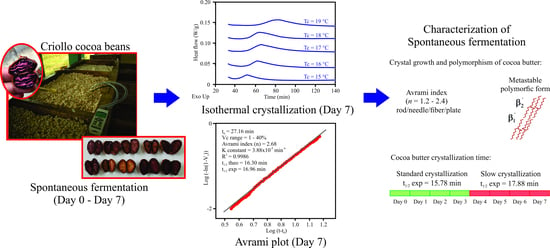
Kinetics Crystallization and Polymorphism of Cocoa Butter throughout the Spontaneous Fermentation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Monitoring Fermentation
2.3. Physico-Chemical Parameters
2.3.1. Titratable Acidity and pH of the Cocoa Beans
2.3.2. Moisture Content
2.3.3. Water Activity
2.4. Crystallization and Melting Profiles of POP and CB Inside Cocoa Bean
2.5. Isothermal Crystallization of Cocoa Butter Inside the Cocoa Bean
2.6. Kinetics Crystallization
2.7. Polymorphism
2.8. Statistical Analysis
3. Results
3.1. Monitoring Fermentation
3.2. Crystallization and Melting Profiles
3.3. Kinetics of Crystallization
4. Discussion
4.1. Monitoring Fermentation
4.2. Crystallization and Melting Profiles of CB and POP
4.3. Kinetics Crystallization
4.4. Polymorphism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badu, M.; Awudza, J.; Budd, P.M.; Yeates, S. Determination of Physical Properties and Crystallization Kinetics of Oil from Allanblackia Seeds and Shea Nuts Under Different Thermal Conditions. Eur. J. Lipid Sci. Technol. 2018, 120, 1700156. [Google Scholar] [CrossRef]
- Liu, W.; Yao, Y.; Li, C. Effect of Tempered Procedures on the Crystallization Behavior of Different Positions of Cocoa Butter Products. Food Chem. 2022, 370, 131002. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, W.; Zhang, D.; Li, R.; Zhou, H.; Li, C.; Wang, S. Dynamic Changes in the Triacylglycerol Composition and Crystallization Behavior of Cocoa Butter. LWT 2020, 129, 109490. [Google Scholar] [CrossRef]
- Ewens, H.; Metilli, L.; Simone, E. Analysis of the Effect of Recent Reformulation Strategies on the Crystallization Behaviour of Cocoa Butter and the Structural Properties of Chocolate. Curr. Res. Food Sci. 2021, 4, 105–114. [Google Scholar] [CrossRef]
- Servent, A.; Boulanger, R.; Davrieux, F.; Pinot, M.-N.; Tardan, E.; Forestier-Chiron, N.; Hue, C. Assessment of Cocoa (Theobroma Cacao L.) Butter Content and Composition throughout Fermentations. Food Res. Int. 2018, 107, 675–682. [Google Scholar] [CrossRef]
- Sirbu, D.; Grimbs, A.; Corno, M.; Ullrich, M.S.; Kuhnert, N. Variation of Triacylglycerol Profiles in Unfermented and Dried Fermented Cocoa Beans of Different Origins. Food Res. Int. 2018, 111, 361–370. [Google Scholar] [CrossRef]
- Padar, S.; Jeelani, S.A.K.; Windhab, E.J. Crystallization Kinetics of Cocoa Fat Systems: Experiments and Modeling. J. Am. Oil Chem. Soc. 2008, 85, 1115–1126. [Google Scholar] [CrossRef] [Green Version]
- Ghazani, S.M.; Marangoni, A.G. The Ternary Solid State Phase Behavior of Triclinic POP, POS, and SOS and Its Relationship to CB and CBE Properties. Cryst. Growth Des. 2019, 19, 704–713. [Google Scholar] [CrossRef]
- Norazlina, M.R.; Jahurul, M.H.A.; Hasmadi, M.; Sharifudin, M.S.; Patricia, M.; Lee, J.S.; Amir, H.M.S.; Noorakmar, A.W.; Riman, I. Effects of Fractionation Technique on Triacylglycerols, Melting and Crystallisation and the Polymorphic Behavior of Bambangan Kernel Fat as Cocoa Butter Improver. LWT 2020, 129, 109558. [Google Scholar] [CrossRef]
- Jin, J.; Jin, Q.; Akoh, C.C.; Wang, X. StOSt-Rich Fats in the Manufacture of Heat-Stable Chocolates and Their Potential Impacts on Fat Bloom Behaviors. Trends Food Sci. Technol. 2021, 118, 418–430. [Google Scholar] [CrossRef]
