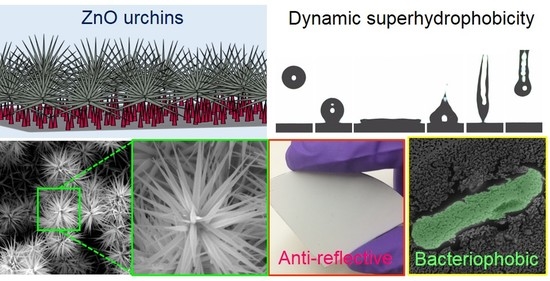
Facile Fabrication of Multifunctional ZnO Urchins on Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Preparation and Cleaning
2.2. Seeding
2.3. ZnO Urchin Growth
2.4. Bacterial Growth Conditions and Sample Preparation
2.5. Scanning Electron Microscopy (SEM)
2.6. Urchin Dimension Analysis
2.7. X-ray Diffraction (XRD)
2.8. Grazing Incidence X-ray Diffraction (GIXRD)
2.9. Contact Angle Measurement and High-Speed Imaging
2.10. Reflectance Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tang, P.; Zhang, W.; Wang, Y.; Zhang, B.; Wang, H.; Lin, C.; Zhang, L. Effect of Superhydrophobic Surface of Titanium on Staphylococcus aureus Adhesion. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial retention on superhydrophobic titanium surfaces fabricated by femtosecond laser ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural Bactericidal Surfaces: Mechanical Rupture of Pseudomonas aeruginosa Cells by Cicada Wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Phong Nguyen, T.H.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical Model of Bacterial Cell Interactions with Nanopatterned Cicada Wing Surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Webster, T.J.; Hedrick, M. Decreased bacteria density on nanostructured polyurethane. J. Biomed. Mater. Res. Part A 2014, 102, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Huang, Y.F.; Jen, Y.J.; Ganguly, A.; Chen, K.H.; Chen, L.C. Anti-reflecting and photonic nanostructures. Mater. Sci. Eng. R Rep. 2010, 69, 1–35. [Google Scholar] [CrossRef]
- Yamada, N.; Kim, O.N.; Tokimitsu, T.; Nakai, Y.; Masuda, H. Optimization of anti-reflection moth-eye structures for use in crystalline silicon solar cells. Prog. Photovolt. Res. Appl. 2011, 19, 134–140. [Google Scholar] [CrossRef]
- Park, H.; Shin, D.; Kang, G.; Baek, S.; Kim, K.; Padilla, W.J. Broadband optical antireflection enhancement by integrating antireflective nanoislands with silicon nanoconical-frustum arrays. Adv. Mater. 2011, 23, 5796–5800. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-C.; Choi, H.J.; Chang, C.-H.; Cohen, R.E.; McKinley, G.H.; Barbastathis, G. Nanotextured Silica Surfaces with Robust Superhydrophobicity and Omnidirectional Broadband Supertransmissivity. ACS Nano 2012, 6, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, W.; Hao, D.; Li, L.; Liu, H.; Lu, Z. Functional nanostructured surfaces in hybrid sol–gel glass in large area for antireflective and super-hydrophobic purposes. J. Mater. Chem. 2012, 22, 17328–17331. [Google Scholar] [CrossRef]
- Tripathy, A.; Sreedharan, S.; Bhaskarla, C.; Majumdar, S.; Peneti, S.K.; Nandi, D.; Sen, P. Enhancing the Bactericidal Efficacy of Nanostructured Multifunctional Surface Using an Ultrathin Metal Coating. Langmuir 2017, 33, 12569–12579. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, characterization, and applications of ZnO nanowires. J. Nanomater. 2012, 2012, 1–22. [Google Scholar] [CrossRef]
- Jones, F.; Tran, H.; Lindberg, D.; Zhao, L.; Hupa, M. Thermal stability of zinc compounds. Energy Fuels 2013, 27, 5663–5669. [Google Scholar] [CrossRef]
- Makarona, E.; Athanassiou, B.; Prionistis, C.; Tegou, E.; Tsamis, C. A cost-efficient solution-based process for the development of ZnO nanostructures: A comprehensive study of the role of the seeding layer formation conditions. Procedia Eng. 2015, 120, 447–450. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Tam, K.H.; Djurišić, A.B.; Chan, C.M.N.; Xi, Y.Y.; Tse, C.W.; Leung, Y.H.; Chan, W.K.; Leung, F.C.C.; Au, D.W.T. Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Films 2008, 516, 6167–6174. [Google Scholar] [CrossRef]
