Edible Brown Seaweed in Gluten-Free Pasta: Technological and Nutritional Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
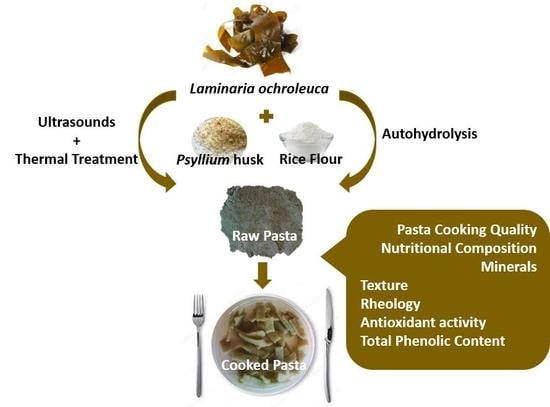
2.2. Fresh Pasta Preparation and Sampling
2.3. Colour Evaluation of Pasta
2.4. Physicochemical Analysis of Pasta
2.5. Phytochemicals and Antioxidant Activity Measurements
2.6. Mechanical Characterization of the Pasta
- Cutting: the firmness of cooked pasta samples was measured following AACC method 66–50.01 [29]. Pasta firmness (N) was determined by measuring the cutting force required to cut three cooked tagliatelle strips using a blade set with guillotine (HDP/BSG) that cut 2.5 mm into the sample at 0.17 mm/s. From this test, adhesiveness (N·s), which is the resistance of the material when the probe is recessing, was also measured.
- Stickiness: pasta stickiness was defined as the maximum peak force required to separate the probe from the sample surface (peak height), and the area under the peak represented the work of adhesion. Three tagliatelle strips were centrally aligned under a circular plexiglass probe (44 mm diameter) on a raised platform and were retained within a circular slot (48 mm diameter) made in a base plate. The samples were compressed for 2 s with an applied force of 9.807 N at 0.5 mm/s. The precision of the stickiness measurement decreased as elapsed time increased. Therefore, the time for stickiness measurements was set at 15 min after draining.
- Extensibility: cooked pasta extensibility characteristics were determined using a Kieffer Dough and Gluten Extensibility Rig (A/KIE). Sample loading and test were conducted as follows: a tagliatelle strip was placed across the grooved region on the sample plate. The hook probe was positioned under the strip and then raised upward at 2.0 mm/s, stretching the strip until rupture. From this tension test, two parameters were obtained: the maximum resistance to extension (Rmax, N) and the extensibility until rupture (ERmax, mm).
2.7. Statistical Analysis
3. Results and Discussion
3.1. Pasta Cooking Quality
3.2. Colour Stability upon Cooking
3.3. Physicochemical Analysis of Pasta
3.4. Effect of Laminaria Processing on the Presence of Phytochemicals in the Pasta
3.5. Mechanical Properties of Pasta
3.5.1. Rheology Characterization of Pasta Samples
3.5.2. Texture Properties of Pasta Samples
3.6. Limitations and Future Work
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, R.; Grigor, J.; Winger, R.; Win, A. Functional food product development-opportunities and challenges for food manufacturers. Trends Food Sci. Technol. 2013, 30, 27–37. [Google Scholar] [CrossRef]
- Küster-Boluda, I.; Vidal-Capilla, I. Consumer attitudes in the election of functional foods. Span. J. Mark-ESIC 2017, 21, 65–79. [Google Scholar] [CrossRef]
- Santeramo, F.G.; Carluccib, D.; De Devitiisa, B.; Secciaa, A.; Stasia, A.; Viscecchiaa, R.; Nardone, G. Emerging trends in European food, diets and food industry. Food Res. Int. 2018, 104, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; Verbeke, W. Consumer evaluation, use and health relevance of health claims in the European Union. Food Q. Prefer. 2019, 74, 88–99. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Pontonio, E.; Filannino, P.; Rizzello, C.G.; De Angelis, M.; Di Cagnoa, R. How to improve the gluten-free diet: The state of the art from a food science perspective. Food Res. Int. 2018, 11, 22–32. [Google Scholar] [CrossRef]
