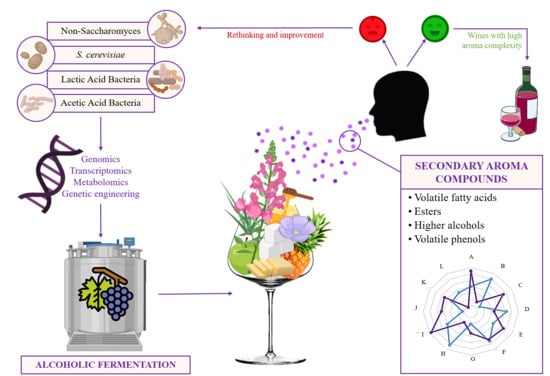
Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile
Abstract
:1. Introduction
1.1. Secondary Wine Aroma
1.2. Fermentation Implication on Wine Secondary Aroma
1.3. Microorganisms Implied in Wine Aroma
2. Compounds Involved in Secondary or Fermentative Aroma
2.1. Volatile Fatty Acids
2.2. Higher Alcohols
2.3. Esters
2.4. Volatile Phenols
3. Saccharomyces Cerevisiae
4. Non-Saccharomyces Species
4.1. Yeasts
4.1.1. Major Yeasts
Hanseniaspora/Kloeckera
Candida
4.1.2. Minor Yeasts
Rhodotorula
Pichia
Torulaspora
4.2. Bacteria
4.2.1. Lactic Acid Bacteria
4.2.2. Acetic Acid Bacteria
5. Strain Dependent Variability and Genetics Influence on Aroma Profile
6. Future Perspectives and New Approaches
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAB | Lactic Acid Bacteria |
| YAN | Yeast Assimilable Nitrogen |
| AAB | Acetic Acid Bacteria |
| 2PA | 2-Phenylethyl Acetate |
| MLF | Malolactic Fermentation |
| GC/MS | Gas Chromatography-Mass Spectrometry |
| QTL | Quantitative Trait Loci |
| HPLC | High Performance Liquid Chromatography |
| GMOs | Genetic Modified Organisms |
References
- Pereira, A.G.; Fraga, M.; Garcia-Oliveira, P.; Carpena, M.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Barros, L.; Ferreira, I.C.F.R.; Prieto, M.A.; Simal-Gandara, J. Management of Wine Aroma Compounds: Principal Basis and Future Perspectives. In Winemaking-Stabilization, Aging Chemistry and Biochemistry; IntechOpen: London, UK, 2020. [Google Scholar]
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An Approach of the Madeira Wine Chemistry. Beverages 2020, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Jeromel, A.; Korenika, A.-M.J.; Tomaz, I. 6—An Influence of Different Yeast Species on Wine Aroma Composition. In Fermented Beverages; Grumezescu, A.M., Holban, A.M.B.T.-F.B., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 171–285. ISBN 978-0-12-815271-3. [Google Scholar]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef] [PubMed]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A. Volatile flavour of wine: Correlation between instrumental analysis and sensory perception. Food Nahr. 1998, 42, 351–363. [Google Scholar] [CrossRef]
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [Green Version]
- Ribâereau-Gayon, P.; Dubourdieu, D.; Donáeche, B. Handbook of Enology: Volume 1; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Marín-San Román, S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Advancement in analytical techniques for the extraction of grape and wine volatile compounds. Food Res. Int. 2020, 137, 109712. [Google Scholar] [CrossRef]
- Blanco-Padilla, A.; Soto, K.M.; Hernández Iturriaga, M.; Mendoza, S. Food antimicrobials nanocarriers. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Van Wyk, N.; Grossmann, M.; Wendland, J.; Von Wallbrunn, C.; Pretorius, I.S. The Whiff of Wine Yeast Innovation: Strategies for Enhancing Aroma Production by Yeast during Wine Fermentation. J. Agric. Food Chem. 2019, 67, 13496–13505. [Google Scholar] [CrossRef]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Carpena, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Wine aging technology: Fundamental role of wood barrels. Foods 2020, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; García, J.F.; Sun, D.W. Advances in Wine Aging Technologies for Enhancing Wine Quality and Accelerating Wine Aging Process. Crit. Rev. Food Sci. Nutr. 2014, 54, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zohre, D.E.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process. Biochem. 2002, 38, 319–324. [Google Scholar] [CrossRef]
- Tenorio, C.; Gutie, A.R. Analysis of yeast population during spontaneous alcoholic fermentation: Effect of the age of the cellar and the practice of inoculation. Int. J. Food Microbiol. 2005, 103, 49–56. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [Green Version]
- Ciani, M.; Comitini, F. Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Rossouw, D.; Bauer, F.F. Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol. 2016, 55, 32–46. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii for secondary fermentation in sparkling wine production. Food Microbiol. 2018, 74, 100–106. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, D.; Duan, C.; Yan, G. Synergistic effect enhances 2-phenylethyl acetate production in the mixed fermentation of Hanseniaspora vineae and Saccharomyces cerevisiae. Process. Biochem. 2020, 90, 44–49. [Google Scholar] [CrossRef]
- Shi, W.K.; Wang, J.; Chen, F.S.; Zhang, X.Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT 2019, 116, 1–9. [Google Scholar] [CrossRef]
- Maturano, Y.P.; Mestre, M.V.; Kuchen, B.; Toro, M.E.; Mercado, L.A.; Vazquez, F.; Combina, M. Optimization of fermentation-relevant factors: A strategy to reduce ethanol in red wine by sequential culture of native yeasts. Int. J. Food Microbiol. 2019, 289, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.; Tsaltas, D. Contribution of Yeast in Wine Aroma and Flavour. In Yeast—Industrial Applications; IntechOpen: London, UK, 2017; pp. 117–134. [Google Scholar]
- Marsit, S.; Dequin, S. Diversity and adaptive evolution of Saccharomyces wine yeast: A review. FEMS Yeast Res. 2015, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Longin, C.; Petitgonnet, C.; Guilloux-Benatier, M.; Rousseaux, S.; Alexandre, H. Application of flow cytometry to wine microorganisms. Food Microbiol. 2017, 62, 221–231. [Google Scholar] [CrossRef]
- National Center for Biotechnology NCBI Taxonomy Browser. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi (accessed on 15 November 2020).
