Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside
Abstract
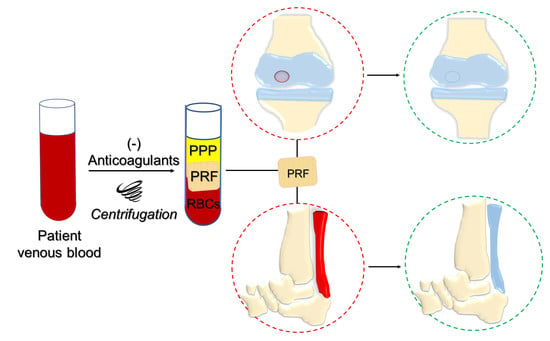
:1. Introduction
2. PRF-GFs in Chondrogenesis
3. PRF in Cartilage Tissue Engineering
3.1. In Vitro Studies
3.2. Pre-Clinical Implantation
3.3. Clinical Trials
4. PRF-GFs in Tenogenesis
5. PRF in Tendon Tissue Engineering
5.1. In Vitro Studies
5.2. Pre-Clinical Studies
5.3. Clinical Studies
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zhang, Y. Autologous liquid platelet rich fibrin: A novel drug delivery system. Acta Biomater. 2018, 75, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une opportunité en paro-implantologie: Le PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.B. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006c, 101, e51–e55. [Google Scholar] [CrossRef]
- Fioravanti, C.; Frustaci, I.; Armellin, E.; Condò, R.; Arcuri, C.; Cerroni, L. Autologous blood preparations rich in platelets, fibrin and growth factors. Oral Implantol. 2016, 8, 96–113. [Google Scholar] [CrossRef]
- Caloprisco, G.; Borean, A.; De Angeli, S.; Gaio, G.B.; Boito, K.; Del Pup, L.; Pavan, E.; Casale, V.; Varinelli, I. New method to produce hemocomponents for regenerative use from peripheral blood: Integration among platelet growth factors monocytes and stem cells. Transfus. Apher. Sci. 2010, 42, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Caloprisco, G.; Borean, A. Leucocyte-platelet haemocomponents for topical use: Regenerative potentiality. Acta Neurochir. Suppl. 2011, 108, 209–211. [Google Scholar] [CrossRef]
- Di Liddo, R.; Bertalot, T.; Borean, A.; Pirola, I.; Argentoni, A.; Schrenk, S.; Cenzi, C.; Capelli, S.; Conconi, M.T.; Parnigotto, P.P. Leucocyte and Platelet-rich Fibrin: A carrier of autologous multipotent cells for regenerative medicine. J. Cell Mol. Med. 2018, 22, 1840–1854. [Google Scholar] [CrossRef]
- Barbon, S.; Stocco, E.; Grandi, F.; Rajendran, S.; Borean, A.; Pirola, I.; Capelli, S.; Bagno, A.; Tavano, R.; Contran, M.; et al. Biofabrication of a novel leukocyte-fibrin-platelet membrane as a cells and growth factors delivery platform for tissue engineering applications. J. Tissue Eng. Regen. Med. 2018, 12, 1891–1906. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef]
- Tunalı, M.; Özdemir, H.; Küçükodacı, Z.; Akman, S.; Fıratlı, E. In vivo evaluation of titanium-prepared platelet-rich fibrin (T-PRF): A new platelet concentrate. Br. J. Oral Maxillofac. Surg. 2013, 51, 438–443. [Google Scholar] [CrossRef]
- Pinto, N.R.; Pereda, A.; Jiménez, P.; Del Corso, M.; Kang, B.S.; Wang, H.L.; Quirynen, M.; Dohan Ehrenfest, D.M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors and fibrin architecture of a Leukocyte- and Platelet-Rich Fibrin (L-PRF) clot and membrane. Part 2: Macroscopic, photonic microscopy and Scanning Electron Microscopy analysis of 4 kinds of L-PRF clots and membranes. POSEIDO 2014, 2, 141–154. [Google Scholar]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H.; Isobe, K.; Watanabe, T.; Okudera, T.; Nakamura, M.; Suzuki, M.; Ryu, J.; Kitamura, Y.; Okudera, H.; Okuda, K.; et al. Quality Assessment of Platelet-Rich Fibrin-Like Matrix Prepared from Whole Blood Samples after Extended Storage. Biomedicines 2017, 5, 57. [Google Scholar] [CrossRef]
- De Pascale, M.R.; Sommese, L.; Casamassimi, A.; Napoli, C. Platelet derivatives in regenerative medicine: An update. Transfus. Med. Rev. 2015, 29, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Agrawal, A.A. Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World J. Clin. Cases 2017, 5, 159–171. [Google Scholar] [CrossRef]