- Nascimento, A.B.; Fiates, G.M.R.; dos Anjos, A.; Teixeira, E. Gluten-free is not enough—Perception and suggestions of celiac consumers. Int. J. Food Sci. Nutr. 2014, 65, 394–398. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Kim, S.K.; Bhatnagar, I. Properties of Medicinal Food-Laminaria. In Marine Medicinal Foods—Implications and Applications, Macro and Microalgae; Kim, S.K., Ed.; Academic Press: Oxford, UK, 2011; Volume 64, pp. 85–96, doi:10.1016/B978-0-12-387669-0.00007-7.Food-Laminaria. In Marine Medicinal Foods—Implications and Applications, Macro and Microalgae; Kim, S.K., Ed.; Academic Press: Oxford, UK, 2011; Volume 64, pp. 85–96. [Google Scholar] [CrossRef]
- Fernandes, F.; Barbosa, M.; Oliveira, A.P.; Azevedo, I.C.; Sousa-Pinto, I.; Valentão, P.; Andrade, P.B. The pigments of kelps (Ochrophyta) as part of the flexible response to highly variable marine environments. J. Appl. Phycol. 2016, 28, 3689–3696. [Google Scholar] [CrossRef]
- Flórez, N.; Gonzalez-Muñoz, M.J.; Ribeiro, D.; Fernandes, E.; Dominguez, H.; Freitas, M. Algae Polysaccharides’ Chemical Characterization and their Role in the Inflammatory Process. Curr. Med. Chem. 2017, 24, 149–175. [Google Scholar] [CrossRef]
- Parada, J.; Pérez-Correa, J.R.; Pérez-Jiménez, J. Design of low glycemic response foods using polyphenols from seaweed. J. Func. Foods 2018, 56, 33–39. [Google Scholar] [CrossRef]
- Cofrades, S.; Serdaroğlu, M.; Jiménez-Colmenero, F. Design of Healthier Foods and Beverages Containing Whole Algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; Volume 256, pp. 609–633. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Kouba, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Bouga, M.; Combet, E. Emergence of Seaweed and Seaweed-Containing Foods in the UK: Focus on Labeling, Iodine Content, Toxicity and Nutrition. Foods 2015, 4, 240–253. [Google Scholar] [CrossRef]
- Marfaing, H. Qualités nutritionnelles des algues, leur présent et futur sur la scène alimentaire. Cah Nutr. Diet 2017, 52, 257–268. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T.; Yunnie, A.L.E.; Vance, T. The rise of Laminaria ochroleuca in the Western English Channel (UK) and comparisons with its competitor and assemblage dominant Laminaria hyperborea. Mar Ecol. 2015, 36, 1033–1044. [Google Scholar] [CrossRef]
- Fradinho, P.; Flórez-Fernández, N.; Sousa, I.; Raymundo, A.; Domínguez, H.; Torres Pérez, M.D. Environmentally friendly processing of Laminaria ochroleuca for soft food applications with bioactive properties. J. Appl. Phycol. 2019. [Google Scholar] [CrossRef]
- Cofrades, S.; López-López, I.; Solas, M.T.; Bravo, L.; Jiménez-Colmenero, F. Influence of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008, 79, 767–776. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Del Olmo, A.; Picon, A.; Nuñez, M. Cheese supplementation with five species of edible seaweeds: Effect on microbiota, antioxidant activity, colour, texture and sensory characteristics. Int. Dairy J. 2018, 84, 36–45. [Google Scholar] [CrossRef]
- Palermo, M.A.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.; Shah, W. Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 2005, 91, 327–331. [Google Scholar] [CrossRef]
- Hager, A.S.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E.K. Starch properties, in vitro digestibility and sensory evaluation of fresh egg pasta produced from oat, teff and wheat flour. J. Cereal Sci. 2013, 58, 156–163. [Google Scholar] [CrossRef]
- Palavecino, P.M.; Ribotta, P.D.; León, A.E.; Bustos, M.C. Gluten-free sorghum pasta: Starch digestibility and antioxidant capacity compared with commercial products. J. Sci. Food Agric. 2019, 99, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.O. Estudos de Incorporação de Microalgas em Massa Alimentícia Isenta de Glúten. Master’s Thesis, Instituto Superior de Agronomia, Universidade de Lisboa, Portugal, 2018. Available online: https://www.repository.utl.pt/handle/10400.5/17818 (accessed on 8 October 2019).