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef]
- Louw, L.; Tredoux, A.G.J.; Van Rensburg, P.; Kidd, M.; Naes, T.; Nieuwoudt, H.H. Fermentation-derived aroma compounds in varietal young wines from South Africa. S. Afr. J. Enol. Vitic. 2010, 31, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, D.; Peinado, R.A.; Medina, M.; Moreno, J. Higher alcohols concentration and its relation with the biological aging evolution. Eur. Food Res. Technol. 2006, 222, 629–635. [Google Scholar] [CrossRef]
- Tokpohozin, S.E.; Fischer, S.; Becker, T. Selection of a new Saccharomyces yeast to enhance relevant sorghum beer aroma components, higher alcohols and esters. Food Microbiol. 2019, 83, 181–186. [Google Scholar] [CrossRef]
- Stribny, J.; Gamero, A.; Pérez-Torrado, R.; Querol, A. Saccharomyces kudriavzevii and Saccharomyces uvarum differ from Saccharomyces cerevisiae during the production of aroma-active higher alcohols and acetate esters using their amino acidic precursors. Int. J. Food Microbiol. 2015, 205, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S.; Curtin, C.D.; Chambers, P.J. Designing wine yeast for the future. In Advances in Fermented Foods and Beverages: Improving Quality, Technologies and Health Benefits; Woodhead Publishing: Cambridge, UK, 2015; pp. 197–226. ISBN 9781782420248. [Google Scholar]
- Jouhten, P.; Ponomarova, O.; Gonzalez, R.; Patil, K.R. Saccharomyces cerevisiae metabolism in ecological context. FEMS Yeast Res. 2016, 16, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagtap, U.B.; Jadhav, J.P.; Bapat, V.A.; Pretorius, I.S. Synthetic biology stretching the realms of possibility in wine yeast research. Int. J. Food Microbiol. 2017, 252, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M.R.; Greig, D. Saccharomyces cerevisiae: A nomadic yeast with no niche? FEMS Yeast Res. 2015, 15, 1–6. [Google Scholar] [CrossRef]
- Suárez-Lepe, J.A.; Morata, A. New trends in yeast selection for winemaking. Trends Food Sci. Technol. 2012, 23, 39–50. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial terroir and food innovation: The case of yeast biodiversity in wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef]
- Hirst, M.B.; Richter, C.L. Review of aroma formation through metabolic pathways of Saccharomyces cerevisiae in beverage fermentations. Am. J. Enol. Vitic. 2016, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Di Gianvito, P.; Perpetuini, G.; Tittarelli, F.; Schirone, M.; Arfelli, G.; Piva, A.; Patrignani, F.; Lanciotti, R.; Olivastri, L.; Suzzi, G.; et al. Impact of Saccharomyces cerevisiae strains on traditional sparkling wines production. Food Res. Int. 2018, 109, 552–560. [Google Scholar] [CrossRef]
- Capece, A.; Granchi, L.; Guerrini, S.; Mangani, S.; Romaniello, R.; Vincenzini, M.; Romano, P. Diversity of Saccharomyces cerevisiae strains isolated from two Italian wine-producing regions. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Comitini, F.; Capece, A.; Ciani, M.; Romano, P. New insights on the use of wine yeasts. Curr. Opin. Food Sci. 2017, 13, 44–49. [Google Scholar] [CrossRef]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional microbial signatures positively correlate with differential wine phenotypes: Evidence for a microbial aspect to terroir. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.M.; Dequin, S.; Mouret, J.R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Morales, P. Wine secondary aroma: Understanding yeast production of higher alcohols. Microb. Biotechnol. 2017, 10, 1449–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Wang, J.; Ji, X.; Liu, R.; Chen, F. Selection of non-Saccharomyces yeasts for orange wine fermentation based on their enological traits and volatile compounds formation. J. Food Sci. Technol. 2018, 55, 4001–4012. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.; Manzanares, P.; Gil, V.; Pinaga, F. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef]
- Carrau, F. Native yeasts for low input winemaking: Searching for wine diversity and increased complexity. In Advances in Applied Microbiology V. 111; Academic Press: Cambridge, MA, USA, 2006; pp. 33–39. [Google Scholar]