- Ray, J.; MacNaughtan, W.; Chong, P.S.; Vieira, J.; Wolf, B. The Effect of Limonene on the Crystallization of Cocoa Butter. J. Am. Oil Chem. Soc. 2012, 89, 437–445. [Google Scholar] [CrossRef]
- Declerck, A.; Nelis, V.; Danthine, S.; Dewettinck, K.; Van der Meeren, P. Characterisation of Fat Crystal Polymorphism in Cocoa Butter by Time-Domain NMR and DSC Deconvolution. Foods 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Dahlenborg, H.; Millqvist-Fureby, A.; Bergenståhl, B. Effect of Shell Microstructure on Oil Migration and Fat Bloom Development in Model Pralines. Food Struct. 2015, 5, 51–65. [Google Scholar] [CrossRef]
- Talbot, G. Chocolate and Cocoa Butter-Structure and Composition. In Cocoa Butter and Related Compounds; Garti, N., Widlak, N.R., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 1–34. ISBN 978-0-9830791-2-5. [Google Scholar]
- Sasaki, M.; Ueno, S.; Sato, K. Polymorphism and Mixing Phase Behavior of Major Triacylglycerols of Cocoa Butter. In Cocoa Butter and Related Compounds; Garti, N., Widlak, N.R., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 151–172. [Google Scholar]
- Loisel, C.; Keller, G.; Lecq, G.; Bourgaux, C.; Ollivon, M. Phase Transitions and Polymorphism of Cocoa Butter. J. Am. Oil Chem. Soc. 1998, 75, 425–439. [Google Scholar] [CrossRef]
- Devos, N.; Reyman, D.; Sanchez-Cortés, S. Chocolate Composition and Its Crystallization Process: A Multidisciplinary Analysis. Food Chem. 2020, 342, 128301. [Google Scholar] [CrossRef]
- Fernandes, V.A.; Müller, A.J.; Sandoval, A.J. Thermal, Structural and Rheological Characteristics of Dark Chocolate with Different Compositions. J. Food Eng. 2013, 116, 97–108. [Google Scholar] [CrossRef]
- Svanberg, L.; Ahrné, L.; Lorén, N.; Windhab, E. Effect of Sugar, Cocoa Particles and Lecithin on Cocoa Butter Crystallisation in Seeded and Non-Seeded Chocolate Model Systems. J. Food Eng. 2011, 104, 70–80. [Google Scholar] [CrossRef]
- Sonwai, S.; Podchong, P.; Rousseau, D. Crystallization Kinetics of Cocoa Butter in the Presence of Sorbitan Esters. Food Chem. 2017, 214, 497–506. [Google Scholar] [CrossRef]
- Pirouzian, H.R.; Konar, N.; Palabiyik, I.; Oba, S.; Toker, O.S. Pre-Crystallization Process in Chocolate: Mechanism, Importance and Novel Aspects. Food Chem. 2020, 321, 126718. [Google Scholar] [CrossRef]
- Chen, J.; Ghazani, S.M.; Stobbs, J.A.; Marangoni, A.G. Tempering of Cocoa Butter and Chocolate Using Minor Lipidic Components. Nat. Commun. 2021, 12, 5018. [Google Scholar] [CrossRef]
- Hernández, M.d.P.L.; Núñez, J.C.; Gómez, M.S.H.; Tovar, M.D.L. Physicochemical and Microbiological Dynamics of the Fermentation of the Ccn51 Cocoa Material in Three Maturity Stages. Rev. Bras. Frutic. 2019, 41, 1–13. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Prandi, B.; Palla, G.; Sforza, S. Influence of Fermentation Level and Geographical Origin on Cocoa Bean Oligopeptide Pattern. Food Chem. 2016, 211, 431–439. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Andrade, E.H.D.A.; do Nascimento, L.D.; de Siqueira, F.C.; Lopes, A.S. Profile of Volatile Compounds of On-Farm Fermented and Dried Cocoa Beans Inoculated with Saccharomyces Cerevisiae KY794742 and Pichia Kudriavzevii KY794725. Molecules 2021, 26, 344. [Google Scholar] [CrossRef]