- Fradinho, P.; Raymundo, A.; Sousa, I.; Domínguez, H.; Torres Pérez, M.D. Psyllium and Laminaria partnership—An overview of possible food gel applications. Appl. Sci. 2019, 9, 4356. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of Analysis, 11th ed.; Method 66–50.01. Pasta and Noodle Cooking Quality—Firmness; AACC International: St. Paul, MN, USA, 2000. [Google Scholar] [CrossRef]
- Fradinho, P.; Sousa, I.; Raymundo, A. Functional and thermorheological properties of rice flour gels for gluten-free pasta applications. Int. J. Food Sci. Tech. 2019, 54, 1109–1120. [Google Scholar] [CrossRef]
- ISO 20483. Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- FAO. Food Energy—Methods of Analysis and Conversion Factors; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; p. 93. [Google Scholar]
- Norma Portuguesa NP 4168. Cereais e Derivados—Determinação do Teor de Matéria Gorda Total; Instituto Português da Qualidade: Lisboa, Portugal, 1991. [Google Scholar]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal. Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- AOAC. Method 991.43. Total, Soluble, and Insoluble Dietary Fiber in Foods, 4th Revision. In AOAC Official Methods of Analysis, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Lee, S.C.; Rodriguez, F.; Storey, M. Determination of soluble and insoluble dietary fiber in Psyllium-containing cereal products. J. AOAC Int. 1995, 78, 724–729. [Google Scholar]
- Sant′Anna, V.; Christiano, F.D.P.; Marczak, L.D.F.; Tessaro, I.C.; Thys, R.C.S. The effect of the incorporation of grape marc powder in fettuccini pasta properties. LWT Food Sci. Technol. 2014, 58, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Marti, A.; Pagani, M.A. What can play the role of gluten in gluten free pasta? Trends Food Sci. Technol. 2013, 31, 63–71. [Google Scholar] [CrossRef]
- Bonomi, F.; D′Egidio, M.G.; Iametti, S.; Marengo, M.; Marti, A.; Pagani, M.A.; Ragg, E.M. Structure-quality relationship in commercial pasta: A molecular glimpse. Food Chem. 2012, 135, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Raymundo, A.; Fradinho, P.; Nunes, M.C. Effect of Psyllium fibre content on the textural and rheological characteristics of biscuit and biscuit dough. Bioact. Carbohydr. Dietary Fibre. 2014, 3, 96–105. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Chlorella vulgaris and Spirulina maxima biomass incorporation in pasta products. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M.; Leffler, D.A. Nutritional consequences of celiac disease and the gluten-free diet. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional Differences Between a Gluten-free Diet and a Diet Containing Equivalent Products with Gluten. Plant Foods Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef]
- Allen, B.; Orfila, C. The Availability and Nutritional Adequacy of Gluten-Free Bread and Pasta. Nutrients 2018, 10, 1370. [Google Scholar] [CrossRef] [Green Version]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of Pressurized Liquid Extraction (PLE) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). J. Pharm. Biomed. Anal. 2019, 164, 86–92. [Google Scholar] [CrossRef]
- Carcea, M.; Narducci, V.; Turfani, V.; Giannini, V. Polyphenols in Raw and Cooked Cereals/Pseudocereals/Legume Pasta and Couscous. Foods 2017, 6, 80. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Goñi, I.; Valdivieso, L.; Garcia-Alonso, A. Nori seaweed consumption modifies glycemic response in healthy volunteers. Nutr. Res. 2000, 20, 1367–1375. [Google Scholar] [CrossRef]
- Chang, H.C.; Wu, L.C. Texture and quality properties of Chinese fresh egg noodles formulated with green seaweed (Monostroma nitidum) powder. J. Food Sci. 2008, 73, S398–S404. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EC) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R1169&from=pt (accessed on 25 September 2019).