- Benito, Á.; Calder, F.; Benito, S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Martin, V.; Giorello, F.; Farin, L.; Minteguiaga, M.; Salzman, V.; Boido, E.; Aguilar, P.S.; Gaggero, C.; Dellacassa, E.; Mas, A.; et al. De Novo Synthesis of Benzenoid Compounds by the Yeast Hanseniaspora vineae Increases the Flavor Diversity of Wines. J. Agric. Food Chem. 2016, 64, 4574–4583. [Google Scholar] [CrossRef] [Green Version]
- Martin, V.; Boido, E.; Giorello, F.; Mas, A.; Dellacassa, E.; Carrau, F. Effect of yeast assimilable nitrogen on the synthesis of phenolic aroma compounds by Hanseniaspora vineae strains. Yeast 2016, 33, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Moreira, N.; Mendes, F.; de Guedes Pinho, P.; Hogg, T.; Vasconcelos, I. Heavy sulphur compounds, higher alcohols and esters production profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii grown as pure and mixed cultures in grape must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Mendes-Faia, A.; Lage, P.; Mira, N.P.; Mendes-Ferreira, A. Genomic expression program of Saccharomyces cerevisiae along a mixed-culture wine fermentation with Hanseniaspora guilliermondii. Microb. Cell Fact. 2015, 14, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, Y.; Zhang, B.Q.; Duan, C.Q.; Yan, G.L. Effects of different pre-fermentation cold maceration time on aroma compounds of Saccharomyces cerevisiae co-fermentation with Hanseniaspora opuntiae or Pichia kudriavzevii. LWT Food Sci. Technol. 2018, 92, 177–186. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Xu, Y.H.; Tao, Y.S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- De Benedictis, M.; Bleve, G.; Tristezza, M.; Tufariello, M.; Grieco, F. An optimized procedure for the enological selection of non-Saccharomyces starter cultures. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 2011, 99, 189–200. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; del Portillo, M.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of fermentation and wines produced by inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Englezos, V.; Torchio, F.; Cravero, F.; Marengo, F.; Giacosa, S.; Gerbi, V.; Rantsiou, K.; Rolle, L.; Cocolin, L. Aroma profile and composition of Barbera wines obtained by mixed fermentations of Starmerella bacillaris (synonym Candida zemplinina) and Saccharomyces cerevisiae. LWT Food Sci. Technol. 2016, 73, 567–575. [Google Scholar] [CrossRef]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Calabretti, A.; La Cara, F.; Sorrentino, A.; Di Stasio, M.; Santomauro, F.; Rastrelli, L.; Gabrielli, L.; Limone, F.; Volpe, M.G. Characterization of volatile fraction of typical Irpinian wines fermented with a new starter yeast. World J. Microbiol. Biotechnol. 2012, 28, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Zhu, X.L.; Mu, H.; Ma, Y.; Ullah, N.; Tao, Y.S. A novel extracellular glycosidase activity from Rhodotorula mucilaginosa: Its application potential in wine aroma enhancement. Lett. Appl. Microbiol. 2016, 62, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Li, A.H.; Dizy, M.; Ullah, N.; Sun, W.X.; Tao, Y.S. Evaluation of aroma enhancement for “Ecolly” dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017, 228, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Martínez-garcía, R.; García-martínez, T.; Puig-pujol, A.; Carlos, J.; Moreno, J. Changes in sparkling wine aroma during the second fermentation under CO2 pressure in sealed bottle. Food Chem. 2017, 237, 1030–1040. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; Moine, V.; Thibon, C.; Bely, M. Enhanced 3-sulfanylhexan-1-ol production in sequential mixed fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae reveals a situation of synergistic interaction between two industrial strains. Front. Microbiol. 2016, 7, 293. [Google Scholar] [CrossRef] [Green Version]
- Egue, L.A.N.; Bouatenin, J.K.M.; Florent, K.N.; Koussemon-camara, M. Microbial Pathogenesis Virulence factors and determination of antifungal susceptibilities of Candida species isolated from palm wine and sorghum beer. Microb. Pthogenes 2018, 124, 5–10. [Google Scholar] [CrossRef]
- Morrison-whittle, P.; Lee, S.A.; Fedrizzi, B.; Goddard, M.R. Co-evolution as Tool for Diversifying Flavor and Aroma Profiles of Wines. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Soden, A.; Francis, I.L.; Oakey, H.; Henschke, P.A. Effects of co-fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Aust. J. Grape Wine Res. 2000, 6, 21–30. [Google Scholar] [CrossRef]
