1. Introduction
The phloem vascular tissue is the predominant passageway for photosynthetically derived nutrients to be propagated around the body of the plant. Within the phloem lies the sieve tube conduit responsible for the transport of not only sugars, but also for the transmission of signals in the form of mRNA [
1], amino acids [
2] or electrical action potentials [
3]. Thus, the phloem is the critical energy transmission pipeline needed for overall plant homeostasis, as well as the coordination of defenses in such events as insect attack [
4], viral outbreak [
5], and drought stress [
6]. Although we are gaining a better understanding about both the control and motive force behind fluid movement within phloem sieve tubes, many questions remain unresolved in terms of its mechanism of action. Previous research on sugar translocation is often focused on loading near source tissue (e.g., leaves) [
7,
8,
9] or unloading near sink tissue (e.g., roots) [
10]. However, the intervening transport phloem which connects source and sink is often neglected due to the difficulty of accessing this deeply embedded tissue. The importance of the transport phloem for influencing long-distance translocation cannot be understated, as it may act as an exchange point for water and nutrients [
11], which has been modeled to significantly influence pressure profiles [
12].
An important set of experiments that have been performed on transport phloem has used the application of a heat exchanger, or cold-block, to rapidly cool a section of the stem. As early as 1912, experiments applying cold to a small section of the stem have been performed and they demonstrated an inhibitory effect on phloem transport [
13]. Indeed, the inhibition of translocation via stem cooling has been measured via carbon isotope tracing of the phloem in a variety of species [
14]. While testing 86 species of angiosperms, all dicots and 30% of monocots experienced a cold-induced reduction of translocation. Upon cooling a 10 mm section of the stem, chilling sensitive plants showed an immediate halt, but then recovered within 3–5 min of rewarming; previously, morning glory was also shown to recover after warming within seconds [
15]. A more recent study showed that in cow thistle, not only does translocation stop, but a pressure builds up in the sieve tube upstream of the cold-block [
16]. This pressure begins to decline to pre-chill levels within 10 min of chilling. The reversibility of the cold response while the cold treatment is still being applied warrants many hypotheses as to the cause of temporary cold-induced phloem blockage.
A continuous sieve tube is made up of the sieve element cells, partitioned end-to-end by sieve plates. One mechanism of cold-induced blockage is that the sieve plates become blocked after chilling due to the dispersion of p-protein filaments which clog sieve plate pores [
17]. The dispersion of these so-called forisome p-proteins has been demonstrated to expand rapidly following cooling due to a depolarization of the sieve element membrane in bean plants [
18]. However, this mechanism of sieve tube blockage does not fully explain why translocation stops in species which do not possess dispersive p-proteins that are commonly found in legumes [
14] or in poplar [
19]. In addition, forisomes in
Arabidopsis that appeared to cover sieve plates did not seem to inhibit phloem transport according to in vivo imaging [
20]. An alternative explanation is that the plasma membrane of the sieve elements is somehow disrupted due to chilling [
14,
15]. This, in turn, may hinder the ability of the sieve element membrane to retrieve assimilates and water that passively leak out along the transport pathway. Since the retrieval of water and solutes is hypothesized to be essential in maintaining mass flow [
21], it is plausible that a cold-induced disruption of the plasma membrane may impact flow. In addition, it is hypothesized that either aquaporins or solute transporters are specifically disrupted by cold [
16].
Aquaporins are intrinsic membrane-bound proteins which are primarily responsible for the passage of water across the plasmalemma or tonoplast [
22,
23]. A variety of protein isoforms exist [
24,
25], playing a role in transporting not only water, but also CO
2 [
26] and O
2 [
27]. They occur in a variety of sub-types (isoforms), including plasma membrane intrinsic proteins (PIPs) which are the major water-transporting isoforms found in plants [
24]. In response to environmental stress such as cold, aquaporins may react in multiple ways to counteract the loss of the hydraulic conductivity of the tissue that is chilled [
28]. Cold has the impact of reducing aquaporin mRNA transcript levels, while simultaneously increasing its protein abundance [
29]. In addition, aquaporins are more likely to be phosphorylated when exposed to a chilling event, which is a gating mechanism used to open the water channel [
30]. Aquaporins play an important role in mediating the hydraulic conductivity of roots in poplar [
31], and likewise respond to chilling through altered transcript abundance [
32]. However, it is important to note that both the mRNA expression and protein expression of aquaporins in response to cold may depend on the chilling tolerance of the species tested, as well as the duration of the chilling treatment [
33,
34].