- European Commission. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1924&from=PT (accessed on 25 September 2019).
- European Commission. Regulation (EU) No 1047/2012 of 8 November 2012 Amending Regulation (EC) No 1924/2006 with Regard to the List of Nutrition Claims. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R1047&from=PT (accessed on 25 September 2019).
- Theethira, T.G.; Dennis, M. Celiac Disease and the Gluten-Free Diet: Consequences and Recommendations for Improvement. Dig. Dis. 2015, 33, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.I.; Palomeque, T.; Lorite, P. Celiac Disease and Other Autoimmune Disorders. In Autoimmunity—Pathogenesis, Clinical Aspects and Therapy of Specific Autoimmune Diseases; Chatzidionysiou, K., Ed.; IntechOpen: London, UK, 2015; Volume 6, pp. 131–151. [Google Scholar] [CrossRef] [Green Version]
- Lundin, K.E.A.; Alaedini, A. Non-celiac Gluten Sensitivity. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 723–734. [Google Scholar] [CrossRef]
- Henggeler, J.C.; Veríssimo, M.; Ramos, F. Non-coeliac gluten sensitivity: A review of the literature. Trends Food Sci. Tech. 2017, 66, 84–92. [Google Scholar] [CrossRef]
- Orecchio, S.; Amorello, D.; Raso, M.; Barreca, S.; Lino, C.; Di Gaudio, F. Determination of trace elements in gluten-free food for celiac people by ICP-MS. Microchem. J. 2014, 116, 163–172. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, N.; Alalwan, T.A.; et al. Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef] [Green Version]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Arredondo, M.; Núñez, M.T. Iron and copper metabolism. Mol. Asp. Med. 2005, 26, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.M.; Braverman, L.E. Consequences of excess iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Ti, H.; Li, Q.; Zhang, R.; Zhang, M.; Deng, Y.; Wei, Z.; Chi, J.; Zhang, Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in southern China. Food Chem. 2014, 159, 166–174. [Google Scholar] [CrossRef]
- Patel, M.K.; Mishra, A.; Jha, B. Non-targeted Metabolite Profiling and Scavenging Activity Unveil the Nutraceutical Potential of Psyllium (Plantago ovata Forsk). Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Effect of hydrothermal processing on colour, antioxidant and free radical scavenging capacities of edible Irish brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 2485–2493. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N. Influence of Indian Brown Seaweed (Sargassum marginatum) as an Ingredient on Quality, Biofunctional, and Microstructure Characteristics of Pasta. Food Sci. Tech. Int. 2009, 15, 0471–0479. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z., Jr.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Fois, S.; Campus, M.; Piu, P.P.; Siliani, S.; Sanna, M.; Roggio, T.; Catzeddu, P. Fresh Pasta Manufactured with Fermented Whole Wheat Semolina: Physicochemical, Sensorial, and Nutritional Properties. Foods 2019, 8, 422. [Google Scholar] [CrossRef] [Green Version]