- Deus, V.L.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. Understanding Amino Acids and Bioactive Amines Changes during On-Farm Cocoa Fermentation. J. Food Compos. Anal. 2021, 97, 103776. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Composition of Methylxanthines, Polyphenols, Key Odorant Volatiles and Minerals in 22 Cocoa Beans Obtained from Different Geographic Origins. LWT 2022, 153, 112395. [Google Scholar] [CrossRef]
- Calvo, A.M.; Botina, B.L.; García, M.C.; Cardona, W.A.; Montenegro, A.C.; Criollo, J. Dynamics of Cocoa Fermentation and Its Effect on Quality. Sci. Rep. 2021, 11, 16746. [Google Scholar] [CrossRef]
- Moreno-Zambrano, M.; Ullrich, M.S.; Hütt, M.-T. Exploring Cocoa Bean Fermentation Mechanisms by Kinetic Modelling. R. Soc. Open Sci. 2022, 9, 210274. [Google Scholar] [CrossRef]
- Fang, Y.; Li, R.; Chu, Z.; Zhu, K.; Gu, F.; Zhang, Y. Chemical and Flavor Profile Changes of Cocoa Beans (Theobroma Cacao L.) during Primary Fermentation. Food Sci. Nutr. 2020, 8, 4121–4133. [Google Scholar] [CrossRef]
- Martini, S. Application of DSC, Pulsed NMR, and Other Analytical Techniques to Study the Crystallization Kinetics of Lipids Models, Oil, Fats, and Their Blends in the Field of Food Technology. In Differential Scanning Calorimetry. Applications in Fat and oil Technology; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2015; pp. 163–195. [Google Scholar]
- Jin, J.; Jin, Q.; Wang, X.; Akoh, C.C. Improving Heat and Fat Bloom Stabilities of “Dark Chocolates” by Addition of Mango Kernel Fat-Based Chocolate Fats. J. Food Eng. 2019, 246, 33–41. [Google Scholar] [CrossRef]
- Hubbes, S.-S.; Danzl, W.; Foerst, P. Crystallization Kinetics of Palm Oil of Different Geographic Origins and Blends Thereof by the Application of the Avrami Model. LWT 2018, 93, 189–196. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Wesdorp, L.H. Nucleation and Crystalline Growth Kinetics. In Structure and Properties of Fat Crystal Networks; CRC Press: Boca Raton, FL, USA, 2013; pp. 27–99. [Google Scholar]
- Sangwal, K.; Sato, K. Nucleation and Crystallization Kinetics of Fats. In Structure-Function Analysis of Edible Fats; Elsevier: London, UK, 2018; pp. 21–72. ISBN 978-0-12-814041-3. [Google Scholar]
- Davis, T.R.; Dimick, P.S. Isolation and Thermal Characterization of High-Melting Seed Crystals Formed during Cocoa Butter Solidification. J. Am. Oil Chem. Soc. 1989, 66, 1488–1493. [Google Scholar] [CrossRef]
- MacNaughtan, W.; Farhat, I.A.; Himawan, C.; Starov, V.M.; Stapley, A.G.F. A Differential Scanning Calorimetry Study of the Crystallization Kinetics of Tristearin-Tripalmitin Mixtures. J. Am. Oil Chem. Soc. 2006, 83, 15. [Google Scholar] [CrossRef]
- Simoes, S.; Lelaj, E.; Rousseau, D. The Presence of Crystalline Sugar Limits the Influence of Emulsifiers on Cocoa Butter Crystallization. Food Chem. 2021, 346, 128848. [Google Scholar] [CrossRef]
- Santander, M.; Vaillant, F.; Sinuco, D.; Rodríguez, J.; Escobar, S. Enhancement of Fine Flavour Cocoa Attributes under a Controlled Postharvest Process. Food Res. Int. 2021, 143, 110236. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. 1998. Available online: https://www.aoac.org/ (accessed on 4 May 2022).
