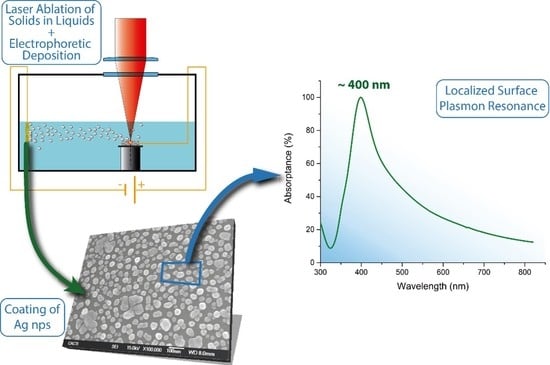
Synthesis and Deposition of Ag Nanoparticles by Combining Laser Ablation and Electrophoretic Deposition Techniques
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233–257. [Google Scholar] [CrossRef]
- Bok, Y.A.; Duoss, E.B.; Motala, M.J.; Guo, X.; Park, S.I.; Xiong, Y.; Yoon, J.; Nuzzo, R.G.; Rogers, J.A.; Lewis, J.A. Omnidirectional printing of flexible, stretchable, and spanning silver microelectrodes. Science 2009, 323, 1590–1593. [Google Scholar]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale 2014, 6, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Raiz, S.; Naseem, S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Buonsanti, R.; Llordes, A.; Aloni, S.; Helms, B.A.; Milliron, D.J. Tunable infraredabsorption and visible transparency of colloidal aluminum-doped zinc oxidenanocrystals. Nano Lett. 2011, 11, 4706–4710. [Google Scholar] [CrossRef]
- Serna, R.; Suarez-Garcia, A.; Afonso, C.N.; Babonneau, R. Optical evidence for reactive processes when embedding Cu nanoparticles in Al2O3 by pulsed laserdeposition. Nanotechnology 2006, 17, 4588–4593. [Google Scholar] [CrossRef]
- Liu, X.H.; Hou, L.X.; Wang, J.F.; Liu, B.; Yu, Z.S.; Ma, L.Q.; Yang, S.P.; Fu, G.S. Plasmonic-enhanced polymer solar cells with high efficiency by addition of silver nanoparticles of different sizes in different layers. Sol. Energy 2014, 110, 627–635. [Google Scholar] [CrossRef]
- Ho, W.J.; Lee, Y.Y.; Su, S.Y. External quantum efficiency response of thin silicon solar cell based on plasmonic scattering of indium and silver nanoparticles. Nanoscale Res. Lett. 2014, 9, 483. [Google Scholar] [CrossRef]
- Albitera, E.; Valenzuela, M.A.; Alfaro, S.; Valverde-Aguilar, G.; Martínez-Pallares, F.M. Photocatalytic deposition of Ag nanoparticles on TiO2: Metal precursor effect on the structural and photoactivity properties. J. Saudi Chem. Soc. 2015, 19, 563–573. [Google Scholar] [CrossRef]
- Liu, F.X.; Tang, C.J.; Zhan, P.; Chen, Z.; Ma, H.T.; Wang, Z.L. Released plasmonic electric field of ultrathin tetrahedral-amorphous-carbon films coated Ag nanoparticles for SERS. Sci. Rep. 2013, 4, 4494. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Saha, S.K. Fabrication of SERS substrate using nanoporous anodic alumina template decorated by silver nanoparticles. Chem. Phys. Lett. 2010, 497, 89–93. [Google Scholar] [CrossRef]
- Lee, S.M.; Choi, K.C.; Kim, D.H.; Jeon, D.Y. Localized surface plasmon enhanced cathodoluminescence from Eu3+-doped phosphor near the nanoscaled silverparticles. Opt. Express 2010, 19, 13209–13217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, J.; Fang, Y. Photoluminescence from colloidal silver nanoparticles. J. Lumin. 2008, 128, 1635–1640. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A: Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Hussain, J.I.; Kumar, S.; Hashmi, A.A.; Khan, Z. Silver nanoparticles: Preparation, characterization, and kinetics. Adv. Mater. Lett. 2011, 2, 188–194. [Google Scholar] [CrossRef]
- Guzmán, M.G.; Dille, J.; Godet, S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. IJCBE 2009, 2, 104–111. [Google Scholar]
- Athanasiou, C.E.; Bellouard, Y. A monolithic micro-tensile tester for investigating silicon dioxide polymorph micromechanics, fabricated and operated using a femtosecond laser. Micromachines 2015, 6, 1365–1386. [Google Scholar] [CrossRef]
- Zhang, J.; Gecevičius, M.; Beresna, M.; Kazansky, P.G. Seemingly unlimited lifetime data storage in nanostructured glass. Phys. Rev. Lett. 2014, 112, 033901. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Lusquiños, F.; Riveiro, A.; Comesaña, R.; Pou, J. Hydroxylapatite nanoparticles obtained by fiber laser induced fracture. Appl. Surf. Sci. 2009, 255, 5382–5385. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Rodríguez-González, B.; del Val, J.; Comesaña, R.; Lusquiños, F.; Pou, J. Laser-assisted production of spherical TiO2 nanoparticles in water. Nanotechnology 2011, 22, 195606. [Google Scholar] [CrossRef] [PubMed]
- Boutinguiza, M.; Comesaña, R.; Lusquiños, F.; Riveiro, A.; del Val, J.; Pou, J. Palladium nanoparticles produced by CW and pulsed laser ablation in water. Appl. Surf. Sci. 2014, 302, 19–23. [Google Scholar] [CrossRef]
- Okada, K. Plasma-enhanced chemical vapor deposition of nanocrystalline diamond. Sci. Technol. Adv. Mater. 2007, 8, 624–634. [Google Scholar] [CrossRef]