- Vijaykrishnaraj, M.; Kumar, S.B.; Prabhasankar, P. Green mussel (Perna canaliculus) as a marine ingredient to enrich gluten free pasta: Product quality, microstructure and biofunctional evaluation. J. Food Meas. Charact. 2015, 9, 76–85. [Google Scholar] [CrossRef]
- Mendis, E.; Kim, S.K. Present and Future Prospects of Seaweeds in Developing Functional Foods. In Advances in Food and Nutrition Research; Kim, S.K., Ed.; Academic Press: Oxford, UK, 2011; Volume 64, pp. 1–15. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Potential of intensification techniques for the extraction and depolymerization of fucoidan. Algal. Res. 2018, 30, 128–148. [Google Scholar] [CrossRef]
- Abu-Ghannam, N.; Shannon, E. Seaweed Carotenoid, Fucoxanthin, as Functional Food. In Microbial Functional Foods and Nutraceuticals; Gupta, V.K., Treichel, H., Shapaval, V., Oliveira, L.A., Tuohy, M.G., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2017; Chapter 3; pp. 39–64. [Google Scholar] [CrossRef] [Green Version]
- Larrosa, V.; Lorenzo, G.; Zaritzky, N.; Califano, A. Improvement of the texture and quality of cooked gluten-free pasta. LWT Food Sci. Tech. 2016, 70, 96–103. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1980; p. 672. [Google Scholar]
- Tudorica, C.M.; Kuri, V.; Brennan, C.S. Nutritional and Physicochemical Characteristics of Dietary Fiber Enriched Pasta. J. Agric. Food Chem. 2002, 50, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.J.; Kim, Y.; Lee, S. Functional characterization of extruded rice noodles with corn bran: Xanthophyll content and rheology. J. Cereal Sci. 2014, 60, 311–316. [Google Scholar] [CrossRef]
- Bouasla, A.; Wojtowicz, A.; Zidoune, M.N. Gluten-free precooked rice pasta enriched with legumes flours: Physical properties, texture, sensory attributes and microstructure. LWT Food Sci. Tech. 2017, 75, 569–577. [Google Scholar] [CrossRef]





| Raw Pasta | Cooked Pasta | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | PL | PP | Control | PL | PP | |||
| Moisture | (g/100 g) | 50.3 ± 0.2 d | 49.2 ± 0.4 d,e | 48.3 ± 0.5 e | 65.4 ± 1.4 b | 62.9 ± 0.9 c | 67.6 ± 1.1 a | |
| Ash | (g/100 g, d.b) | 0.5 ± 0.0 e | 1.3 ± 0.1 b | 1.9 ± 0.1 a | 0.4 ± 0.0 e | 0.8 ± 0.0 d | 1.1 ± 0.1 c | |
| Lipids | 2.2 ± 0.2 a | 0.7 ± 0.0 c | 1.7 ± 0.3 a,b | 0.6 ± 0.2 c | 0.9 ± 0.1 c | 1.1 ± 0.1 b,c | ||
| Protein | 7.7 ± 0.2 c | 8.2 ± 0.2 a | 8.3 ± 0.2 a | 7.7 ± 0.0 b,c | 8.2 ± 0.1 a,b | 8.1 ± 0.0 a,b,c | ||
| Fibre | Insoluble | - | - | - | 4.8 ± 0.4 b | 4.4 ± 0.4 b | 7.1 ± 0.1 a | |
| Soluble | - | - | - | 0.9 ± 0.3 b | 1.4 ± 0.5 b | 2.4 ± 0.2 a | ||
| Total | - | - | - | 6.1 ± 0.7 b | 5.6 ± 0.8 b | 9.5 ± 0.3 a | ||
