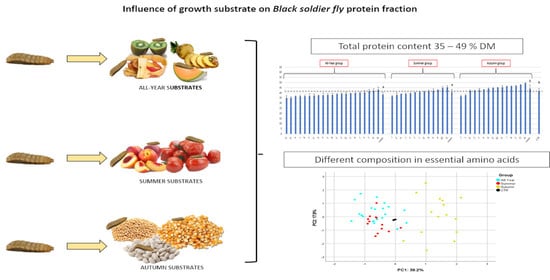
Effect of the Rearing Substrate on Total Protein and Amino Acid Composition in Black Soldier Fly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Insect Rearing Conditions and Substrate Selection
2.3. Proximate Composition of Rearing Substrates and BSF Prepupae
2.4. Amino Acid Profile of BSF Prepupae
2.4.1. Sample Preparation
2.4.2. UPLC/ESI-MS Analysis
2.4.3. Tryptophan Determination
2.5. Statistical Analysis
3. Results and Discussion
3.1. Rearing Substrate Composition and Related BSF Biomass
3.2. Effect of the Rearing Substrate Composition on BSF Prepupae Total Protein Content
3.3. BSF Amino Acid Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer-Rochow, V.B. Can insects help to ease the problem of world food shortage? Search 1975, 6, 261–262. [Google Scholar]
- van Huis, A.; van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and agriculture Organization of the United Nation: Rome, Italy, 2013; ISBN 978-92-5-107596-8. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, Y.; Zhang, H. Review of the nutrition value of edible insects. In Proceedings of the Forest Insects as Food: Humans Bite Back, Chiang Mai, Thailand, 19–21 February 2008. [Google Scholar]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Why for feed and not for human consumption? The black soldier fly larvae. CRFSFS 2020, 19, 2747–2763. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappellozza, S.; Leonardi, M.G.; Savoldelli, S.; Carminati, D.; Rizzolo, A.; Cortellino, G.; Terova, G.; Moretto, E.; Badaile, A.; Concheri, G.; et al. A first attempt to produce proteins from insects by means of a circular economy. Animals 2019, 9, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste: Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Bortolini, S.; Macavei, L.I.; Hadj Saadoun, J.; Foca, G.; Ulrici, A.; Bernini, F.; Malferrari, D.; Setti, L.; Ronga, D.; Maistrello, L. Hermetia illucens (L.) larvae as chicken manure management tool for circular economy. J. Clean. Prod. 2020, 262, 121289. [Google Scholar] [CrossRef]
- Madau, F.A.; Arru, B.; Furesi, R.; Pulina, P. Insect farming for feed and food production from a circular business model perspective. Sustainability 2020, 12, 5418. [Google Scholar] [CrossRef]
- Newton, G.L.; Booram, C.V.; Barker, R.W.; Hale, O.M. Dried Hermetia illucens Larvae Meal as a Supplement for Swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrügg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemicals in black soldier fly larval treatment: A review. Waste Manag. 2018, 82, 302–318. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Zheng, L.; Wang, Y.; Zhang, J.; Yu, Z.; Zhang, Y. Potential biodiesel and biogas production from corncob by anaerobic fermentation and black soldier fly. Bioresour. Technol. 2015, 194, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Gortari, M.C.; Hours, R.A. Biotechnological processes for chitin recovery out of crustacean waste: A mini-review. Electron. J. Biotechnol. 2013, 16, 14. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- Choi, Y.-C.; Choi, J.-Y.; Kim, J.-G.; Kim, M.-S.; Kim, W.-T.; Park, K.-H.; Bae, S.-W.; Jeong, G.-S. Potential Usage of Food Waste as a Natural Fertilizer after Digestion by Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2009, 19, 171–174. [Google Scholar]
- Setti, L.; Francia, E.; Pulvirenti, A.; Gigliano, S.; Zaccardelli, M.; Pane, C.; Caradonia, F.; Bortolini, S.; Maistrello, L.; Ronga, D. Use of black soldier fly (Hermetia illucens (L.), Diptera: Stratiomyidae) larvae processing residue in peat-based growing media. Waste Manag. 2019, 95, 278–288. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, K.; Zhong, W.; Liu, N.; Wu, X.; Li, W.; Zheng, L.; Yu, Z.; Zhang, J. Bioconversion-Composting of Golden Needle Mushroom (Flammulina velutipes) Root Waste by Black Soldier Fly (Hermetia illucens, Diptera: Stratiomyidae) Larvae, to Obtain Added-Value Biomass and Fertilizer. Waste Biomass Valorization 2019, 10, 265–273. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrugg, C.; Roa Gutiérrez, F.; Nguyen, H.D.; Morel, A.; Koottatep, T.; Tockner, K. Black soldier fly larvae for organic waste treatment-prospects and constraints. In Proceedings of the WasteSafe 2011 2nd International Conference on Solid Waste Management in the Developing Countries, Khulna, Bangladesh, 13–15 February 2011. [Google Scholar]
- Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed 2015, 1, 131–139. [Google Scholar] [CrossRef]
- Banks, I.J.; Gibson, W.T.; Cameron, M.M. Growth rates of black soldier fly larvae fed on fresh human faeces and their implication for improving sanitation. Trop. Med. Int. Health 2014, 19, 14–22. [Google Scholar] [CrossRef]
- European Commission Regulation. Commission Regulation (EU) 2017/893-of 24 May 2017-Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU); EU: Brussels, Belgium, 2017. [Google Scholar]
- EFSA Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef] [Green Version]
- Lalander, C.; Senecal, J.; Gros Calvo, M.; Ahrens, L.; Josefsson, S.; Wiberg, K.; Vinnerås, B. Fate of pharmaceuticals and pesticides in fly larvae composting. Sci. Total Environ. 2016, 565, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Purschke, B.; Scheibelberger, R.; Axmann, S.; Adler, A.; Jäger, H. Impact of substrate contamination with mycotoxins, heavy metals and pesticides on the growth performance and composition of black soldier fly larvae (Hermetia illucens) for use in the feed and food value chain. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Leni, G.; Cirlini, M.; Jacobs, J.; Depraetere, S.; Gianotten, N.; Sforza, S.; Dall’Asta, C. Impact of naturally contaminated substrates on alphitobius diaperinus and Hermetia illucens: Uptake and excretion of mycotoxins. Toxins 2019, 11, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, G.; Van Der Fels-Klerx, H.J.; De Rijk, T.C.; Oonincx, D.G.A.B. Aflatoxin B1 tolerance and accumulation in black soldier fly larvae (Hermetia illucens) and yellow mealworms (Tenebrio molitor). Toxins 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Gere, A.; Radványi, D.; Héberger, K. Which insect species can best be proposed for human consumption? Innov. Food Sci. Emerg. Technol. 2019, 52, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Barbi, S.; Macavei, L.I.; Fuso, A.; Luparelli, A.V.; Caligiani, A.; Ferrari, A.M.; Maistrello, L.; Montorsi, M. Valorization of seasonal agri-food leftovers through insects. Sci. Total Environ. 2020, 709, 136209. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing Methods for the Black Soldier Fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Method of Analysis, 16th ed.; Association of official analytical: Washington, DC, USA, 2002. [Google Scholar]
- Janssen, R.H.; Vincken, J.P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Jiménez-Pérez, S.; Morales, F.J. Tryptophan determination in milk-based ingredients and dried sport supplements by liquid chromatography with fluorescence detection. Food Chem. 2006, 98, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, D.C. Design and Analysis of Experiments Eighth Edition. 2012. Available online: http://faculty.business.utsa.edu/manderso/STA4723/readings/Douglas-C.-Montgomery-Design-and-Analysis-of-Experiments-Wiley-2012.pdf (accessed on 20 December 2020).
- Kim, E.; Park, J.; Lee, S.; Kim, Y. Identification and Physiological Characters of Intestinal Bacteria of the Black Soldier Fly, Hermetia illucens. Korean J. Appl. Entomol. 2014, 53, 15–26. [Google Scholar] [CrossRef]
- Jeon, H.; Park, S.; Choi, J.; Jeong, G.; Lee, S.B.; Choi, Y.; Lee, S.J. The intestinal bacterial community in the food waste-reducing larvae of Hermetia illucens. Curr. Microbiol. 2011, 62, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.R.; Ferreira, C. Biochemistry and Molecular Biology of Digestion. In Insect Molecular Biology and Biochemistry; Academic press: Cambridge, MA, USA, 2012; pp. 365–418. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, Y.S.; Seo, S.H.; Yoon, S.H.; Kim, S.J.; Hahn, B.S.; Sim, J.S.; Koo, B.S. Screening and characterization of a novel cellulase gene from the gut microflora of Hermetia illucens using metagenomic library. J. Microbiol. Biotechnol. 2014, 24, 1196–1206. [Google Scholar] [CrossRef] [Green Version]
- Bonelli, M.; Bruno, D.; Brilli, M.; Gianfranceschi, N.; Tian, L.; Tettamanti, G.; Caccia, S.; Casartelli, M. Black soldier fly larvae adapt to different food substrates through morphological and functional responses of the midgut. Int. J. Mol. Sci. 2020, 21, 4955. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Fonseca, K.B. Flies Are What They Eat. 2018. Available online: https://www.researchgate.net/publication/325956717_Flies_are_what_they_eat#fullTextFileContent (accessed on 15 January 2021).