- Ghanaati, S.; Herrera-Vizcaino, C.; Al-Maawi, S.; Lorenz, J.; Miron, R.J.; Nelson, K.; Schwarz, F.; Choukroun, J.; Sader, R. Fifteen Years of Platelet Rich Fibrin in Dentistry and Oromaxillofacial Surgery: How High is the Level of Scientific Evidence? J. Oral Implantol. 2018, 44, 471–492. [Google Scholar] [CrossRef]
- Dülgeroglu, T.C.; Metineren, H. Evaluation of the Effect of Platelet-Rich Fibrin on Long Bone Healing: An Experimental Rat Model. Orthopedics 2017, 40, e479–e484. [Google Scholar] [CrossRef]
- Ved, V.; Bhagat, J.; Gala, V.; Fernandes, G. Platelet Rich Fibrin and Its Role in Regenerative Dentistry: A Mini Review. J. Dent. Sci. Med. 2018, 3, 127. [Google Scholar]
- Sánchez-González, D.J.; Méndez-Bolaina, E.; Trejo-Bahena, N.I. Platelet-Rich Plasma Peptides: Key for Regeneration. Int. J. Pept. 2012, 532519. [Google Scholar] [CrossRef]
- Qureshi, A.H.; Chaoji, V.; Maiguel, D.; Faridi, M.H.; Barth, C.J.; Salem, S.M.; Singhal, M.; Stoub, D.; Krastins, B.; Ogihara, M.; et al. Proteomic and phospho-proteomic profile of human platelets in basal, resting state: Insights into integrin signaling. PLoS ONE 2009, 4, e7627. [Google Scholar] [CrossRef]
- Molloy, T.; Wang, Y.; Murrell, G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003, 33, 381–394. [Google Scholar] [CrossRef]
- Brandl, A.; Angele, P.; Roll, C.; Prantl, L.; Kujat, R.; Kinner, B. Influence of the growth factors PDGF-BB, TGF-beta1 and bFGF on the replicative aging of human articular chondrocytes during in vitro expansion. J. Orthop. Res. 2010, 28, 354–360. [Google Scholar] [CrossRef]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef]
- Kabiri, A.; Esfandiari, E.; Esmaeili, A.; Hashemibeni, B.; Pourazar, A.; Mardani, M. Platelet-rich plasma application in chondrogenesis. Adv. Biomed. Res. 2014, 3, 138. [Google Scholar] [CrossRef]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z.; Nehrer, S. Platelet-Rich Plasma Supports Proliferation and Redifferentiation of Chondrocytes during In Vitro Expansion. Front. Bioeng. Biotechnol. 2017, 5, 75. [Google Scholar] [CrossRef]
- Gaissmaier, C.; Koh, J.L.; Weise, K. Growth and differentiation factors for cartilage healing and repair. Injury 2008, 39, S88–S96. [Google Scholar] [CrossRef] [PubMed]
- Akeda, K.; An, H.S.; Okuma, M.; Attawia, M.; Miyamoto, K.; Thonar, E.J.; Lenz, M.E.; Sah, R.L.; Masuda, K. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthr. Cartil. 2006, 14, 1272–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Feng, Y.; Zhang, C.Q.; Chen, S.B.; Cheng, X.G. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int. Orthop. 2009, 34, 589–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milano, G.; Sanna Passino, E.; Deriu, L.; Careddu, G.; Manunta, L.; Manunta, A.; Saccomanno, M.F.; Fabbriciani, C. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: An experimental study in a sheep model. Osteoarthr. Cartil. 2010, 18, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Civinini, R.; Nistri, L.; Martini, C.; Redl, B.; Ristori, G.; Innocenti, M. Growth factors in the treatment of early osteoarthritis. Clin. Cases Miner. Bone Metab. 2013, 10, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, X.; Xu, L.; Zhang, Y.; Yin, Q.; Wang, F. PDGF regulates chondrocyte proliferation through activation of the GIT1- and PLCγ1-mediated ERK1/2 signaling pathway. Mol. Med. Rep. 2014, 10, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Montaseri, A.; Busch, F.; Mobasheri, A.; Buhrmann, C.; Aldinger, C.; Rad, J.S.; Shakibaei, M. IGF-1 and PDGF-bb suppress IL-1β-induced cartilage degradation through down-regulation of NF-κB signaling: Involvement of Src/PI-3K/AKT pathway. PLoS ONE 2011, 6, e28663. [Google Scholar] [CrossRef]