| Minerals | Na | (mg/100 g, d.b) | 74.1 ± 2.8 e | 145.7 ± 3.1 c | 229.1 ± 4.8 a | 70.0 ± 3.3 e | 110.4 ± 2.6 d | 159.4 ± 0.3 b |
| K | 325.3 ± 3.3 e | 558.6 ± 3.7 b | 761.1 ± 3.4 a | 272.4 ± 4.4 f | 394.4 ± 7.7 d | 495.2 ± 8.8 c | ||
| Ca | 7.4 ± 1.6 c | 14.6 ± 1.0 b | 50.3 ± 1.9 a | 9.9 ± 1.8 c | 15.0 ± 0.5 b | 47.3 ± 1.8 a | ||
| Mg | 28.3 ± 0.2 e | 38.9 ± 0.4 c | 55.1 ± 1.1 a | 27.6 ± 0.5 e | 34.3 ± 0.1 d | 51.9 ± 0.6 b | ||
| P | 115.8 ± 2.3 b | 123.2 ± 1.1 a | 121.9 ± 0.5 a | 109.5 ± 0.7 c | 110.0 ± 1.5 c | 116.9 ± 0.9 b | ||
| S | 109.0 ± 4.2 c | 126.2 ± 1.6 b | 144.8 ± 1.4 a | 104.7 ± 1.8 c | 121.3 ± 2.8 b | 150.3 ± 3.0 a | ||
| Fe | 0.7 ± 0.4 b | 0.5 ± 0.3 b | 0.5 ± 0.2 b | 1.2 ± 0.1 b | 3.9 ± 0.7 a | 1.5 ± 0.4 b | ||
| Cu | 0.3 ± 0.3 b | 1.1 ± 0.4 a,b | 1.7 ± 0.5 a | 0.3 ± 0.1 b | 0.4 ± 0.2 b | 0.6 ± 0.0 b | ||
| Zn | 1.1 ± 0.0 a | 1.6 ± 0.3 a | 1.4 ± 0.3 a | 1.3 ± 0.1 a | 1.7 ± 0.1 a | 1.6 ± 0.4 a | ||
| Mn | 0.8 ± 0.1 a | 0.8 ± 0.1 a | 0.8 ± 0.1 a | 0.8 ± 0.1 a | 0.9 ± 0.2 a | 0.7 ± 0.1 a | ||
| I | 0.1 ± 0.0 e | 0.8 ± 0.0 a | 0.5 ± 0.0 c | 0.1 ± 0.0 e | 0.6 ± 0.0 b | 0.4 ± 0.0 d | ||
| Minerals (mg/100 g) | Control | % RDAControl | PL | % RDAPL | PP | % RDAPP |
|---|---|---|---|---|---|---|
| K | 94.3 | 4.7 | 146.3 | 7.3 | 160.4 | 8.0 |
| Ca | 3.4 | 0.4 | 5.6 | 0.7 | 15.3 | 1.9 |
| Mg | 9.5 | 2.5 | 12.7 | 3.4 | 16.8 | 4.5 |
| P | 37.9 | 5.4 | 40.8 | 5.8 | 37.9 | 5.4 |
| Fe | 0.4 | 3.0 | 1.4 | 10.3 | 0.5 | 3.5 |
| Cu | 0.1 | 10.4 | 0.1 | 14.8 | 0.2 | 19.4 |
| Zn | 0.4 | 4.5 | 0.6 | 6.3 | 0.5 | 5.2 |
| Mn | 0.3 | 13.8 | 0.3 | 16.7 | 0.2 | 11.3 |
| I | 0.0 | 15.0 | 0.2 | 155.6 | 0.1 | 92.2 |
| Control | PL | PP | |||
|---|---|---|---|---|---|
| Raw Pasta | Firmness (N) |  | 3.2 ± 0.5 b | 3.6 ± 0.6 b | 5.8 ± 0.4 a |
| Adhesiveness (N·s) | −0.2 ± 0.1 a | −0.5 ± 0.1 b | −0.5 ± 0.1 b | ||
| Cohesiveness | 0.5 ± 0.0 a | 0.5 ± 0.0 a | 0.3 ± 0.0 b | ||
| Cooked Pasta | Firmness (N) |  | 2.8 ± 0.3 a | 2.4 ± 0.3 b | 2.3 ± 0.3 b |
| Stickiness (N) |  | 1.5 ± 0.4 a | 0.7 ± 0.2 b | 1.4 ± 0.4 a | |
| Rmax (N) |  | 0.6 ± 0.1 a,b | 0.7 ± 0.1 a | 0.6 ± 0.1 b | |
| ERmax (mm) | 4.6 ± 1.4 a | 5.6 ± 1.3 b | 5.5 ± 1.1 b | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fradinho, P.; Raymundo, A.; Sousa, I.; Domínguez, H.; Torres, M.D. Edible Brown Seaweed in Gluten-Free Pasta: Technological and Nutritional Evaluation. Foods 2019, 8, 622. https://doi.org/10.3390/foods8120622
Fradinho P, Raymundo A, Sousa I, Domínguez H, Torres MD. Edible Brown Seaweed in Gluten-Free Pasta: Technological and Nutritional Evaluation. Foods. 2019; 8(12):622. https://doi.org/10.3390/foods8120622
Chicago/Turabian StyleFradinho, Patrícia, Anabela Raymundo, Isabel Sousa, Herminia Domínguez, and María Dolores Torres. 2019. "Edible Brown Seaweed in Gluten-Free Pasta: Technological and Nutritional Evaluation" Foods 8, no. 12: 622. https://doi.org/10.3390/foods8120622