Previous cold-block experiments on phloem transport have mainly focused on translocation rates using isotope tracing [
14,
15,
35] or pressure [
16]. However, no studies to date have shown the effect of aquaporin cold response within the sieve tubes. Despite work that shows how aquaporin cellular location, protein and mRNA transcript abundance change in accordance to environmental stress such as cold [
36], it is unknown how these parameters change within phloem sieve tubes. Previous work on aquaporins shows that a different pattern of localization occurs between the PIP1 and PIP2 isoforms [
37]. This work showed that PIP1s are predominantly found within internal compartments, whereas PIP2s are found to occur mainly in the plasma membrane of the sieve tubes in poplar. Although no known role has been described for PIPs occurring within internal compartments, the endomembrane likely serves as a reservoir for containing PIPs until they are needed at the plasma membrane [
38,
39,
40]. This shows that aquaporins on the plasma membrane may dynamically regulate their abundance within the sieve tubes to compensate for changes in water potential. Thus, the first objective of this study was to ascertain if localization patterns and protein abundance changed in accordance to cold-block treatment using immunohistochemistry. Next, we sought to determine mRNA transcript abundance using reverse transcription polymerase chain reaction (qrtPCR) and how this was changed according to cold-block treatment. According to the work of [
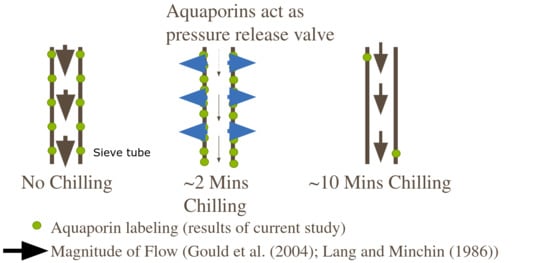
16], there was a transient increase in sieve tube pressure immediately after cold-block treatment began in cow thistle. In this previous work, after 2 min of chilling, the sieve tube pressure began recovering to pre-chill levels. The location of these previously studied effects was upstream (towards the photosynthetic source) of the cold-block. Thus, we hypothesized that aquaporins increase in protein labeling abundance at the cold-block site as well as mobilize in greater quantities in the plasma membrane to release water quickly from the sieve tubes following a chilling event. In addition, we expected mRNA transcript abundance to increase as well from stem tissue located around the site of the cold-block application. We found that mRNA transcript abundance increased for certain isoforms of PIP1 and PIP2. Meanwhile, immunohistochemistry showed that the PIP2 signal increased within 2 min in the plasma membrane of sieve tubes, but then decreased after 10 min of cold application. We discuss the implication that aquaporins are acting to regulate the sieve tube pipeline following a cold disturbance.
2. Materials and Methods
2.1. Plant Materials
Initial dormant balsam poplar (
Populus balsamifera L.) cuttings were taken from the river valley, adjacent to the University of Alberta, Edmonton, Canada (53°31′45.06′′ N, 113°31′2.88′′ W) on 29 March 2017. Cuttings of 10-cm length were prepared as described by [
41]. Cuttings originated from the separate branches of trees connected to the same root stock (thus, the genetic origin was from a single parent plant). Briefly, cuttings were soaked in tap water for two days (water was replaced between each day). Cuttings were then transferred to an equal part perlite, vermiculite, Sunshine soil mix #4 (Sun Gro Horticulture, Agawam, MA, USA). Plants were allowed to break bud within a growth chamber set at 18–21 °C and a 16 h photoperiod for 54 days before being transferred to a greenhouse (18–30 °C) for the rest of the growing season. On 31 August 2017, plants of approximately 1.3-m height were transferred to an outside growing area for their overwintering dormancy period. On 15 January, 2018, these overwintered plants were re-potted in fresh Sunshine Soil Mix #4 and transferred to a temperature-controlled growth chamber with 19 °C and 21 °C night/day temperatures, respectively, and a 16 h photoperiod. Photosynthetic photon flux was 363 umol m
−2s
−1. Trees were well watered on a daily basis, and a 20-8-8 NPK fertilizer at 200 ppm was applied on a biweekly basis. Experimental sampling began on 26 April 2018.