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D Printing of Multilayered Orodispersible Films with In-Process Drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Agoda-Tandjawa, G.; Dieudé-Fauvel, E.; Girault, R.; Baudez, J.-C. Using Water Activity Measurements to Evaluate Rheological Consistency and Structure Strength of Sludge. Chem. Eng. J. 2013, 228, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Foubert, I.; Vanrolleghem, P.A.; Thas, O.; Dewettinck, K. Influence of Chemical Composition on the Isothermal Cocoa Butter Crystallization. J. Food Sci. 2006, 69, E478–E487. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Rangel-Vargas, E.; Dibildox-Alvarado, E.; Charó-Alonso, M.A. Crystallization of Cocoa Butter with and without Polar Lipids Evaluated by Rheometry, Calorimetry and Polarized Light Microscopy. Eur. J. Lipid Sci. Technol. 2005, 107, 641–655. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC Isothermal Polymer Crystallization Kinetics Measurements and the Use of the Avrami Equation to Fit the Data: Guidelines to Avoid Common Problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Briceño-Montelongo, M.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Reyes-Hernández, J. Crystallization Kinetics of Palm Stearin in Blends with Sesame Seed Oil. J. Am. Oil Chem. Soc. 2000, 77, 297–310. [Google Scholar] [CrossRef]
- Brereton, R.G. Applied Chemometrics for Scientist, 1st ed.; John Wiley and Sons: Chichester, UK, 2007. [Google Scholar]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Gloria, M.B.A.; Martins, L.H.d.S.; Lopes, A.S. Chemical Implications and Time Reduction of On-Farm Cocoa Fermentation by Saccharomyces Cerevisiae and Pichia Kudriavzevii. Food Chem. 2021, 338, 127834. [Google Scholar] [CrossRef] [PubMed]
- Visintin, S.; Ramos, C.L.; Batista, N.N.; Dolci, P.; Schwan, R.F.; Cocolin, L. Impact of Saccharomyces Cerevisiae and Torulaspora Delbrueckii Starter Cultures on Cocoa Beans Fermentation. Int. J. Food Microbiol. 2017, 257, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of Aromatic Compounds Precursors during Fermentation of Criollo and Forastero Cocoa. Heliyon 2019, 5, e01157. [Google Scholar] [CrossRef] [Green Version]
- Deus, V.L.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. Influence of Cocoa Clones on the Quality and Functional Properties of Chocolate—Nitrogenous Compounds. LWT 2020, 134, 110202. [Google Scholar] [CrossRef]
- Bayés-García, L.; Yoshikawa, S.; Aguilar-Jiménez, M.; Ishibashi, C.; Ueno, S.; Calvet, T. Heterogeneous Nucleation Effects of Talc Particles on Polymorphic Crystallization of Cocoa Butter. Cryst. Growth Des. 2022, 22, 213–227. [Google Scholar] [CrossRef]