- Abinaya, C.; Mayandi, J.; Osborne, J.; Frost, M.; Ekstrum, C.; Pearce, J.M. Inhibition of growth of S. epidermidis by hydrothermally synthesized ZnO nanoplates. Mater. Res. Express 2017, 4, 075401. [Google Scholar] [CrossRef]
- Li, Z.; Yang, R.; Yu, M.; Bai, F.; Li, C.; Wang, Z.L.; Li, Z.; Yang, R.; Yu, M.; Bai, F.; et al. Cellular Level Biocompatibility and Biosafety of ZnO Nanowires Cellular Level Biocompatibility and Biosafety of ZnO Nanowires. J. Phys. Chem. C 2008, 112, 20114–20117. [Google Scholar] [CrossRef]
- Li, P.; Liu, H.; Lu, B.; Wei, Y. Formation mechanism of 1D ZnO nanowhiskers in aqueous solution. J. Phys. Chem. C 2010, 114, 21132–21137. [Google Scholar] [CrossRef]
- McBride, R.A.; Kelly, J.M.; McCormack, D.E. Growth of well-defined ZnO microparticles by hydroxide ion hydrolysis of zinc salts. J. Mater. Chem. 2003, 13, 1196–1201. [Google Scholar] [CrossRef]
- Wang, J.; Hou, S.; Zhang, L.; Chen, J.; Xiang, L. Ultra-rapid formation of ZnO hierarchical structures from dilution-induced supersaturated solutions. CrystEngComm 2014, 16, 7115–7123. [Google Scholar] [CrossRef]
- You, T.; Yan, J.; Zhang, Z.; Li, J.; Tian, J.; Yun, J.; Zhao, W. Fabrication and optical properties of needle-like ZnO array by a simple hydrothermal process. Mater. Lett. 2012, 66, 246–249. [Google Scholar] [CrossRef]
- Safa, S. Enhanced UV-detection properties of carbon nanotube impregnated ZnO nanourchins. Optik (Stuttg) 2015, 126, 2194–2198. [Google Scholar] [CrossRef]
- Ko, Y.H.; Yu, J.S. Tunable growth of urchin-shaped ZnO nanostructures on patterned transparent substrates. CrystEngComm 2012, 14, 5824–5829. [Google Scholar] [CrossRef]
- Imani, R.; Drašler, B.; Kononenko, V.; Romih, T.; Eleršič, K.; Jelenc, J.; Junkar, I.; Remškar, M.; Drobne, D.; Kralj-Iglič, V.; et al. Growth of a Novel Nanostructured ZnO Urchin: Control of Cytotoxicity and Dissolution of the ZnO Urchin. Nanoscale Res. Lett. 2015, 10, 441. [Google Scholar] [CrossRef]
- Banerjee, D.; Lao, J.Y.; Wang, D.Z.; Huang, J.Y.; Steeves, D.; Kimball, B.; Ren, Z.F. Synthesis and photoluminescence studies on ZnO nanowires. Nanotechnology 2004, 15, 404–409. [Google Scholar] [CrossRef]
- Wasik, P.; Redeker, C.; Dane, T.G.; Seddon, A.M.; Wu, H.; Briscoe, W.H. Hierarchical Surface Patterns upon Evaporation of a ZnO Nanofluid Droplet: Effect of Particle Morphology. Langmuir 2018, 34, 1645–1654. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.X.; Zeng, X.Q.; Ren, T.H.; Briscoe, W.H. Self-assembly in an evaporating nanofluid droplet: Rapid transformation of nanorods into 3D fibre network structures. Soft Matter 2014, 10, 5243–5248. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Tan, D.H.; Montano, M.; Goldberger, J.; Somorjai, G.; Yang, P. General route to vertical ZnO nanowire arrays using textured ZnO seeds. Nano Lett. 2005, 5, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Z.; Li, M.; Feng, Y.; Li, W.; Lv, S.; Liao, J. Synthesizing vertical porous ZnO nanowires arrays on Si/ITO substrate for enhanced photocatalysis. Ceram. Int. 2018, 44, 1291–1295. [Google Scholar] [CrossRef]
- Gacusan, J.; Kobayashi, N.P.; Sanghadasa, M.; Meyyappan, M. Controlled growth of vertical ZnO nanowires on copper substrate. Appl. Phys. Lett. 2013, 102, 083105. [Google Scholar]
- Wu, C.; Shen, L.; Yu, H.; Huang, Q.; Zhang, Y.C. Synthesis of Sn-doped ZnO nanorods and their photocatalytic properties. Mater. Res. Bull. 2011, 46, 1107–1112. [Google Scholar] [CrossRef]