- Soetemans, L.; Gianotten, N.; Bastiaens, L. Agri-food side-stream inclusion in the diet of alphitobius diaperinus. Part 2: Impact on larvae composition. Insects 2020, 11, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Leni, G.; Caligiani, A.; Sforza, S. Killing method affects the browning and the quality of the protein fraction of Black Soldier Fly (Hermetia illucens) prepupae: A metabolomics and proteomic insight. Food Res. Int. 2018, 115, 116–125. [Google Scholar] [CrossRef] [PubMed]






| All-Year (n = 21) | Summer (n = 13) | Autumn (n = 15) | CTR | |
|---|---|---|---|---|
| Lipid | 0.78 ± 0.35 | 3.28 ± 2.12 | 3.18 ± 1.46 | 3.37 ± 0.1 |
| Ashes | 6.66 ± 3.20 | 4.87 ± 0.71 | 4.02 ± 1.59 | 5.9 ± 0.5 |
| Fibres | 23.1 ± 17.51 | 56.86 ± 14.79 | 56.69 ± 6.53 | 41 ± 2.5 |
| Polyphenols | 0.03 ± 0.02 | 0.09 ± 0.02 | 0.04 ± 0.01 | Nd |
| Protein | 5.07 ± 1.20 | 9.97 ± 2.71 | 16.03 ± 6.57 | 17.28 ± 1 |
| Available carbohydrates | 64.36 ± 20.07 | 24.93 ± 15.61 | 17.05 ± 10.72 | 32.61 ± 1 |
| Total prepupae biomass (g) | 21.2 ± 2 | 39.8 ± 3 | 57.1 ± 5 | 52.0 ± 4 |
| All-Year (n = 21) | Summer (n = 13) | Autumn (n = 15) | CTR (n = 3) | |
|---|---|---|---|---|
| His | 33.50 a | 37.86 ab | 39.00 b | 37.00 ab |
| Ile | 42.89 a | 43.11 a | 44.10 a | 45.39 a |
| Leu | 71.24 a | 77.66 bc | 79.88 c | 74.00 ab |
| Val | 60.49 a | 60.25 a | 66.09 b | 62.43 a |
| Lys | 62.22 a | 62.93 a | 51.73 b | 62.44 a |
| Cys | 18.17 a | 20.42 a | 20.14 a | 20.70 a |
| Met | 18.37 a | 17.54 a | 19.16 a | 18.40 a |
| Phe | 41.69 a | 40.03 a | 46.47 b | 42.53 ab |
| Tyr | 61.45 a | 63.16 ab | 69.67 b | 64.13 ab |
| Thr | 39.50 ab | 37.36 a | 40.58 b | 39.30 ab |
| Trp | 14.80 a | 15.76 a | 15.84 a | 17.60 a |
| Reference Protein FAO 2011 (mg/g Protein) | Average Values Found in BSF Prepupae Protein (mg/g Protein) | Lowest Values Found in BSF Prepupae Protein (mg/g Protein) | Highest Values Found in BSF Prepupae Protein (mg/g Protein) | |
|---|---|---|---|---|
| His | 16 | 36 | 23 | 46 |
| Ile | 30 | 44 | 39 | 50 |
| Leu | 61 | 76 | 67 | 89 |
| Lys | 48 | 58 | 47 | 72 |
| Cys + Met | 23 | 38 | 32 | 45 |
| Phe + Tyr | 41 | 107 | 91 | 127 |
| Thr | 25 | 39 | 33 | 44 |
| Trp | 6.6 | 15 | 7 | 19 |
| Val | 40 | 62 | 56 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuso, A.; Barbi, S.; Macavei, L.I.; Luparelli, A.V.; Maistrello, L.; Montorsi, M.; Sforza, S.; Caligiani, A. Effect of the Rearing Substrate on Total Protein and Amino Acid Composition in Black Soldier Fly. Foods 2021, 10, 1773. https://doi.org/10.3390/foods10081773
Fuso A, Barbi S, Macavei LI, Luparelli AV, Maistrello L, Montorsi M, Sforza S, Caligiani A. Effect of the Rearing Substrate on Total Protein and Amino Acid Composition in Black Soldier Fly. Foods. 2021; 10(8):1773. https://doi.org/10.3390/foods10081773
Chicago/Turabian StyleFuso, Andrea, Silvia Barbi, Laura Ioana Macavei, Anna Valentina Luparelli, Lara Maistrello, Monia Montorsi, Stefano Sforza, and Augusta Caligiani. 2021. "Effect of the Rearing Substrate on Total Protein and Amino Acid Composition in Black Soldier Fly" Foods 10, no. 8: 1773. https://doi.org/10.3390/foods10081773