2.2. Cold-Block Experiment
An aluminum cold-block measuring 10 mm × 10 mm × 13 mm (l × w × h) was fabricated from the University of Alberta Physics Machine Shop to encapsulate a small section of the stem (
Figure 1A). The block was divided into two symmetrical pieces so that it could be easily placed and removed from the intact test stem. Each side of the block had 5-mm holes drilled out for the passage of room temperature or cold water. Three blocks in total were designed to accommodate 3-, 4- or 5-mm diameter stems. The block was fastened in the middle of the internode region (
Figure 1B) of the stem. Thermal paste (Céramique, Arctic Silver Inc., Visalia, CA, USA) was applied to the stem to allow for good thermal contact between the stem and cold-block. To ascertain the temperature experienced at the cold-block, a thermocouple wire was inserted into the cold-block contacting both the stem and block (blue wire,
Figure 1B). Two small screws were used to tighten the block to the stem. Inlet and outlet plastic tubing was used to deliver water to and from the cold-block. A submersible pump (Algreen Products, Cambrige, Ontario, Canada) with a rate of 757 L per hour was used to pump water into the block from a small reservoir of either room temperature or ice water. Temperature values were recorded for the warm and ice water reservoirs as well as cold-block (
Table 1). A stop-cock was used to quickly change water flow from room to ice water reservoirs or visa-versa.
Cold-block measurements were carefully undertaken to minimize the chance that phloem tissue would change because of disturbance. Of crucial importance was the minimization of phloem-induced disturbance via vibration. Even minor shaking may cause the cessation of phloem transport [
42]. Thus, any measurements taken in the subsequent experiments were allowed to settle 60 min after the cold-block was fastened to the stem to allow the translocation system to equilibrate to vibrational disturbance, as suggested by [
43]. When the cold-block was fastened to the stem, care was taken to not move or shake the plant. To make the plant accustomed to the vibration of water moving through the cold-block, room temperature water was ran through the cold-block for the initial 60 min equilibration time. After this time, cold water was run through the cold-block. As the xylem and phloem are believed to be hydraulically linked, stomatal conductance measurements were taken before and after cold water was run through the block to determine the best sampling times for subsequent experiments.
Stomatal conductance measurements were taken using a SC-1 Porometer (Decagon Devices, Pullman, Washington, DC, USA) for N = 24 test plants. For each plant, conductance measurements were taken every five minutes on the distal leaf located closest to the cold-block (
Figure 1B). A total of 12 measurements were taken during the initial 60 min equilibration time (room temperature), after which cold water was run through the block. Another 12 measurements were taken for 55 min after cold-block application. The data for these measurements are summarized (
Figure 2). The average stomatal conductance was calculated for each plant prior to cold application. The conductance value for each time sampled after cold application was then subtracted by this average pre-cold conductance value and converted to an absolute value. Note that on average, stomatal conductance was lower post cold-block application (data not shown). These stomatal conductance measurements, in addition to the phloem pressure response times generated from the Gould et al. [
16] study, were used to justify sampling times for immunolabeling and mRNA analysis. Our results indicated that deviation of stomatal conductance relative to pretreatment levels peaked after 5 min of cold application. In addition, pressure drop measurements from Gould et al. [
16] showed that a phloem translocation stoppage occurred during the first two minutes of the cold-block application. According to our results, after 10 min of the stem cooling, stomatal conductance fell closer to pre-chilled levels, and according to Gould et al. [
16], so did pressure. Previous cold-block studies have also shown a recovery of translocation after the stem was rewarmed to pre-chill temperatures. Therefore, four temperature treatments were established for use in immunolabeling and mRNA expression sampling. These treatments were, (1) control treatment: 60 min room temperature water, (2) 2-min chilling: 60 min room temperature followed by 2 min cold water, (3) 10-min chilling: 60 min room temperature followed by 10 min cold water and (4) rewarm: 60 min room temperature, and then 10 min cold, followed by 10 min room temperature water.