- Solís-Pomar, F.; Martínez, E.; Meléndrez, M.F.; Pérez-Tijerina, E. Growth of vertically aligned ZnO nanorods using textured ZnO films. Nanoscale Res. Lett. 2011, 6, 524. [Google Scholar] [CrossRef] [PubMed]
- Gurav, A.B.; Latthe, S.S.; Vhatkar, R.S.; Lee, J.G.; Kim, D.Y.; Park, J.J.; Yoon, S.S. Superhydrophobic surface decorated with vertical ZnO nanorods modified by stearic acid. Ceram. Int. 2014, 40, 7151–7160. [Google Scholar] [CrossRef]
- Lee, Y.; Zhang, Y.; Ng, S.L.G.; Kartawidjaja, F.C.; Wang, J. Hydrothermal growth of vertical ZnO nanorods. J. Am. Ceram. Soc. 2009, 92, 1940–1945. [Google Scholar] [CrossRef]
- Wąsik, P.; Seddon, A.M.; Wu, H.; Briscoe, W.H. Dendritic surface patterns from Bénard-Marangoni instabilities upon evaporation of a reactive ZnO nanofluid droplet: A fractal dimension analysis. J. Colloid Interface Sci. 2019, 536, 493–498. [Google Scholar] [CrossRef]
- Wu, H.; Briscoe, W.H. Morphogenesis of polycrystalline dendritic patterns from evaporation of a reactive nanofluid sessile drop. Phys. Rev. Mater. 2018, 2, 045601. [Google Scholar] [CrossRef]
- Hu, J.Q.; Li, Q.; Meng, X.M.; Lee, C.S.; Lee, S.T. Thermal reduction route to the fabrication of coaxial Zn/ZnO nanocables and ZnO nanotubes. Chem. Mater. 2003, 15, 305–308. [Google Scholar] [CrossRef]
- Riaz, M.; Fulati, A.; Amin, G.; Alvi, N.H.; Nur, O.; Willander, M. Buckling and elastic stability of vertical ZnO nanotubes and nanorods. J. Appl. Phys. 2009, 106, 034309. [Google Scholar] [CrossRef]
- Sun, Y.; Riley, D.J.; Ashfbld, M.N.R. Mechanism of ZnO nanotube growth by hydrothermal methods on ZnO film-coated Si substrates. J. Phys. Chem. B 2006, 110, 15186–15192. [Google Scholar] [CrossRef]
- Katwal, G.; Paulose, M.; Rusakova, I.A.; Martinez, J.E.; Varghese, O.K. Rapid Growth of Zinc Oxide Nanotube-Nanowire Hybrid Architectures and Their Use in Breast Cancer-Related Volatile Organics Detection. Nano Lett. 2016, 16, 3014–3021. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Z. Synthesis and photoluminescence of well aligned ZnO nanotube arrays by a simple chemical solution method. J. Phys. Conf. Ser. 2009, 152, 012021. [Google Scholar] [CrossRef] [Green Version]
- Wahab, R.; Ansari, S.G.; Kim, Y.S.; Seo, H.K.; Kim, G.S.; Khang, G.; Shin, H.S. Low temperature solution synthesis and characterization of ZnO nano-flowers. Mater. Res. Bull. 2007, 42, 1640–1648. [Google Scholar] [CrossRef]
- Gokarna, A.; Parize, R.; Kadiri, H.; Nomenyo, K.; Patriarche, G.; Miska, P.; Lerondel, G. Highly crystalline urchin-like structures made of ultra-thin zinc oxide nanowires. RSC Adv. 2014, 4, 47234–47239. [Google Scholar] [CrossRef]
- Hieu, H.N.; Vuong, N.M.; Jung, H.; Jang, D.M.; Kim, D.; Kim, H.; Hong, S.-K. Optimization of a zinc oxide urchin-like structure for high-performance gas sensing. J. Mater. Chem. 2012, 22, 1127–1134. [Google Scholar] [CrossRef]
- Taheri, M.; Abdizadeh, H.; Golobostanfard, M.R. Formation of urchin-like ZnO nanostructures by sol-gel electrophoretic deposition for photocatalytic application. J. Alloys Compd. 2017, 725, 291–301. [Google Scholar] [CrossRef]
- Shen, G.; Bando, Y.; Lee, C.J. Synthesis and evolution of novel hollow ZnO urchins by a simple thermal evaporation process. J. Phys. Chem. B 2005, 109, 10578–10583. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.; Bechelany, M.; Utke, I.; Erni, R.; Hosseini, D.; Michler, J.; Philippe, L. Urchin-inspired zinc oxide as building blocks for nanostructured solar cells. Nano Energy 2012, 1, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Newton, M.C.; Warburton, P.A. ZnO tetrapod nanocrystals. Mater. Today 2007, 10, 50–54. [Google Scholar] [CrossRef]
