ‘Fuji’ apples | Extended shelf-life. Retarding enzymatic browning, adecrease of weight loss, inhibition of microbial growth and maintenance of firmness | [100] |
| Xanthan gum-enriched with cinnamic acid | Fresh-cut pears | Prevention of browning, lower polyphenol oxidase activity, and extension of the shelf-life | [101] |
| Proteins | |||
| Gelatin-tea polyphenol | Golden pomfret fillets | Reduction of the weight loss, pH lowering, and microbial growth inhibition. Retarding myofibril degradation during cold storage | [102] |
| Gelatin-chitosan | Golden pomfret fillets | Inhibition of myofibril degradation during cold storage | [103] |
| Gelatin-oregano EO | Rainbow trout fillets | Decrease of total volatile basic nitrogen, peroxide value, thiobarbituric acid, and microbial growth | [104] |
| Gelatin-CMC-chitin nanofibers-Trachyspermum ammi EO (Ajowan) | Refrigerated raw beef | Antimicrobial effect against psychrotrophic bacteria, Pseudomonas spp., Staphylococcus aureus, LAB, molds, and yeasts. Maintenance of chemical profile, color, and sensory properties | [105] |
| Whey protein | Frozen Atlantic salmon | Decrease of lipid oxidation of fish fillets. Increase of whiteness of cooked samples | [106] |
| Whey protein-oregano or clove EO | Chicken breast fillets | Antimicrobial effect against aerobic mesophilic bacteria, Enterobacteriaceae, total aerobic psychrotrophic bacteria, LAB, and Pseudomonas spp. | [107] |
| Whey protein-lactoperoxidase system-α-tocopherol | Pike-perch fillets | Antibacterial and antioxidant properties directed towards shelf-life extension | [108] |
| Lipids | |||
| Candelilla wax | Strawberry | Antifungal activity against Rhizopus stolonifera and extension of postharvest shelf-life | [109] |
| Candelixa wax-ellagic acid | Avocado | Antimicrobial activity against Colletotrichum gloeosporioides and improvement of quality and shelf-life | [110] |
| Carnauba wax-cassava starch | Fresh-cut apples | Positive impact on water solubility and respiration rate. Improve film mechanical properties | [111] |
| Shellac and Aloe vera gel (resins) | Tomatoes | Improved permeability of O2, CO2, and water vapor. Senescence delay and shelf-life extension | [112] |
| Spectroscopic Technique | Wavelength Limits | Type of Transition | Advantages | Limitations |
|---|---|---|---|---|
| Fluorescence | 250–750 nm | Bonding electrons in molecules | Rapid, high accuracy, sensitivity, relatively low cost | Limited to samples containing fluorophores, sample surface technique |
| Visible | 380–750 nm | Bonding electrons in molecules | Accuracy, sensitivity, relatively low cost, suitable to measure color | Effect of scattering and path length |
| Near-infrared | 750–2500 nm | Overtones and combinations of fundamental bands | Less sample preparation requirement, high sensitivity to physical structure, and presence of water | Requires reliable reference methods, low specificity, overlapped and complex spectra, dry samples preferred |
| Hyperspectral imaging | 400–1000 nm (Most common) | - | Providing spatial information (pixel-to-pixel signal) | Huge amount of data and data processing, costs |
| Mid-infrared | 2500–25000 nm | Fundamental stretching, bending, and rotating | High sensitivity to chemical compositions, distinct absorption peaks | Water interference and low light penetration |
| Raman | 750–1064 nm | Vibrational transitions | Provides structural and qualitative information, low sensitivity to water | Interference from biological fluorescence background signals, a small part of the sample is irradiated (laser spot), low sensitivity, due to weak scattering |
| Nuclear magnetic resonance | 1–1000 m | Nuclei orientation into a magnetic field | Accuracy, determination of precise structures, minimal sample preparation, spatial information (magnetic resonance imaging: MRI) | Expensive equipment, low sensitivity, overlapping signal, especially when analyzing complex mixtures |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassoun, A.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J.; Özogul, F.; Özogul, Y.; Çoban, Ö.E.; Guðjónsdóttir, M.; Barba, F.J.; Marti-Quijal, F.J.; et al. Use of Spectroscopic Techniques to Monitor Changes in Food Quality during Application of Natural Preservatives: A Review. Antioxidants 2020, 9, 882. https://doi.org/10.3390/antiox9090882
Hassoun A, Carpena M, Prieto MA, Simal-Gandara J, Özogul F, Özogul Y, Çoban ÖE, Guðjónsdóttir M, Barba FJ, Marti-Quijal FJ, et al. Use of Spectroscopic Techniques to Monitor Changes in Food Quality during Application of Natural Preservatives: A Review. Antioxidants. 2020; 9(9):882. https://doi.org/10.3390/antiox9090882
Chicago/Turabian StyleHassoun, Abdo, Maria Carpena, Miguel A. Prieto, Jesus Simal-Gandara, Fatih Özogul, Yeşim Özogul, Özlem Emir Çoban, María Guðjónsdóttir, Francisco J. Barba, Francisco J. Marti-Quijal, and et al. 2020. "Use of Spectroscopic Techniques to Monitor Changes in Food Quality during Application of Natural Preservatives: A Review" Antioxidants 9, no. 9: 882. https://doi.org/10.3390/antiox9090882