- Aumpai, K.; Tan, C.P.; Huang, Q.; Sonwai, S. Production of Cocoa Butter Equivalent from Blending of Illipé Butter and Palm Mid-Fraction. Food Chem. 2022, 384, 132535. [Google Scholar] [CrossRef]
- Marangoni, A.G.; McGauley, S.E. Relationship between Crystallization Behavior and Structure in Cocoa Butter. Cryst. Growth Des. 2003, 3, 95–108. [Google Scholar] [CrossRef]
- Bayés-García, L.; Aguilar-Jiménez, M.; Calvet, T.; Koyano, T.; Sato, K. Crystallization and Melting Behavior of Cocoa Butter in Lipid Bodies of Fresh Cacao Beans. Cryst. Growth Des. 2019, 19, 4127–4137. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, B.; Zhang, H.; Guo, Y.; Dang, L.; Liu, Z.; Shu, Q.; Wang, Z. Solid–Liquid Phase Equilibrium and Phase Behaviors for Binary Mixtures Composed of Tripalmitoylglycerol (PPP), 1,3-Dipalmitoyl-2-Oleoyl-Glycerol (POP), and 1,2-Dioleoyl-3-Palmitoyl-Glycerol (POO). Ind. Eng. Chem. Res. 2019, 58, 10044–10052. [Google Scholar] [CrossRef]
- Smith, K.W.; Cain, F.W.; Talbot, G. Kinetic Analysis of Nonisothermal Differential Scanning Calorimetry of 1,3-Dipalmitoyl-2-Oleoylglycerol. J. Agric. Food Chem. 2005, 53, 3031–3040. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Mello, N.A.; Cardoso, L.P.; Badan Ribeiro, A.P.; Bicas, J.L. The Effects of Limonene on the Crystallization of Palm Oil. LWT 2020, 133, 110079. [Google Scholar] [CrossRef]
- Miyasaki, E.K.; dos Santos, C.A.; Vieira, L.R.; Ming, C.C.; Calligaris, G.A.; Cardoso, L.P.; Gonçalves, L.A.G. Acceleration of Polymorphic Transition of Cocoa Butter and Cocoa Butter Equivalent by Addition of D-limonene. Eur. J. Lipid Sci. Technol. 2016, 118, 716–723. [Google Scholar] [CrossRef]
- Martini, S.; Carelli, A.A.; Lee, J. Effect of the Addition of Waxes on the Crystallization Behavior of Anhydrous Milk Fat. J. Am. Oil Chem. Soc. 2008, 85, 1097–1104. [Google Scholar] [CrossRef]
- Rashid, N.A. Crystallisation Kinetics of Palm Stearin, Palm Kernel Olein and Their Blends. Food Sci. Technol. 2012, 46, 571–573. [Google Scholar]
- Gomez, N.A.; Sanchez, K.; Arguello, H. Non-Destructive Method for Classification of Cocoa Beans from Spectral Information. In Proceedings of the 2019 XXII Symposium on Image, Signal Processing and Artificial Vision (STSIVA), Bucaramanga, Colombia, 24–26 April 2019; pp. 1–5. [Google Scholar]
- Cevallos-Cevallos, J.M.; Gysel, L.; Maridueña-Zavala, M.G.; Molina-Miranda, M.J. Time-Related Changes in Volatile Compounds during Fermentation of Bulk and Fine-Flavor Cocoa (Theobroma Cacao) Beans. J. Food Qual. 2018, 2018, 1758381. [Google Scholar] [CrossRef] [Green Version]
- Talbot, G. Chocolate Temper. In Industrial Chocolate, Manufacture and Use; Blackwell Publishing: Oxford, UK, 2009; pp. 261–275. ISBN 978-1-4051-3949-6. [Google Scholar]