- Modi, G. Zinc oxide tetrapod: A morphology with multifunctional applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 033002. [Google Scholar] [CrossRef]
- Neykova, N.; Brož, A.; Remeš, Z.; Hruška, K.; Kalbáčová, M.; Kromka, A.; Vaněček, M. ZnO hedgehog-like structures for control cell cultivation. Appl. Surf. Sci. 2012, 258, 3485–3489. [Google Scholar] [CrossRef]
- Bhaskarla, C.; Das, M.; Verma, T.; Kumar, A.; Mahadevan, S.; Nandi, D. Roles of Lon protease and its substrate MarA during sodium salicylate-mediated growth reduction and antibiotic resistance in Escherichia coli. Microbiology 2016, 162, 764–776. [Google Scholar] [CrossRef] [Green Version]
- Sutton, S. Measurement of microbial cells by optical density. J. Valid. Technol. 2011, 17, 46–49. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashiotis, G.; Deschildre, A.; Nawaz, Z.; Wright, J.P.; Karkoulis, D.; Picca, F.E.; Kieffer, J. The fast azimuthal integration Python library: PyFAI. J. Appl. Crystallogr. 2015, 48, 510–519. [Google Scholar] [CrossRef]
- De Gennes, P.-G.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena; Springer: New York, NY, USA, 2004; ISBN 978-1-4419-1833-8. [Google Scholar]
- Efroimsky, M.; Gamberg, L.; Gitman, D.; Lazarian, A.; Smirnov, B. Wetting of Real Surfaces: De Gruyter Studies in Mathematical Physics 19; Walter de Gruyter GmbH: Berlin, Germany, 2013; ISBN 978-311-02-58-530. [Google Scholar]
- Mittal, K. Contact Angle Wettability and Adhesion; Koninklijke Brill NV: Leiden, The Netherlands, 2009; ISBN 978-90-04-16932-6. [Google Scholar]
- Wu, X.; Bai, H.; Li, C.; Lu, G.; Shi, G. Controlled one-step fabrication of highly oriented ZnO nanoneedle/nanorods arrays at near room temperature. Chem. Commun. 2006, 15, 1655–1657. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef] [Green Version]
- Clanet, C.; Béguin, C.; Richard, D.; Quéré, D. Maximal deformation of an impacting drop. J. Fluid Mech. 2004, 517, 199–208. [Google Scholar] [CrossRef]
- Han, Z.W.; Wang, Z.; Feng, X.M.; Li, B.; Mu, Z.Z.; Zhang, J.Q.; Niu, S.C.; Ren, L.Q. Antireflective surface inspired from biology: A review. Biosurface Biotribol. 2016, 2, 137–150. [Google Scholar] [CrossRef]
- Diao, Z.; Dirks, J.-H.; Spatz, J. Bio-inspired, nanostructured anti-reflective surfaces for laser applications. In Proceedings of the 2016 Conference on Lasers and Electro-Optics (CLEO), San Jose, CA, USA, 5–10 June 2016. [Google Scholar]
- Boden, S.A.; Bagnall, D.M. Tunable reflection minima of nanostructured antireflective surfaces. Appl. Phys. Lett. 2008, 93, 133108. [Google Scholar] [CrossRef] [Green Version]
- Zada, I.; Zhang, W.; Sun, P.; Imtiaz, M.; Abbas, W.; Zhang, D. Multifunctional, Angle Dependent Antireflection, and Hydrophilic Properties of SiO2 Inspired by Nano-Scale Structures of Cicada Wings. Appl. Phys. Lett. 2017, 111, 153701. [Google Scholar] [CrossRef]
- Chen, Y.C.; Huang, Z.S.; Yang, H. Cicada-Wing-Inspired Self-Cleaning Antireflection Coatings on Polymer Substrates. ACS Appl. Mater. Interfaces 2015, 7, 25495–25505. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, J.; Ryu, M.; Seniutinas, G.; Balčytis, A.; Maximova, K.; Wang, X.; Zamengo, M.; Ivanova, E.P.; Juodkazis, S. Nanostructured antireflective and thermoisolative cicada wings. Langmuir 2016, 32, 4698–4703. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Sen, P. Dragonfly wing inspired multifunctional antireflective superhydrophobic surfaces. In Proceedings of the 2016 3rd International Conference on Emerging Electronics (ICEE), Mumbai, India, 27–30 December 2016; pp. 1–3. [Google Scholar]