2.3. Fixative and RNA Stabilizer Solutions
Two solutions were made for preserving the cellular structures for immunolabeling or mRNA analysis. For immunolabeling, the stem samples were kept in chilled Formalin Acetic Acid (FAA). For mRNA analysis, the stem samples were kept in homemade RNA stabilizer solution; the solution was made by adding 117 g of ammonium sulfate and 5.56 mL of 0.75 M sodium citrate to 6.67 mL of 0.5 M EDTA adjusted to a pH of 5.2 in 250 mL of nuclease-free water.
2.4. Sampling for Immunolabeling and mRNA Expression
Sampling for each tree occurred between 11:00–11:30 each day over an 86-day period between 24 April 2018, and 13 July 2018. The sampling was taken precisely where the cold-block was applied to the stem of the tree (i.e., 13 mm of the stem covered by the block). After the treatment time was completed, the cold-block was carefully and quickly removed. The stem was then quickly cut below the nearest basal leaf, and then the section of the stem with the cold-block was trimmed using a fresh razor blade. This 13-mm stem section was cut in half and dropped into one of two solutions (a) 4 °C chilled FAA for immunolabeling or (b) RNA stabilizer for mRNA expression data. After 30 min, the samples for immunolabeling were transferred into fresh chilled FAA, and once more after 6 h. The samples for mRNA analysis were transferred to a −20 °C freezer until the start of qtPCR. For immunolabeling, a total of 6 controls and 4 plants from each experimental treatment were examined, for a total of 18.
2.5. Sectioning and Immunolabeling
The stem sections were kept in FAA fixative for at least 72 h prior to embedding. Organs were then paraffin embedded in a Leica TP 1020 tissue processor (Leica Microsystems, Wetzlar, Germany) before going into wax block molds. Longitudinal or transverse sections of 7 µm thickness were made on a rotary microtome and transferred directly onto Probe-on Plus (Fisher Scientific, Pittsburgh, Pennsylvania) slides flooded with deionized water. The slides were kept on a hotplate set to 50 °C until the sections flattened out on the water and were then placed in a drying oven for at least 24 h at 37 °C.
Immunolabeling was performed following the methodology of [
44], previously described in [
37]. Antibodies used to target aquaporins in
Arabidopsis (
AtPIP1;3) were used and targeted the 42 N-terminal sequence of this protein; this antibody had confirmed reactivity with balsam poplar aquaporin PIP1s according to Western Blot analysis [
37]. For PIP2s, antibodies were raised against the conserved 10-sequence C-terminal of this protein (with confirmed reactivity in balsam poplar, as previously demonstrated [
37]). Given the conserved nature of our PIP2 antibody, it targeted all PIP2s indiscriminately during the immunolabeling process.
To summarize the immunolabeling methodology, the slides were dewaxed in Safeclear® Xylene Substitute (Fisher Scientific), rehydrated in an ethanol series and then washed in phosphate buffered saline (PBS). After a step in post-fixative and another wash in PBS, the slides were transferred to blocking solution (BS) and then washed briefly in low-salt water washing buffer (LWB) before primary antibodies were applied. Approximately 80–100 µL of AtPIP1;3 and PIP2 aquaporin antibodies were applied to the slides. After 16–24 h of incubation at 4 °C in a dark humid container, the slides were washed in LWB and secondary antibodies were applied. Approximately 80–100 µL of Pre-absorbed [1/500] Alexa Fluor (Fisher Scientific) 488 conjugated goat anti-mouse and Alexa Fluor 568 conjugated goat anti-chicken were applied to the slides for 2 h at 37 °C. Secondary antibodies were then removed with LWB, quickly washed in double-distilled H2O and mounted in Slow Fade Gold (Fisher Scientific). Cover slips were sealed in using nail polish.
2.6. Microscopy and Image Analysis
Confocal microscopy was performed on a Zeiss LSM 700 (Carl Zeiss AG, Oberkochen, Germany) operated on Zen Black 2011 edition software. A 63x oil immersion lens was used to capture images. Laser power was set to 5.5% to excite Alexa Fluor 488 (PIP2) and to 2% to excite Alexa Fluor 568 (PIP1). Camera gain was 700 for both color channels and the pinhole diameter was set to 1 Airy unit.