- Longobardi, L.; O’Rear, L.; Aakula, S.; Johnstone, B.; Shimer, K.; Chytil, A.; Horton, W.A.; Moses, H.L.; Spagnoli, A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J. Bone Miner. Res. 2006, 21, 626–636. [Google Scholar] [CrossRef]
- Goodrich, L.R.; Hidaka, C.; Robbins, P.D.; Evans, C.H.; Nixon, A.J. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J. Bone Joint Surg. Br. 2007, 89, 672–685. [Google Scholar] [CrossRef] [Green Version]
- Boehm, A.K.; Seth, M.; Mayr, K.G.; Fortier, L.A. Hsp90 mediates insulin-like growth factor 1 and interleukin-1beta signaling in an age-dependent manner in equine articular chondrocytes. Arthritis Rheum. 2007, 56, 2335–2343. [Google Scholar] [CrossRef]
- Loeser, R.F.; Carlson, C.S.; Del Carlo, M.; Cole, A. Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum. 2002, 46, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Blaney Davidson, E.N.; Kraan, P.M.; Berg, W.B. TGF-beta and osteoarthritis. Osteoarthr. Cartil. 2007, 15, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Green, J.D.; Tollemar, V.; Dougherty, M.; Yan, Z.; Yin, L.; Ye, J.; Collier, Z.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. Multifaceted signaling regulators of chondrogenesis: Implications in cartilage regeneration and tissue engineering. Genes Dis. 2015, 2, 307–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Gong, Y.; Ren, L.; Varshney, R.R.; Cai, D.; Wang, D.A. In vitro engineered cartilage using synovium-derived mesenchymal stem cells with injectable gellan hydrogels. Acta Biomater. 2010, 6, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Wang, J.; Shen, C.; Xia, S.; Guo, T.; Dong, L.; Zhang, C.; Chen, J.; Zhao, J.; Zhang, J. Improved cartilage regeneration utilizing mesenchymal stem cells in TGF-beta1 gene-activated scaffolds. Tissue Eng. Part A 2009, 15, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Rey-Rico, A.; Venkatesan, J.K. Transforming growth factor Beta-releasing scaffolds for cartilage tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Grandi, F.; Gamba, P.G.; Borgio, L.; Del Gaudio, C.; Dalzoppo, D.; Lora, S.; Rajendran, S.; Porzionato, A.; et al. Partially oxidized polyvinyl alcohol as a promising material for tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Nummenmaa, E.; Hämäläinen, M.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Effects of FGF-2 and FGF receptor antagonists on MMP enzymes, aggrecan, and type II collagen in primary human OA chondrocytes. Scand. J. Rheumatol. 2015, 44, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.N.; Batschkus, S.; Schimmel, S.; Bode, C.; Schminke, B.; Miosge, N. The Influence of TGF-β3, EGF, and BGN on SOX9 and RUNX2 Expression in Human Chondrogenic Progenitor Cells. J. Histochem. Cytochem. 2019, 67, 117–127. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Liu, F.; Li, Y.; Chandra, A.; Levin, L.S.; Beier, F.; Enomoto-Iwamoto, M.; Qin, L. Reduced EGFR signaling enhances cartilage destruction in a mouse osteoarthritis model. Bone Res. 2014, 2, 14015. [Google Scholar] [CrossRef] [Green Version]