- Rocha, M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine Assessment of the identification of the would-be impact odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Gambetta, J.M.; Jeffery, D.W.; Grbin, P.R.; Jiranek, V. Lower-alcohol wines produced by Metschnikowia pulcherrima and Saccharomyces cerevisiae co-fermentations: The effect of sequential inoculation timing. Int. J. Food Microbiol. 2020, 329, 108651. [Google Scholar] [CrossRef] [PubMed]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef]
- Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Co. Front. Microbiol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Otero, R.R.C.; Pe, A.I.B. Optimization of a rapid method for studying the cellular location of b -glucosidase activity in wine yeasts. J. Appl. Microbiol. 2005, 558–564. [Google Scholar] [CrossRef]
- Prior, K.J.; Bauer, F.F.; Divol, B. The utilisation of nitrogenous compounds by commercial non-Saccharomyces yeasts associated with wine. J. Food Microbiol. 2019, 79, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Renault, P.; Miot-Sertier, C.; Marullo, P.; Hernández-Orte, P.; Lagarrigue, L.; Lonvaud-Funel, A.; Bely, M. Genetic characterization and phenotypic variability in Torulaspora delbrueckii species: Potential applications in the wine industry. Int. J. Food Microbiol. 2009, 134, 201–210. [Google Scholar] [CrossRef]
- Ferrando, N.; Araque, I.; Ortís, A.; Thornes, G.; Bautista-Gallego, J.; Bordons, A.; Reguant, C. Evaluating the effect of using non-Saccharomyces on Oenococcus oeni and wine malolactic fermentation. Food Res. Int. 2020, 138, 1–9. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A. Lactic Acid Bacteria and Malolactic Fermentation in Wine. In Biotechnology of Lactic Acid Bacteria: Novel Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 231–247. [Google Scholar]
- Cappello, M.S.; Zapparoli, G.; Logrieco, A.; Bartowsky, E.J. Linking wine lactic acid bacteria diversity with wine aroma and flavour. Int. J. Food Microbiol. 2017, 243, 16–27. [Google Scholar] [CrossRef]
- Dicks, L.M.T.; Dellaglio, F.; Collins, M.D. Proposal to reclassify Leuconostoc oenos as Oenococcus oeni [corrig.] gen. nov., comb. nov. Int. J. Syst. Bacteriol. 1995, 45, 395–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez-Ozaeta, I.; Lavilla, M.; Amárita, F. Technological characterisation of potential malolactic starters from Rioja Alavesa winemaking region. LWT 2020, 134, 1–8. [Google Scholar] [CrossRef]
- Badotti, F.; Moreira, A.P.B.; Tonon, L.A.C.; de Lucena, B.T.L.; de Gomes, F.C.O.; Kruger, R.; Thompson, C.C.; de Morais, M.A.; Rosa, C.A.; Thompson, F.L. Oenococcus alcoholitolerans sp. nov., a lactic acid bacteria isolated from cachaça and ethanol fermentation processes. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 2014, 106, 1259–1267. [Google Scholar] [CrossRef]
- Endo, A.; Okada, S. Oenococcus kitaharae sp. nov., a non-acidophilic and non-malolactic-fermenting oenococcus isolated from a composting distilled shochu residue. Int. J. Syst. Evol. Microbiol. 2006, 56, 2345–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pripis-Nicolau, L.; De Revel, G.; Bertrand, A.; Lonvaud-Funel, A. Methionine catabolism and production of volatile sulphur compounds by Oenococcus œni. J. Appl. Microbiol. 2004, 96, 1176–1184. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum strategies and performances of malolactic starter lactobacillus plantarum M10: Impact on chemical and sensorial characteristics of fiano wine. Microorganisms 2020, 8, 516. [Google Scholar] [CrossRef] [Green Version]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT Food Sci. Technol. 2016, 73, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Orte, P.; Cersosimo, M.; Loscos, N.; Cacho, J.; Garcia-Moruno, E.; Ferreira, V. Aroma development from non-floral grape precursors by wine lactic acid bacteria. Food Res. Int. 2009, 42, 773–781. [Google Scholar] [CrossRef]
- Devi, A.; Anu-Appaiah, K.A. Mixed malolactic co-culture (Lactobacillus plantarum and Oenococcus oeni) with compatible Saccharomyces influences the polyphenolic, volatile and sensory profile of Shiraz wine. LWT 2021, 135, 110246. [Google Scholar] [CrossRef]