- Garbolino, C.; Bartoccini, M.; Flöter, E. The Influence of Emulsifiers on the Crystallisation Behaviour of a Palm Oil-based Blend. Eur. J. Lipid Sci. Technol. 2005, 107, 616–626. [Google Scholar] [CrossRef]





| Type of Crystal Growth and Nucleation Expected | |
|---|---|
| 3 + 1 = 4 | Spherical crystals grow from sporadic nuclei |
| 3 + 0 = 3 | Spherical crystals grow from instantaneous nuclei |
| 2 + 1 = 3 | Crystals grow plate-like from sporadic nuclei |
| 2 + 0 = 2 | Crystals grow plate-like from instantaneous nuclei |
| 1 + 1 = 2 | Crystals grow as rod/needle/fiber from sporadic nuclei |
| 1 + 0 = 1 | Crystals grow as rod/needle/fiber from instantaneous nuclei. |
| Sample | Crystallization | Melting | Polymorphic Form | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | Enthalpy (J/g) | (°C) | (°C) | (°C) | Enthalpy (J/g) | ||
| CB | 15.45 | 12.93 | 2.99 | 48.42 | 15.05 | 19.65 | 26.24 | 69.01 | γ |
| POP | 12.07 | 10.40 | 4.55 | 54.91 | 12.75 | 15.75 | 19.34 | 76.49 | γ |
| Crystallization Temperature (°C) | Sample | (min−n) | (min) | (min) | (min) | ||
|---|---|---|---|---|---|---|---|
| 15 | CB | 2.53 | 2.87 × 10−3 | 13.19 | 8.77 | 8.75 | 0.9990 |
| POP | 3.07 | 2.33 × 10−5 | 5.89 | 28.54 | 28.57 | 0.9996 | |
| 16 | CB | 2.91 | 5.97 × 10−4 | 14.59 | 11.30 | 11.42 | 0.9993 |
| POP | 2.94 | 1.94 × 10−5 | 11.56 | 35.36 | 35.05 | 0.9920 | |
| 17 | CB | 2.99 | 3.01 × 10−4 | 16.20 | 13.28 | 13.29 | 0.9992 |
| POP | 2.84 | 2.56 × 10−5 | 14.33 | 36.18 | 36.70 | 0.9999 | |
| 18 | CB | 3.42 | 9.70 × 10−5 | 15.61 | 13.36 | 13.74 | 0.9997 |
| POP | 2.35 | 6.04 × 10−5 | 19.27 | 53.29 | 52.98 | 0.9998 | |
| 19 | CB | 3.73 | 8.54 × 10−5 | 17.85 | 15.90 | 16.35 | 0.9993 |
| POP | 2.61 | 1.06 × 10−5 | 35.27 | 70.13 | 72.17 | 0.9989 |
| Crystallization Temperature (°C) | Sample | (min−n) | (min) | (min) | ||
|---|---|---|---|---|---|---|
| 15 | Non-fermented | 2.13 | 1.11 × 10−2 | 6.99 | 6.55 | 0.9992 |
| Day 0 | 2.09 | 8.68 × 10−3 | 8.09 | 8.31 | 0.9984 | |
| Day 1 | 2.66 | 1.04 ×10−3 | 11.55 | 12.33 | 0.9993 | |
| Day 2 | 2.58 | 1.53 × 10−3 | 10.73 | 11.26 | 0.9994 | |
| Day 3 | 1.76 | 2.44 × 10−2 | 6.69 | 7.10 | 0.9918 | |
| Day 4 | 2.51 | 1.75 × 10−3 | 10.81 | 10.52 | 0.9995 | |
| Day 5 | 2.76 | 8.06 × 10−4 | 11.57 | 11.90 | 0.9998 | |
| Day 6 | 2.04 | 3.02 × 10−3 | 14.34 | 12.70 | 0.9999 | |
| Day 7 | 2.34 | 3.32 × 10−3 | 9.83 | 9.75 | 0.9994 | |
| 16 | Non-fermented | 1.51 | 2.56 × 10−2 | 8.93 | 7.77 | 0.9921 |
| Day 0 | 1.78 | 1.59 × 10−2 | 8.31 | 8.05 | 0.9973 | |
| Day 1 | 2.13 | 2.38 × 10−3 | 14.54 | 14.98 | 0.9991 | |
| Day 2 | 2.49 | 1.65 × 10−3 | 11.25 | 11.36 | 0.9998 | |
| Day 3 | 2.00 | 1.42 × 10−2 | 6.96 | 6.93 | 0.9992 | |
| Day 4 | 2.67 | 1.10 × 10−3 | 11.14 | 10.80 | 0.9977 | |