- Iacob, S.; Cs-Szabo, G. Biglycan regulates the expression of EGF receptors through EGF signaling pathways in human articular chondrocytes. Connect. Tissue Res. 2010, 51, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Yudoh, K.; Masuko, K. The potential role of vascular endothelial growth factor (VEGF) in cartilage: How the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthr. Cartil. 2008, 16, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Sheu, S.Y.; Hsu, L.H.; Yang, K.C.; Tseng, C.C.; Kuo, T.F. Intra-articular Injection of platelet-rich fibrin releasates in combination with bone marrow-derived mesenchymal stem cells in the treatment of articular cartilage defects: An in vivo study in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.R.; Carvalho, M.R.; Oliveira, J.M.; Reis, R.L. Tissue Engineering Strategies for Osteochondral Repair. Adv. Exp. Med. Biol. 2018, 1059, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. Sci. 1994, 331, 889. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, M.J.; Grad, S.; Eglin, D.; Alini, M. Cells and biomaterials in cartilage tissue engineering. Regen. Med. 2009, 4, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Graceffa, V.; Vinatier, C.; Guicheux, J.; Stoddart, M.; Alini, M.; Zeugolis, D.I. Chasing Chimeras—The elusive stable chondrogenic phenotype. Biomaterials 2019, 192, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Cole, B.J.; Kercher, J.S.; Rodkey, W.G.; Briggs, K.K. Microfracture. Cartilage 2010, 1, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Bella, C.; Fosang, A.; Donati, D.M.; Wallace, G.G.; Choong, P.F. 3D Bioprinting of Cartilage for Orthopedic Surgeons: Reading between the Lines. Front. Surg. 2015, 2, 39. [Google Scholar] [CrossRef]
- Onofrillo, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; O’Connor, A.J.; Scott, M.; Wallace, G.G.; Choong, P.F.M.; Di Bella, C. Biofabrication of human articular cartilage: A path towards the development of a clinical treatment. Biofabrication 2018, 10, 045006. [Google Scholar] [CrossRef]
- Abd El Raouf, M.; Wang, X.; Miusi, S.; Chai, J.; Mohamed AbdEl-Aal, A.B.; Nefissa Helmy, M.M.; Ghanaati, S.; Choukroun, J.; Choukroun, E.; Zhang, Y.; et al. Injectable-platelet rich fibrin using the low speed centrifugation concept improves cartilage regeneration when compared to platelet-rich plasma. Platelets 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bahmanpour, S.P.; Ghasemi, M.P.; Sadeghi-Naini, M.M.; Kashani, I.R.P. Effects of Platelet-Rich Plasma & Platelet-Rich Fibrin with and without Stromal Cell-Derived Factor-1 on Repairing Full-Thickness Cartilage Defects in Knees of Rabbits. Iran. J. Med. Sci. 2016, 41, 507–517. [Google Scholar]
- Zhu, Y.; Yuan, M.; Meng, H.Y.; Wang, A.Y.; Guo, Q.Y.; Wang, Y.; Peng, J. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthr. Cartil. 2013, 21, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis. Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.S.; Ho, H.O.; Liang, Y.C.; Ko, P.H.; Sheu, M.T.; Chen, C.H. Incorporation of exudates of human platelet-rich fibrin gel in biodegradable fibrin scaffolds for tissue engineering of cartilage. J. Biomed. Mater. Res Part B 2012, 100, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Chen, C.H.; Chan, W.P.; Chiu, L.H.; Ho, W.P.; Hsieh, F.J.; Chen, Y.T.; Yang, T.L. Single-Stage Cartilage Repair Using Platelet-Rich Fibrin Scaffolds with Autologous Cartilaginous Grafts. Am. J. Sports Med. 2017, 45, 3128–3142. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Kuo, T.F.; Yang, T.L.; Tsuang, Y.H.; Lin, M.F.; Chang, C.H.; Lin, Y.H.; Chan, W.P. Platelet-Rich Fibrin Facilitates Rabbit Meniscal Repair by Promoting Meniscocytes Proliferation, Migration, and Extracellular Matrix Synthesis. Int. J. Mol. Sci. 2017, 18, 1722. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.G.d.; Fernandes, B.L.; Rebelatto, C.L.K.; Aguiar, A.M.d.; Fracaro, L.; Brofman, P.R.S. Proliferation and differentiation of stem cells in contact with eluate from fibrin-rich plasma membrane. Rev. Bras. Ortop. 2017, 53, 45–52. [Google Scholar] [CrossRef]
- Kuo, T.F.; Lin, M.F.; Lin, Y.H.; Lin, Y.C.; Su, R.J.; Lin, H.W.; Chan, W.P. Implantation of platelet-rich fibrin and cartilage granules facilitates cartilage repair in the injured rabbit knee: Preliminary report. Clinics 2011, 66, 1835–1838. [Google Scholar] [CrossRef]
- Kazemi, D.; Fakhrjou, A.; Dizaji, V.M.; Alishahi, M.K. Effect of autologous platelet rich fibrin on the healing of experimental articular cartilage defects of the knee in an animal model. Biomed. Res. Int. 2014, 2014, 486436. [Google Scholar] [CrossRef]
- Kazemi, D.; Fakhrjou, A. Leukocyte and Platelet Rich Plasma (L-PRP) Versus Leukocyte and Platelet Rich Fibrin (L-PRF) For Articular Cartilage Repair of the Knee: A Comparative Evaluation in an Animal Model. Iran. Red Crescent Med. J. 2015, 17, e19594. [Google Scholar] [CrossRef]
- Goodrich, L.R.; Chen, A.C.; Werpy, N.M.; Williams, A.A.; Kisiday, J.D.; Su, A.W.; Cory, E.; Morley, P.S.; McIlwraith, C.W.; Sah, R.L.; et al. Addition of Mesenchymal Stem Cells to Autologous Platelet-Enhanced Fibrin Scaffolds in Chondral Defects: Does It Enhance Repair? J. Bone Joint Surg. Am. 2016, 98, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Satake, H.; Suzuki, T.; Honma, R.; Naganuma, Y.; Takakubo, Y.; Takagi, M. Comparison of the Effects of Osteochondral Autograft Transplantation with Platelet-Rich Plasma or Platelet-Rich Fibrin on Osteochondral Defects in a Rabbit Model. Am. J. Sports Med. 2017, 45, 3280–3288. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, D.; Shams Asenjan, K.; Dehdilani, N.; Parsa, H. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin: Macroscopic and histological assessments. Bone Joint Res. 2017, 6, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.Y.; Wang, C.H.; Pao, Y.H.; Fu, Y.T.; Liu, C.H.; Yao, C.H.; Kuo, T.F. The effect of platelet-rich fibrin on autologous osteochondral transplantation: An in vivo porcine model. Knee 2017, 24, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.K.; Sheu, S.Y.; Wang, C.Y.; Chuang, M.H.; Chung, P.C.; Luo, Y.S.; Huang, J.J.; Ohashi, F.; Akiyoshi, H.; Kuo, T.F. The effect of adipose-derived mesenchymal stem cells and chondrocytes with platelet-rich fibrin releasates augmentation by intra-articular injection on acute osteochondral defects in a rabbit model. Knee 2018, 25, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yang, Y.; Yang, T.; Dai, T.; Shao, X.; Xu, H.; An, R.; Liu, Y.; Liu, B. The use of allogenic adipose-derived stem cells in combination with platelet-rich fibrin for the treatment of cartilage defects in rabbit ear. American journal of translational research. Am. J. Transl. Res. 2018, 10, 1900–1907. [Google Scholar]