- Costello, P.J.; Siebert, T.E.; Solomon, M.R.; Bartowsky, E.J. Synthesis of fruity ethyl esters by acyl coenzyme A: Alcohol acyltransferase and reverse esterase activities in Oenococcus oeni and Lactobacillus plantarum. J. Appl. Microbiol. 2013, 114, 797–806. [Google Scholar] [CrossRef]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Rauhut, D.; Du Toit, M. Influence of pH and ethanol on malolactic fermentation and volatile aroma compound composition in white wines. LWT Food Sci. Technol. 2011, 44, 2077–2086. [Google Scholar] [CrossRef]
- Lytra, G.; Miot-Sertier, C.; Moine, V.; Coulon, J.; Barbe, J.C. Influence of must yeast-assimilable nitrogen content on fruity aroma variation during malolactic fermentation in red wine. Food Res. Int. 2020, 135, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, H.; Jiang, D.; Zhang, Y.; Zhang, S.; Sun, S. Effect of Saccharomyces cerevisiae, Torulaspora delbrueckii and malolactic fermentation on fermentation kinetics and sensory property of black raspberry wines. Food Microbiol. 2020, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Su, W.; Mu, Y.; Jiang, L.; Mu, Y. Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res. Int. 2020, 138, 109800. [Google Scholar] [CrossRef] [PubMed]
- Antalick, G.; Perello, M.C.; De Revel, G. Characterization of fruity aroma modifications in red wines during malolactic fermentation. J. Agric. Food Chem. 2012, 60, 12371–12383. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Luo, Y.; Zhou, Y.; Bianba, C.; Guo, H.; Zhao, Y.; Fu, H. Exploring microbial dynamics associated with flavours production during highland barley wine fermentation. Food Res. Int. 2020, 130, 108971. [Google Scholar] [CrossRef]
- Roda, A.; Lucini, L.; Torchio, F.; Dordoni, R.; De Faveri, D.M.; Lambri, M. Metabolite profiling and volatiles of pineapple wine and vinegar obtained from pineapple waste. Food Chem. 2017, 229, 734–742. [Google Scholar] [CrossRef]
- Jiang, L.; Su, W.; Mu, Y.; Mu, Y. Major Metabolites and Microbial Community of Fermented Black Glutinous Rice Wine with Different Starters. Front. Microbiol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Bartolomé Suáldea, B.; Victoria Moreno-Arribas, M. Malolactic Fermentation. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 85–98. ISBN 9780128144008. [Google Scholar]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; La Hens, D.V.; Hollmann, A.; Delfederico, L.; Caballero, A.; Tymczyszyn, E.E.; Semorile, L. Comparative vinification assays with selected Patagonian strains of Oenococcus oeni and Lactobacillus plantarum. LWT Food Sci. Technol. 2017, 77, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Brizuela, N.; Tymczyszyn, E.E.; Semorile, L.C.; Valdes La Hens, D.; Delfederico, L.; Hollmann, A.; Bravo-Ferrada, B. Lactobacillus plantarum as a malolactic starter culture in winemaking: A new (old) player? Electron. J. Biotechnol. 2019, 38, 10–18. [Google Scholar] [CrossRef]
- Balmaseda, A.; Rozès, N.; Leal, M.Á.; Bordons, A.; Reguant, C. Impact of changes in wine composition produced by non-Saccharomyces on malolactic fermentation. Int. J. Food Microbiol. 2021, 337, 108954. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.T.; Schopp, L.M.; Edwards, C.G.; Osborne, J.P. Impact of Pediococcus spp. on pinot noir wine quality and growth of Brettanomyces. Am. J. Enol. Vitic. 2016, 67, 188–198. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, B.; Kaur, G.; Chakraborty, D.; Kaur, K. Application of recombinant Pediococcus acidilactici BD16 (fcs+/ech+) in malolactic fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 3015–3028. [Google Scholar] [CrossRef] [PubMed]
- Matei, F.; Kosseva, M.R. Microbiology of Fruit Wine Production. In Science and Technology of Fruit Wine Production; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 73–103. ISBN 9780128010341. [Google Scholar]
- Rollán, G.C.; Farías, M.E.; De Nadra, M.C.M. Characterization of two extracellular proteases from Leuconostoc oenos. World J. Microbiol. Biotechnol. 1995, 11, 153–155. [Google Scholar] [CrossRef]