| Day 5 | 2.27 | 1.09 × 10−3 | 17.24 | 16.43 | 0.9929 | |
| Day 6 | 1.67 | 7.13 × 10−3 | 15.43 | 14.27 | 0.9903 | |
| Day 7 | 2.80 | 6.37 × 10−4 | 11.14 | 12.60 | 0.9992 | |
| 17 | Non-fermented | 1.92 | 6.82 × 10−3 | 11.14 | 10.58 | 0.9965 |
| Day 0 | 2.08 | 7.22 × 10−3 | 8.97 | 9.17 | 0.9991 | |
| Day 1 | 1.82 | 3.10 × 10−3 | 15.64 | 12.35 | 0.9924 | |
| Day 2 | 2.54 | 9.54 × 10−4 | 13.41 | 13.30 | 0.9986 | |
| Day 3 | 2.43 | 2.93 × 10−3 | 9.51 | 9.93 | 0.9998 | |
| Day 4 | 2.94 | 5.68 × 10−4 | 11.20 | 11.43 | 0.9999 | |
| Day 5 | 2.47 | 1.77 × 10−3 | 11.24 | 11.90 | 0.9985 | |
| Day 6 | 2.45 | 9.05 × 10−4 | 15.07 | 15.19 | 0.9990 | |
| Day 7 | 2.35 | 1.49 × 10−3 | 13.61 | 13.87 | 0.9999 | |
| 18 | Non-fermented | 2.10 | 3.61 × 10−3 | 12.23 | 11.73 | 0.9952 |
| Day 0 | 1.79 | 6.78 × 10−3 | 13.21 | 13.48 | 0.9936 | |
| Day 1 | 1.73 | 8.77 × 10−3 | 12.56 | 13.28 | 0.9984 | |
| Day 2 | 2.94 | 2.51 × 10−4 | 14.84 | 15.78 | 0.9994 | |
| Day 3 | 2.55 | 1.98 × 10−3 | 9.91 | 10.14 | 0.9999 | |
| Day 4 | 2.82 | 6.48 × 10−4 | 11.89 | 12.33 | 0.9998 | |
| Day 5 | 2.55 | 8.59 × 10−4 | 13.74 | 14.24 | 0.9993 | |
| Day 6 | 2.59 | 5.78 × 10−4 | 15.40 | 15.43 | 0.9993 | |
| Day 7 | 1.98 | 3.45 × 10−3 | 14.63 | 13.94 | 0.9964 | |
| 19 | Non-fermented | 2.17 | 3.01 × 10−3 | 12.29 | 12.28 | 0.9997 |
| Day 0 | 1.60 | 8.14 × 10−3 | 16.01 | 14.08 | 0.9862 | |
| Day 1 | 1.20 | 9.38 × 10−3 | 12.02 | 12.24 | 0.9991 | |
| Day 2 | 2.66 | 4.20 × 10−4 | 16.12 | 17.18 | 0.9989 | |
| Day 3 | 2.09 | 2.75 × 10−3 | 14.09 | 15.68 | 0.9985 | |
| Day 4 | 2.94 | 1.72 × 10−4 | 16.90 | 17.88 | 0.9981 | |
| Day 5 | 2.29 | 1.66 × 10−3 | 13.89 | 14.55 | 0.9995 | |
| Day 6 | 2.57 | 4.73 × 10−4 | 17.12 | 17.85 | 0.9996 | |
| Day 7 | 2.68 | 3.88 × 10−4 | 16.30 | 16.96 | 0.9986 |
| Crystallization Temperature (°C) | Sample | Enthalpy (J/g) | (°C) | (°C) | (°C) | Polymorphic Form |
|---|---|---|---|---|---|---|
| 15 | CB | 89.53 | 19.82 | 25.80 | 28.59 | |
| POP | 105.92 | 20.88 | 24.70 | 27.34 | α | |
| 16 | CB | 87.16 | 20.48 | 26.27 | 28.80 | |
| POP | 105.06 | 21.38 | 25.92 | 27.71 | ||
| 17 | CB | 84.06 | 20.79 | 26.69 | 28.93 | |
| POP | 104.67 | 23.18 | 26.66 | 28.29 | ||
| 18 | CB | 75.80 | 21.42 | 27.01 | 29.04 | |
| POP | 103.20 | 23.46 | 26.17 | 27.87 | ||
| 19 | CB | 69.73 | 22.14 | 27.42 | 29.30 | |
| POP | 80.23 | 24.25 | 26.56 | 28.09 |
| Crystallization Temperature (°C) | Sample | Enthalpy (J/g) | (°C) | (°C) | (°C) | Polymorphic Form |
|---|---|---|---|---|---|---|
| 15 | Non-fermented | 23.42 | 20.31 | 26.19 | 29.29 | |
| Day 0 | 33.81 | 20.66 | 26.32 | 30.22 | ||
| Day 1 | 17.53 | 19.14 | 24.72 | 28.17 | ||
| Day 2 | 34.89 | 20.26 | 25.73 | 28.76 | ||
| Day 3 | 33.33 | 19.55 | 24.84 | 28.17 | ||