- Güler, İ.; Billur, D.; Aydin, S.; Kocatürk, S. Efficacy of platelet-rich fibrin matrix on viability of diced cartilage grafts in a rabbit model. Laryngoscope 2015, 125, E104–E111. [Google Scholar] [CrossRef]
- Göral, A.; Aslan, C.; Bolat Küçükzeybek, B.; Işık, D.; Hoşnuter, M.; Durgun, M. Platelet-Rich Fibrin Improves the Viability of Diced Cartilage Grafts in a Rabbit Model. Aesthet. Surg. J. 2016, 36, NP153–NP162. [Google Scholar] [CrossRef]
- Cook, J.L.; Hung, C.T.; Kuroki, K.; Stoker, A.M.; Cook, C.R.; Pfeiffer, F.M.; Sherman, S.L.; Stannard, J.P. Animal models of cartilage repair. Bone Joint Res. 2014, 3, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.R.; Szczodry, M.; Bruno, S. Animal models for cartilage regeneration and repair. Tissue Eng. Part B Rev. 2010, 16, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Buda, R.; Cavallo, M.; Castagnini, F.; Cenacchi, A.; Natali, S.; Vannini, F.; Giannini, S. Treatment of Hemophilic Ankle Arthropathy with One-Step Arthroscopic Bone Marrow-Derived Cells Transplantation. Cartilage 2015, 6, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Radossi, P.; Rajendran, S.; Dalzoppo, D.; Bortolami, M.; Bagno, A.; Grandi, F.; Gamba, P.G.; Parnigotto, P.P.; et al. Autologous chondrocytes as a novel source for neo-chondrogenesis in haemophiliacs. Cell Tissue Res. 2016, 366, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Papalia, R.; Diaz Balzani, L.; Torre, G.; Tirindelli, M.C.; Nobile, C.; Maffulli, N.; Denaro, V. Intraoperative application Platelet rich fibrin, postoperative injections OF PRP or microfracture only for osteochondral lesions of the knee: A five-year retrospective evaluation. J. Biol. Regul. Homeost. Agents 2016, 30, 41–49. [Google Scholar] [PubMed]
- D’Antimo, C.; Biggi, F.; Borean, A.; Di Fabio, S.; Pirola, I. Combining a novel leucocyte-platelet-concentrated membrane and an injectable collagen scaffold in a single-step AMIC procedure to treat chondral lesions of the knee: A preliminary retrospective study. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, M.; Riedel, F.; Wurm, J.; Bran, G.M. Cartilage Scales Embedded in Fibrin Gel. Facial Plast. Surg. 2017, 33, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Nourissat, G.; Berenbaum, F.; Duprez, D. Tendon injury: From biology to tendon repair. Nat. Rev. Rheumatol. 2015, 11, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Mast, B.A. Healing in other tissues. Surg. Clin. North Am. 1997, 77, 529–547. [Google Scholar] [CrossRef]
- Branford, O.A.; Klass, B.R.; Grobbelaar, A.O.; Rolfe, K.J. The growth factors involved in flexor tendon repair and adhesion formation. J. Hand Surg. Eur. Vol. 2014, 39, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Chang, J. Clinical implications of growth factors in flexor tendon wound healing. J. Hand Surg. Am. 2004, 29, 551–563. [Google Scholar] [CrossRef]
- Tozer, S.; Duprez, D. Tendon and ligament: Development, repair and disease. Birth Defects Res. C Embryo Today 2005, 75, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, R.; Zelzer, E.; Volk, T. Connecting muscles to tendons: Tendons and musculoskeletal development in flies and vertebrates. Development 2010, 137, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.I.; Rodrigues, M.T.; Lee, S.J.; Atala, A.; Yoo, J.J.; Reis, R.L.; Gomes, M.E. Understanding the role of growth factors in modulating stem cell tenogenesis. PLoS ONE 2013, 8, e83734. [Google Scholar] [CrossRef]