- Cai, J.; Qi, L. Recent advances in antireflective surfaces based on nanostructure arrays. Mater. Horiz. 2015, 2, 37–53. [Google Scholar] [CrossRef]
- Liu, S.; Killen, E.; Lim, M.; Gunawan, C.; Amal, R. The effect of common bacterial growth media on zinc oxide thin films: Identification of reaction products and implications for the toxicology of ZnO. RSC Adv. 2014, 4, 4363–4370. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cheung, G.S.; Watson, G.S.; Watson, J.A.; Lin, S.; Schwarzkopf, L.; Green, D.W. The nanotipped hairs of gecko skin and biotemplated replicas impair and/or kill pathogenic bacteria with high efficiency. Nanoscale 2016, 8, 18860–18869. [Google Scholar] [CrossRef]
- Tripathy, A.; Pahal, S.; Mudakavi, R.J.; Raichur, A.M.; Varma, M.M.; Sen, P. Impact of Bioinspired Nanotopography on the Antibacterial and Antibiofilm Efficacy of Chitosan. Biomacromolecules 2018, 19, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Zanni, E.; Bruni, E.; Chandraiahgari, C.R.; De Bellis, G.; Santangelo, M.G.; Leone, M.; Bregnocchi, A.; Mancini, P.; Sarto, M.S.; Uccelletti, D. Evaluation of the antibacterial power and biocompatibility of zinc oxide nanorods decorated graphene nanoplatelets: New perspectives for antibiodeteriorative approaches. J. Nanobiotechnol. 2017, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Okyay, T.O.; Bala, R.K.; Nguyen, H.N.; Atalay, R.; Bayam, Y.; Rodrigues, D.F. Antibacterial properties and mechanisms of toxicity of sonochemically grown ZnO nanorods. RSC Adv. 2015, 5, 2568–2575. [Google Scholar] [CrossRef]
- Rago, I.; Chandraiahgari, C.R.; Bracciale, M.P.; De Bellis, G.; Zanni, E.; Cestelli Guidi, M.; Sali, D.; Broggi, A.; Palleschi, C.; Sarto, M.S.; et al. Zinc oxide microrods and nanorods: Different antibacterial activity and their mode of action against Gram-positive bacteria. RSC Adv. 2014, 4, 56031–56040. [Google Scholar] [CrossRef]
- Li, X.; Chen, T. Enhancement and suppression effects of a nanopatterned surface on bacterial adhesion. Phys. Rev. E 2016, 93, 052419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Y.; Gleichauf, K.; Lou, J.; Li, Q. Nanostructure on taro leaves resists fouling by colloids and bacteria under submerged conditions. Langmuir 2011, 27, 10035–10040. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.K.; Webb, H.K.; Fadeeva, E.; Chichkov, B.N.; Wu, A.H.F.; Lamb, R.; Wang, J.Y.; Crawford, R.J.; Ivanova, E.P. Air-directed attachment of coccoid bacteria to the surface of superhydrophobic lotus-like titanium. Biofouling 2012, 28, 539–550. [Google Scholar] [CrossRef]
- Stallard, C.P.; McDonnell, K.A.; Onayemi, O.D.; O’Gara, J.P.; Dowling, D.P. Evaluation of protein adsorption on atmospheric plasma deposited coatings exhibiting superhydrophilic to superhydrophobic properties. Biointerphases 2012, 7, 31. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathy, A.; Wąsik, P.; Sreedharan, S.; Nandi, D.; Bikondoa, O.; Su, B.; Sen, P.; Briscoe, W.H. Facile Fabrication of Multifunctional ZnO Urchins on Surfaces. Colloids Interfaces 2018, 2, 74. https://doi.org/10.3390/colloids2040074
Tripathy A, Wąsik P, Sreedharan S, Nandi D, Bikondoa O, Su B, Sen P, Briscoe WH. Facile Fabrication of Multifunctional ZnO Urchins on Surfaces. Colloids and Interfaces. 2018; 2(4):74. https://doi.org/10.3390/colloids2040074
Chicago/Turabian StyleTripathy, Abinash, Patryk Wąsik, Syama Sreedharan, Dipankar Nandi, Oier Bikondoa, Bo Su, Prosenjit Sen, and Wuge H. Briscoe. 2018. "Facile Fabrication of Multifunctional ZnO Urchins on Surfaces" Colloids and Interfaces 2, no. 4: 74. https://doi.org/10.3390/colloids2040074