- Bekal, S.; Van Beeumen, J.; Samyn, B.; Garmyn, D.; Henini, S.; Diviès, C.; Prévost, H. Purification of Leuconostoc mesenteroides citrate lyase and cloning and characterization of the citCDEFG gene cluster. J. Bacteriol. 1998, 180, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Mateo, E.; Torija, M.J.; Mas, A.; Bartowsky, E.J. Acetic acid bacteria isolated from grapes of South Australian vineyards. Int. J. Food Microbiol. 2014, 178, 98–106. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Xia, D.; Gibson, R.L.; Fleet, G.H.; Henschke, P.A. Spoilage of bottled red wine by acetic acid bacteria. Lett. Appl. Microbiol. 2003. [Google Scholar] [CrossRef] [Green Version]
- Nurgel, C.; Pickering, G.J.; Inglis, D.L. Sensory and chemical characteristics of Canadian ice wines. J. Sci. Food Agric. 2004, 84, 1675–1684. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. Acetic acid bacteria spoilage of bottled red wine—A review. Int. J. Food Microbiol. 2008, 125, 60–70. [Google Scholar] [CrossRef]
- Valera, M.J.; Torija, M.J.; Mas, A.; Mateo, E. Acetobacter malorum and Acetobacter cerevisiae identification and quantification by Real-Time PCR with TaqMan-MGB probes. Food Microbiol. 2013, 36, 30–39. [Google Scholar] [CrossRef]
- Valera, M.J.; Laich, F.; González, S.S.; Torija, M.J.; Mateo, E.; Mas, A. Diversity of acetic acid bacteria present in healthy grapes from the Canary Islands. Int. J. Food Microbiol. 2011, 151, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Shamala, T.R.; Sreekantiah, K.R. Microbiological and biochemical studies on traditional Indian palm wine fermentation. Food Microbiol. 1988, 5, 157–162. [Google Scholar] [CrossRef]
- Hommel, R.K. Gluconobacter. In Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2014; Volume 2, pp. 99–105. ISBN 9780123847331. [Google Scholar]
- Amaresan, N.; Annapurna, K.; Sankaranarayanan, A.M.; Senthil Kumar, K.K. (Eds.) Mitesh Dwivedi Gluconobacter. In Beneficial Microbes in Agro-Ecology; Academic Press: Cambridge, MA, USA, 2020; pp. 521–544. ISBN 978-0-12-823414-3. [Google Scholar]
- Hyma, K.E.; Saerens, S.M.; Verstrepen, K.J.; Fay, J.C. Divergence in wine characteristics produced by wild and domesticated strains of Saccharomyces cerevisiae. FEMS Yeast Res. 2011, 11, 540–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belda, I.; Gobbi, A.; Ruiz, J.; de Celis, M.; Ortiz-Álvarez, R.; Acedo, A.; Santos, A. Microbiomics to Define Wine Terroir. In Comprehensive Foodomics; Cifuentes, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 438–451. ISBN 978-0-12-816396-2. [Google Scholar]
- Tilloy, V.; Cadière, A.; Ehsani, M.; Dequin, S. Reducing alcohol levels in wines through rational and evolutionary engineering of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2015, 213, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Salinas, F.; Cubillos, F.A.; Soto, D.; Garcia, V.; Bergström, A.; Warringer, J.; Ganga, M.A.; Louis, E.J.; Liti, G.; Martinez, C. The Genetic Basis of Natural Variation in Oenological Traits in Saccharomyces cerevisiae. PLoS ONE 2012, 7, e49640. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Torrado, R.; Querol, A.; Guillamón, J.M. Genetic improvement of non-GMO wine yeasts: Strategies, advantages and safety. Trends Food Sci. Technol. 2015, 45, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Zhu, J.; Niu, Z.; Shi, G.; Liu, Z.; Li, K.; Guo, X. Genetic diversity and association study of aromatics in grapevine. J. Am. Soc. Hortic. Sci. 2017, 142, 225–231. [Google Scholar] [CrossRef]
- Mendes, I.; Sanchez, I.; Franco-Duarte, R.; Camarasa, C.; Schuller, D.; Dequin, S.; Sousa, M.J. Integrating transcriptomics and metabolomics for the analysis of the aroma profiles of Saccharomyces cerevisiae strains from diverse origins. BMC Genom. 2017, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, C.; García-Martínez, J.; Pérez-Ortín, J.E.; Mendes-Ferreira, A. Comparative transcriptomic analysis reveals similarities and dissimilarities in Saccharomyces cerevisiae wine strains response to nitrogen availability. PLoS ONE 2015, 10, e0122709. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Wang, Y.; Ye, D.; Duan, L.; Duan, C.; Yan, G. Effect of the addition of branched-chain amino acids to non-limited nitrogen synthetic grape must on volatile compounds and global gene expression during alcoholic fermentation. Aust. J. Grape Wine Res. 2018, 24, 197–205. [Google Scholar] [CrossRef]
- Katarína, F.; Katarína, M.; Katarína, Ď.; Ivan, Š.; Fedor, M. Influence of yeast strain on aromatic profile of Gewürztraminer wine. LWT Food Sci. Technol. 2014, 59, 256–262. [Google Scholar] [CrossRef]