- Herchenhan, A.; Bayer, M.L.; Eliasson, P.; Magnusson, S.P.; Kjaer, M. Insulin-like growth factor I enhances collagen synthesis in engineered human tendon tissue. Growth Horm. IGF Res. 2015, 25, 13–19. [Google Scholar] [CrossRef]
- Dahlgren, L.A.; Mohammed, H.O.; Nixon, A.J. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J. Orthop. Res. 2005, 23, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissell, L.; Tibrewal, S.; Sahni, V.; Khan, W.S. Growth factors and platelet rich plasma in anterior cruciate ligament reconstruction. Curr. Stem Cell Res. Ther. 2015, 10, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J.; Nixon, A.J. Biochemical and site-specific effects of insulin-like growth factor I on intrinsic tenocyte activity in equine flexor tendons. Am. J. Vet. Res. 1997, 58, 103–109. [Google Scholar] [PubMed]
- Lynch, S.E.; Colvin, R.B.; Antoniades, H.N. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J. Clin. Investig. 1989, 84, 640–646. [Google Scholar] [CrossRef]
- Petersen, W.; Pufe, T.; Kurz, B.; Mentlein, R.; Tillmann, B. Angiogenesis in fetal tendon development: Spatial and temporal expression of the angiogenic peptide vascular endothelial cell growth factor. Anat. Embryol. 2002, 205, 263–270. [Google Scholar] [CrossRef]
- Halper, J. Advances in the use of growth factors for treatment of disorders of soft tissues. Adv. Exp. Med. Biol. 2014, 802, 59–76. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; Nurden, A.T.; Zalduendo, M.; de la Fuente, M.; Orive, G.; Azofra, J.; Andia, I. Autologous fibrin matrices: A potential source of biological mediators that modulate tendon cell activities. J. Biomed. Mater. Res. A 2006, 77, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.C.; Arnoczky, S.P.; Caballero, O.; Egerbacher, M. Platelet-Rich Fibrin Constructs Elute Higher Concentrations of Transforming Growth Factor-β1 and Increase Tendon Cell Proliferation Over Time when Compared to Blood Clots: A Comparative In Vitro Analysis. Vet. Surg. 2010, 39, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Russell, R.P.; Apostolakos, J.; Ramos, D.M.; Kumbar, S.G.; Imhoff, A.B.; Arciero, R.A.; Mazzocca, A.D. Properties of Biologic Scaffolds and Their Response to Mesenchymal Stem Cells. Arthroscopy 2014, 30, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Barber, F.A.; Hrnack, S.A.; Snyder, S.J.; Hapa, O. Rotator Cuff Repair Healing Influenced by Platelet-Rich Plasma Construct Augmentation. Arthroscopy 2011, 27, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Barber, F.A. Triple-Loaded Single-Row versus Suture-Bridge Double-Row Rotator Cuff Tendon Repair with Platelet-Rich Plasma Fibrin Membrane: A Randomized Controlled Trial. Arthroscopy 2016, 32, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Bergeson, A.G.; Tashjian, R.Z.; Greis, P.E.; Crim, J.; Stoddard, G.J.; Burks, R.T. Effects of Platelet-Rich Fibrin Matrix on Repair Integrity of At-Risk Rotator Cuff Tears. Am. J. Sports Med. 2012, 40, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Castricini, R.; Longo, U.G.; De Benedetto, M.; Panfoli, N.; Pirani, P.; Zini, R.; Maffulli, N.; Denaro, V. Platelet-Rich Plasma Augmentation for Arthroscopic Rotator Cuff Repair: A Randomized Controlled Trial. Am. J. Sports Med. 2011, 39, 258–265. [Google Scholar] [CrossRef]