For 3D Structured Illumination Microscopy (3D-SIM), images were captured as previously performed in [
37]. Briefly, longitudinal stem sections of 7 µm thickness were adhered to poly-l-lysine coated cover glass (#1.5 thickness). The sections were suspended on a small pool of water above the cover glass and allowed to flatten; heat was applied from a slide warmer below (set to 37 °C) to facilitate adherence of the tissue to the cover glass. Once the cover glass dried overnight, the tissue was processed as detailed as above, with the exception of allowing the secondary antibody to incubate for 4 h at room temperature. Once the tissue was properly labeled with PIP1 and PIP2 antibodies, a drop of Slow Fade Diamond (Fisher Scientific) mounting medium was applied, and the cover glass was sealed in using nail polish to the middle of a standard microscope glass slide. To carry out 3D-SIM microscopy, a Deltavision OMX was used. PIP1 and PIP2 fluorophores were excited using 568 nm and 488 nm laser lines, respectively. Laser power was set to 1%, and the exposure times were set to between 100 and 200 ms.
Image analysis was carried out using Image-Pro Premier Version 9.2 (Media Cybernetics, Rockville, Maryland). Transverse section images from each sampled tree were analyzed to view sieve elements. The sieve elements within each image were manually traced based upon their PIP2 outline. The use of software allowed for three main types of data to be collected for highlighted sieve elements: (1) average signal intensity (luminance µm), (2) internalization of aquaporin signal and (3) sieve element area (µm
2). Internalization of aquaporins was a categorical value (i.e., sieve element appearing with mostly internalized aquaporin signal) which was determined using the margination and heterogeneity tools of Image Pro (as performed in [
37]). Generally, if the sieve elements had margination values of ≤0.56, they were classified as having an internalized aquaporin signal; conversely, if the value was >0.56, their aquaporin signal was classified as membrane-bound.
2.7. qrtPCR Analysis
Gene transcript measurements by quantitative real-time PCR sections of the stem segments corresponding to the cold-block application (~15 mm) were collected and submerged in RNAlater stabilization solution (Ambion, USA) until further processing. The samples were always collected between 10:00 h and 11:30 h to minimize any diurnal effects on aquaporin expression.
Under a binocular microscope, >40 mg of phloem-enriched tissue was dissected by peeling off the interior layer of epidermal peels using fine forceps. Control tissue containing the remaining epidermis and the xylem tissue was also stored at −80 °C. Total RNA was extracted using the CTAB method [
45]. RNA quality was assessed on an agarose gel and quantified with a spectrophotometer (Nanodrop ND-1000, Thermo Scientific, Wilmington, DE, USA). RNA was treated as previously described [
46]. Putative stem-expressed AQP genes were selected, PtPIP1;1 (Potri.010G191900), PtPIP1;4 (Potri.006G098100), PtPIP2;4 (Potri.008G039600), PtPIP2;5 (POPTR_0006s12980) and PtPIP2;8 (POPTR_0009s01940) [
47], and specific primers (
Supplementary Table S1) were designed using the QuantPrime online tool [
48]. PCR efficiency (E) was determined from a five-point cDNA serial dilution, according to: E = 10[−1/slope]. All selected primer pairs showed correlation coefficients of R2 > 0.98 and primer efficiency values ranging between 1.92 and 2.03, and specificity was checked using melting curves. Real-time qPCR was performed on an Applied Biosystems viiA™ Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Relative gene expression was measured according to [
49], using the 2
−ΔΔCT method. The expression values were normalized to the housekeeping gene,
Elongation Factor 1B (Potri.001G224700; [
50,
51]). Relative gene expression was determined as the fold change of an AQP isoform at a given condition relative to its expression under control conditions. Real-time PCR was carried out using three biological replicates each with three technical replicates.
2.8. Data Analysis
A one-factor ANOVA was used to determine whether a significant difference existed between the cold-block treatments from the response variables of signal intensity, aquaporin internalization and sieve element area. Multiple comparisons were made for outcomes determined to be significantly different (p < 0.05) using the Tukey Test. Sigma Plot Version 13.0 (Systat Software, San Jose, California) was used to compute all statistical tests.