- Tronchoni, J.; Curiel, J.A.; Morales, P.; Torres-Pérez, R.; Gonzalez, R. Early transcriptional response to biotic stress in mixed starter fermentations involving Saccharomyces cerevisiae and Torulaspora delbrueckii. Int. J. Food Microbiol. 2017, 241, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Tondini, F.; Lang, T.; Chen, L.; Herderich, M.; Jiranek, V. Linking gene expression and oenological traits: Comparison between Torulaspora delbrueckii and Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2019, 294, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; de Celis, M.; de Toro, M.; Mendes-Ferreira, A.; Rauhut, D.; Santos, A.; Belda, I. Phenotypic and transcriptional analysis of Saccharomyces cerevisiae during wine fermentation in response to nitrogen nutrition and co-inoculation with Torulaspora delbrueckii. Food Res. Int. 2020, 137, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Massonnet, M.; Cantu, D. The genetic basis of grape and wine aroma. Hortic. Res. 2019, 6, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Arribas, M.V.; Polo, M.C. Winemaking biochemistry and microbiology: Current knowledge and future trends. Crit. Rev. Food Sci. Nutr. 2005, 45, 265–286. [Google Scholar] [CrossRef]
- Schmidtke, L.M.; Blackman, J.W.; Agboola, S.O. Production technologies for reduced alcoholic wines. J. Food Sci. 2012, 77. [Google Scholar] [CrossRef]
- Ozturk, B.; Anli, E. Different techniques for reducing alcohol levels in wine: A review. BIO Web Conf. 2014, 3, 02012. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bordiga, M.; Rinaldi, M.; Locatelli, M.; Piana, G.; Travaglia, F.; Coïsson, J.D.; Arlorio, M. Characterization of Muscat wines aroma evolution using comprehensive gas chromatography followed by a post-analytic approach to 2D contour plots comparison. Food Chem. 2013, 140, 57–67. [Google Scholar] [CrossRef]



| Aromatic Class | Main Compounds | Desirable Concentration | Sensorial Properties | Producer Organism | Ref. |
|---|---|---|---|---|---|
| Fatty acids | Acetic acid, pentanoic acid, hexanoic acid, octanoic acid, decanoic acid, 9-Decenoic acid, 3-methylbutanoic acid, sobutyric acid | 200–700 mg/L | In excessive amount: rancid, greasy, and cheesy notes | S. cerevisiae, P. fermentas, C. zemplinina, H. guilliermondii, H. vineae, H. uvarum | [3,19,31] |
| Higher alcohols | 1-Propanol-isobutanol, isoamyl alcohol, 2-Phenylethanol, tyrosol, tryptophol, 2-methylbutanol-1, 3-methyl-1-butanol-1 | <300 mg/L | Floral, honey, and fruity notes (<300 mg/L). Pungent aroma (>400 mg/L) | S. cerevisiae, C. zemplinin, H. uvarum, H. osmophila, H. guilliermondii, P. anomala, P. membranifaciens | [2,3,5,8,19] |
| Esters | Ethyl hexanoate, ethyl octanoate, ethyl decanoate, ethyl acetate, isobutyl acetate, amyl acetate, hexyl acetate, 2PA, isoamyl acetate | 150–200 mg/L | Fruity aroma, including banana or apple, honey, and floral tones | S. cerevisiae, Candida, Hansenula, Pichia | [2,3,7,19] |
| Phenolics | 4-Vinyl guajacol, 4-Vinylphenol | - | Sweet vanillin aroma | LAB | [1,7,31] |
| Yeast | Compounds | Matrix | Aroma (Odour Descriptor) | Ref. |
|---|---|---|---|---|
| H. uvarum and C. stellata | Benzyl alcohol | Cabernet sauvignon wine | Chocolate, fig and tobacco | [56] |
| H. vineae | Beta-phenylethyl acetate | Red wine from Uruguay (Tannat cultivar) | Intense fruity | [54,56] |
| H. vineae | P-hydroxybenzyl | Wine | Fruity, coconut, woody, vanilla | [56] |
| H. guilliermondii | Beta-phenylethyl acetate ester, 2PA | Wine | Rose, honey, fruity and flowery | [53] |
| H. uvarum and H. guilliermondii | 2-phenylethanol | Grape must from Douro, Portugal | Fruity and flowery | [58] |
| H. uvarum | Ethyl acetate | Wine | Fruity | [64] |
| H. uvaum | Terpenes, C13-norisoprenoids, volatile phenols, terpineol and linalool oxide | Ecolly and Cabernet Sauvignon wine | Tropical fruity and floral | [62] |
| H. vinae | 2PA, isoamyl acetate and esters | Chardonnay wine | Banana, pear, apple, citric fruits, guava | [65] |
| H. vinae | Phenyl ethyl acetate | Macabeo must | Fruity, floral and honey | [66] |
| C. pulcherrima | Ethyl acetate, Iso-amyl acetate | Wine | Fruity, sweet and banana-like | [17] |
| C. zemplinina | Hexyl acetate, ethyl hexanoate, ethyl heptanoate, ethyl dodecanoate and ethyl butanoate | Barbera wines | Apple, fruit, herb, sweet or waxy | [67] |