| Day 4 | 32.55 | 19.62 | 25.42 | 29.04 | ||
| Day 5 | 32.94 | 20.65 | 26.14 | 29.59 | ||
| Day 6 | 36.58 | 20.81 | 26.96 | 30.01 | ||
| Day 7 | 22.88 | 19.06 | 24.44 | 27.08 | ||
| 16 | Non-fermented | 18.75 | 21.07 | 26.41 | 29.54 | |
| Day 0 | 17.69 | 22.38 | 26.48 | 28.81 | ||
| Day 1 | 14.92 | 20.02 | 24.90 | 28.22 | ||
| Day 2 | 33.92 | 20.78 | 25.79 | 28.67 | ||
| Day 3 | 27.16 | 19.90 | 25.42 | 28.18 | ||
| Day 4 | 30.88 | 20.14 | 25.69 | 29.07 | ||
| Day 5 | 32.27 | 20.77 | 26.42 | 29.63 | ||
| Day 6 | 31.99 | 21.90 | 27.10 | 30.35 | ||
| Day 7 | 21.21 | 19.81 | 24.64 | 27.18 | ||
| 17 | Non-fermented | 16.19 | 21.40 | 26.69 | 29.86 | |
| Day 0 | 12.38 | 22.05 | 26.56 | 28.94 | ||
| Day 1 | 11.61 | 20.83 | 25.28 | 28.28 | ||
| Day 2 | 33.19 | 21.25 | 25.97 | 28.70 | ||
| Day 3 | 22.61 | 20.81 | 25.82 | 28.34 | ||
| Day 4 | 28.87 | 20.89 | 26.03 | 29.34 | ||
| Day 5 | 31.42 | 21.22 | 26.54 | 29.63 | ||
| Day 6 | 30.35 | 21.17 | 25.78 | 28.49 | ||
| Day 7 | 18.95 | 20.53 | 24.97 | 27.16 | ||
| 18 | Non-fermented | 14.17 | 22.20 | 27.15 | 30.06 | |
| Day 0 | 9.16 | 22.57 | 26.79 | 29.31 | ||
| Day 1 | 7.53 | 21.66 | 25.77 | 28.37 | ||
| Day 2 | 28.42 | 22.11 | 26.26 | 28.90 | ||
| Day 3 | 18.28 | 21.83 | 26.10 | 28.44 | ||
| Day 4 | 26.78 | 21.73 | 26.38 | 29.63 | ||
| Day 5 | 30.53 | 21.78 | 26.79 | 29.64 | ||
| Day 6 | 29.60 | 21.85 | 26.20 | 28.62 | ||
| Day 7 | 16.14 | 21.27 | 25.30 | 27.29 | ||
| 19 | Non-fermented | 11.21 | 23.19 | 27.63 | 30.30 | |
| Day 0 | 6.25 | 23.26 | 27.06 | 29.52 | ||
| Day 1 | 4.01 | 22.42 | 26.42 | 28.65 | ||
| Day 2 | 23.94 | 22.46 | 26.55 | 28.97 | ||
| Day 3 | 13.86 | 22.48 | 26.48 | 28.69 | ||
| Day 4 | 23.45 | 22.12 | 26.83 | 29.66 | ||
| Day 5 | 26.83 | 22.48 | 27.22 | 29.78 | ||
| Day 6 | 27.27 | 22.35 | 26.62 | 28.82 | ||
| Day 7 | 12.12 | 21.82 | 25.98 | 27.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Alayo, E.M.; Torrejón-Valqui, L.; Medina-Mendoza, M.; Cayo-Colca, I.S.; Cárdenas-Toro, F.P. Kinetics Crystallization and Polymorphism of Cocoa Butter throughout the Spontaneous Fermentation Process. Foods 2022, 11, 1769. https://doi.org/10.3390/foods11121769
Castro-Alayo EM, Torrejón-Valqui L, Medina-Mendoza M, Cayo-Colca IS, Cárdenas-Toro FP. Kinetics Crystallization and Polymorphism of Cocoa Butter throughout the Spontaneous Fermentation Process. Foods. 2022; 11(12):1769. https://doi.org/10.3390/foods11121769
Chicago/Turabian StyleCastro-Alayo, Efraín M., Llisela Torrejón-Valqui, Marleni Medina-Mendoza, Ilse S. Cayo-Colca, and Fiorella P. Cárdenas-Toro. 2022. "Kinetics Crystallization and Polymorphism of Cocoa Butter throughout the Spontaneous Fermentation Process" Foods 11, no. 12: 1769. https://doi.org/10.3390/foods11121769