- Kong, Y.C.; Yu, D.P.; Zhang, B.; Fang, W.; Feng, S.Q. Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl. Phys. Lett. 2011, 78, 407–409. [Google Scholar] [CrossRef]
- Brodsky, M.H.; Cardona, M.; Cuomo, J.J. Infrared and Raman spectra of the silicon-hydrogen bonds in amorphous silicon prepared by glow discharge and sputtering. Phys. Rev. B 1977, 16, 3556–3571. [Google Scholar] [CrossRef]
- López, I.; Vázquez, A.; Hernández-Padrón, G.H.; Gómez, I. Electrophoretic deposition (EPD) of silver nanoparticles and their application as surface-enhanced Raman scattering (SERS) substrates. Appl. Surf. Sci. 2013, 280, 715–719. [Google Scholar] [CrossRef]
- Yang, G.W. Laser ablation in liquids: Applications in the synthesis of nanocrystals. Prog. Mater. Sci. 2007, 52, 648–698. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Comesaña, R.; Lusquiños, F.; Riveiro, A.; del Val, J.; Pou, J. Production of silver nanoparticles by laser ablation in open air. Appl. Surf. Sci. 2015, 336, 108–111. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Boutinguiza, M.; del Val, J.; Medina, E.; Rodríguez, D.; Riveiro, A.; Comesaña, R.; Lusquiños, F.; Gil, F.J.; Pou, J. Re-irradiation of silver nanoparticles obtained by laser ablation in water and assessment of their antibacterial effect. Appl. Surf. Sci. 2019, 473, 548–554. [Google Scholar] [CrossRef]
- Mejac, I.; Bryan, W.W.; Lee, T.R.; Tran, C.D. Visualizing the size, shape, morphology, and localized surface plasmon resonance of individual gold nanoshells by near-infrared multispectral imaging microscopy. Anal. Chem. 2009, 81, 6687–6694. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta. 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Schatz, G.C.; Hupp, J.T. Synthesis and optical properties of anisotropic metal nanoparticles. J. Fluoresc. 2004, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Thanga, D.H.; Okazaki, Y.; Nakanishi, M.; Tsuboi, Y.; Tsuji, M. Preparation of silver nanoparticles by laser ablation in polyvinylpyrrolidone solutions. Appl. Surf. Sci. 2008, 254, 5224–5230. [Google Scholar] [CrossRef]
- Krajczewski, J.; Kołataj, K.; Kudelski, A. Plasmonic nanoparticles in chemical analysis. RSC Adv. 2017, 7, 17559–17576. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.P.; Prado, A.P.; Juvêncio Keijok, W.; Ribeiro, M.R.N.; Pontes, M.J.; Nogueira, B.V.; Guimarães, M.C. A helpful method for controlled synthesis of monodisperse gold nanoparticles through response surface modelling. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Rong, W.; Ding, W.; Madler, L.; Ruoff, R.S.; Friedlander, S.K. Mechanical properties of nanoparticle chain aggregates by combined AFM and SEM: Isolated aggregates and networks. Nano Lett. 2006, 6, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry, 3rd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1997; ISBN 9780824793975. [Google Scholar]
- Yang, J.; Ren, F.; Chong, X.; Fan, D.; Chakravarty, S.; Wang, Z.; Chen, R.T.; Wang, A.X. Photonics guided-mode resonance grating with self-assembled silver nanoparticles for surface-enhanced Raman scattering spectroscopy. Photonics 2014, 1, 380–389. [Google Scholar] [CrossRef] [PubMed]







| dhkl (nm) | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Measured | 0.235 | 0.204 | 0.144 | 0.123 | 0.118 | 0.101 |
| Ag JCPDS_ICDD (1993) | 0.236 | 0.204 | 0.145 | 0.123 | 0.118 | 0.102 |
| Ag2O JCPDS_ICDD (1993) | 0.237 | – | 0.143 | – | 0.118 | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Arias, M.; Zimbone, M.; Boutinguiza, M.; Del Val, J.; Riveiro, A.; Privitera, V.; Grimaldi, M.G.; Pou, J. Synthesis and Deposition of Ag Nanoparticles by Combining Laser Ablation and Electrophoretic Deposition Techniques. Coatings 2019, 9, 571. https://doi.org/10.3390/coatings9090571
Fernández-Arias M, Zimbone M, Boutinguiza M, Del Val J, Riveiro A, Privitera V, Grimaldi MG, Pou J. Synthesis and Deposition of Ag Nanoparticles by Combining Laser Ablation and Electrophoretic Deposition Techniques. Coatings. 2019; 9(9):571. https://doi.org/10.3390/coatings9090571
Chicago/Turabian StyleFernández-Arias, Mònica, Massimo Zimbone, Mohamed Boutinguiza, Jesús Del Val, Antonio Riveiro, Vittorio Privitera, Maria G. Grimaldi, and Juan Pou. 2019. "Synthesis and Deposition of Ag Nanoparticles by Combining Laser Ablation and Electrophoretic Deposition Techniques" Coatings 9, no. 9: 571. https://doi.org/10.3390/coatings9090571