| M. pulcherrima | Phenol,2,6-dimethoxy | White wine | Smoky notes | [68] |
| R. mucillaginosa | Terpenic compounds (b-damascenone, geraniol, citronellol, linalool, b-terpineol) | Irpinian wines (Aglianico and Fiano wines) | Floral, sweet and ripened fruit | [69] |
| R. mucillaginosa | Terpenols | Chinese wine | Fruity and floral | [70] |
| R. mucillaginosa | C6 compounds (1-hexanol) and faty acids | Chinese wine | Grass and unpleasant fatty | [70] |
| R. mucillaginosa | 3-hexene-1-ol, neroloxide, acetates and ethyl groups | Ecolly dry white wine | Citrus, sweet/acid fruit, berry, floral | [71] |
| P. anomala | Isoamyl acetate | Wine | Banana | [53] |
| P. kluyveri | 2PA, ethyl octanoate | Sparkling wine | Fruity, rose, sweet, honey flavors and pineapple, pear, soapy | [55,72] |
| T. delbrueckii | Ethyl butyrate, ethyl acetate, ethyl hexanoate and ethyl hexanoate | Sparkling wine | Fruity, sweet, pineapple, green apple, brandy, wine-like, strawberry | [22] |
| T. delbrueckii | Ethyl propanoate, ethyl isobutanoate, ethyl dihydrocinnamate and isobutyl acetate | Sauvignon blanc and Merlot must | Fruitiness and complexity | [26] |
| T. delbrueckii | Isoamyl acetate, hexyl acetate, ethyl hexanoate and ethyl octanoate | Juice from Syrah grapes | Fresh and fruity | [73] |
| T. delbrueckii | 3-sulfanylhexan-1-ol | Sauvignon Blanc grape must | Grapefruit/passion fruit | [74] |
| Bacteria | Compounds | Matrix | Aroma | Ref. |
|---|---|---|---|---|
| Lactic Acid Bacteria | ||||
| Lactobacillus brevis | Methanethiol | Merlot wine | Unpleasant sulfur aroma | [93] |
| 3-(methylsulfanyl) propan-1-ol | Meaty aroma (<10 μM) | |||
| Lactobacillus plantarum | Linalool, 2 phenyl-ethanol, 2,3-butanediol, 4-terpineol and geraniol | Fiano wine | Floral, fruity and spicy aroma | [94] |
| Lactobacillus plantarum | Terpenes, limonene and linalool | Synthetic wine | Flowery-citric aroma | [95] |
| Benzyl alcohol and b-phenyl-ethyl-alcohol | Rose-like odor | |||
| Oenococcus oeni and Lactobacillus spp. | Terpenes, norisoprenoids, phenols and vanillins | Synthetic wine | Alcohol and dried sensory descriptors. Fruity aroma | [96] |
| Oenococcus oeni and Lactobacillus plantarum | 2 phenyl-ethanol, 2,3-butanediol, ethyl-lactate, terpenes and vanillate derivatives | Shiraz wine | Fruity, floral, earthy/nutty aromas | [97] |
| Oenococcus oeni | Ethyl esters | Wine | Fruit-like | [98] |
| Oenococcus oeni | 2-phenylethanol, terpenes, lactic acid ethyl-ester and succinic acid, diethyl-ester | Riesling wine | Rose notes, fruity and floral notes | [99] |
| Oenococcus oeni | Hexanol, 3-methylbutylester, acid esters | Chardonnay wine | Green and herbaceous, banana notes and fruity aroma | |
| Oenococcus oeni | Substituted ethyl esters: i.e., (2S)-2-hydroxy-n-me-thylpentanoic acid | Merlot wine | Black-berry and jammy-fruit notes | [100] |
| Oenococcus oeni | Fruity esters and lower production of alcohols and terpenes | Black raspberry wine | Strong fruity and slight notes of solvent and herbaceous | [101] |
| Leuconostoc | Phenyl-ethyl acetate | Black glutinous rice wine | Sweet, floral aroma | [102] |
| Leuconostoc | 2,3-butanediol | Buttery aroma | ||
| LAB commercial starter | Diacetyl, ethyl acetate, ethyl lactate, mono-ethyl and diethyl succinate | Single-varietal red wines | Fruity, smoked/toasted. | [103] |
| Acetic Acid Bacteria | ||||
| Acetobacter | Ethyl esters | Highland barley wine | Fruity, grape-like aroma | [104] |
| Acetic acid | Vinegar | |||
| Acetobacter aceti | 2PA, 3-methyl butanol, ethyl acetate | Pineapple wine | Floral-fruity aroma | [105] |
| Gluconobacter | Tartaric and citric acid, ethyl esters | Black glutinous rice wine | Acid and fruity aroma | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2021, 10, 51. https://doi.org/10.3390/foods10010051
Carpena M, Fraga-Corral M, Otero P, Nogueira RA, Garcia-Oliveira P, Prieto MA, Simal-Gandara J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods. 2021; 10(1):51. https://doi.org/10.3390/foods10010051
Chicago/Turabian StyleCarpena, Maria, Maria Fraga-Corral, Paz Otero, Raquel A. Nogueira, Paula Garcia-Oliveira, Miguel A. Prieto, and Jesus Simal-Gandara. 2021. "Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile" Foods 10, no. 1: 51. https://doi.org/10.3390/foods10010051