3. Results
Visually, immunolabeling of the stem organ sieve elements in cross-sectional view appeared to have different intensities (
Figure 3). The overall phloem area can be seen stained with aniline blue (
Figure 3A). For antibody labeled specimens, a primary antibody control (no primary antibody applied) showed only background fluorescence using confocal microscopy (
Figure 3B). In contrast, the four experimental treatments showed various intensities of PIP1 (red) and PIP2 (green) (
Figure 3C–F). The sieve elements could be identified through their labeling of PIP2 within the plasma membrane. Of interest were the moderate aquaporin intensities within the sieve elements of the control and rewarm treatment (
Figure 3C,F). In contrast to these, the 2-min chilling treatment (
Figure 3D) had very intense PIP2 antibody signals within sieve element plasma membranes whereas the 10-min chilling treatment had markedly diminished signals (
Figure 3E).
The higher resolution longitudinal stem sections viewed using 3D-SIM (
Figure 4) also showed a similar pattern to cross-sectional confocal images for sieve element aquaporin labeling. Plasma membrane labeling for the non-chilled control treatment showed high levels of PIP2 labeling of the sieve element membranes (green), with some internal membrane labeling of PIP1 (red) (
Figure 4A). After 2 min of chilling, the sieve elements showed strong plasma membrane PIP2 labeling (
Figure 4B). The 10-min chilling treatment showed markedly lower labeling response in the plasma membrane (
Figure 4C, between arrow points); meanwhile, some labeling was shown to occur within the internal membranes (
Figure 4C, asterisk), although the signal was rather weak. After stem segments were rewarmed for 10 min with room temperature water, there was a recovery of the PIP2 signal in the plasma membrane (
Figure 4D, between arrow points) and within the internal membranes (
Figure 4D, asterisk).
To ascertain if there was a quantitative difference in antibody labeling between treatments, image analysis was performed on 30 confocal cross section images taken from each sampled tree from within each treatment (N = 18). Quantitative intensities of the PIP2 signal were significantly greater in the 2-min chilling treatment than the control and 10-min chilling treatment (
Figure 5A; DF = 17, F = 5.273,
p < 0.05). Additionally, the 10-min chilling treatment had a 1.6-fold lower antibody signal than the control treatment and was on average 3.1-fold less than the 2-min chilling treatment. In contrast, the rewarm treatment was not significantly different from the other treatments. Comparing the ratio of PIP2:PIP1 signal intensity, there was no significant difference found between treatments (
Figure 5B; DF = 17, F = 0.717,
p ≥ 0.05). In terms of the sieve element cross-sectional area, there was no significant difference found between treatments (
Figure 5C; DF = 17, F = 2.362,
p ≥ 0.05). Although there was a trend towards greater internalization of aquaporins from the plasma membrane to internal membranes in the experimental treatments vs. the control, no significant difference was found (
Figure 5D; DF = 17, F = 1.138,
p ≥ 0.05).
Using qrtPCR analysis, mRNA transcript abundance was assessed between the different cold-block treatments (
Figure 6). A total of five PIP aquaporin genes and one sucrose transporter (SUT) gene was measured. The reference transcript abundance among the four treatments was made relative to the control (i.e., control transcript abundance was always 1). Overall, PIP2;4 showed the most significant treatment effect as it significantly increased 3-fold in comparison to controls for the 10-min chilling treatment and also saw a more than a 2.5-fold increase for the rewarming treatment (
Figure 6A). Although PIP2;5 did show an increase for the rewarming treatment, this was not statistically significant (
Figure 6B). In comparison, PIP2;8 showed a significant 2-fold increase over controls for the rewarming treatment (
Figure 6C). In contrast to PIP2s, overall, PIP1s did not increase substantially in response to the cold (
Figure 6D,E). However, the PIP1;4 gene did show a significant gain of over 2-fold for the 10-min chilling treatment (
Figure 6E). Finally, the sucrose transporter tested, SUT4, showed a significant ~2.5-fold increase for the rewarming treatment in comparison to the control (
Figure 6F).