- Hasan, S.; Weinberg, M.; Khatib, O.; Jazrawi, L.; Strauss, E.J. The Effect of Platelet-rich Fibrin Matrix on Rotator Cuff Healing in a Rat Model. Int. J. Sports Med. 2016, 37, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S.A.; Delos, D.; Williams, R.J.; Adler, R.S.; Pearle, A.; Warren, R.F. The Effect of Platelet-Rich Fibrin Matrix on Rotator Cuff Tendon Healing: A Prospective, Randomized Clinical Study. Am. J. Sports Med. 2012, 40, 1234–1241. [Google Scholar] [CrossRef]
- Weber, S.C.; Kauffman, J.I.; Parise, C.; Weber, S.J.; Katz, S.D. Platelet-Rich Fibrin Matrix in the Management of Arthroscopic Repair of the Rotator Cuff:A Prospective, Randomized, Double-Blinded Study. Am. J. Sports Med. 2013, 41, 263–270. [Google Scholar] [CrossRef]
- Zumstein, M.A.; Berger, S.; Schober, M.; Boileau, P.; Nyffeler, R.W.; Horn, M.; Dahinden, C.A. Leukocyte- and platelet-rich fibrin (L-PRF) for long-term delivery of growth factor in rotator cuff repair: Review, preliminary results and future directions. Curr. Pharm. Biotechnol. 2012, 13, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Zumstein, M.A.; Rumian, A.; Thélu, C.É.; Lesbats, V.; O’Shea, K.; Schaer, M.; Boileau, P. SECEC Research Grant 2008 II: Use of platelet- and leucocyte-rich fibrin (L-PRF) does not affect late rotator cuff tendon healing: A prospective randomized controlled study. J. Shoulder Elbow Surg. 2016, 25, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Alviti, F.; Gurzì, M.; Santilli, V.; Paoloni, M.; Padua, R.; Bernetti, A.; Bernardi, M.; Mangone, M. Achilles Tendon Open Surgical Treatment with Platelet-Rich Fibrin Matrix Augmentation: Biomechanical Evaluation. J. Foot Ankle Surg. 2017, 56, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Sarrafian, T.L.; Wang, H.; Hackett, E.S.; Yao, J.Q.; Shih, M.S.; Ramsay, H.L.; Turner, A.S. Comparison of Achilles Tendon Repair Techniques in a Sheep Model Using a Cross-linked Acellular Porcine Dermal Patch and Platelet-rich Plasma Fibrin Matrix for Augmentation. J. Foot Ankle Surg. 2010, 49, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Takahara, M.; Narita, A.; Yamakawa, J.; Hashimoto, J.; Ishikawa, H.; Ogino, T. Effect of Platelet-Rich Plasma with Fibrin Matrix on Healing of Intrasynovial Flexor Tendons. J. Hand Surg. Am. 2012, 37, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.Y.; He, M.; Gan, A.W.T.; Chong, A.K.S. The Effects of Autologous Platelet-Rich Fibrin on Flexor Tendon Healing in a Rabbit Model. J. Hand Surg. Am. 2017, 42, 928.e1–928.e7. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.C.; Arnoczky, S.P.; Caballero, O.; Gardner, K.L. Evaluation of the use of an autologous platelet-rich fibrin membrane to enhance tendon healing in dogs. Am. J. Vet. Res. 2011, 72, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, D.; Akizuki, S.; Takizawa, T.; Omae, S.; Kato, H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee 2013, 20, 545–550. [Google Scholar] [CrossRef]
- Saltzman, B.M.; Ukwuani, G.; Makhni, E.C.; Stephens, J.P.; Nho, S.J. The Effect of Platelet-Rich Fibrin Matrix at the Time of Gluteus Medius Repair: A Retrospective Comparative Study. Arthroscopy 2018, 34, 832–841. [Google Scholar] [CrossRef]
- Xin, X.; Yang, S.; Ingle, G.; Zlot, C.; Rangell, L.; Kowalski, J.; Schwall, R.; Ferrara, N.; Gerritsen, M.E. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am. J. Pathol. 2001, 158, 1111–1120. [Google Scholar] [CrossRef]
- Castillo, T.N.; Pouliot, M.A.; Kim, H.J.; Dragoo, J.L. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011, 39, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Boswell, S.G.; Cole, B.J.; Sundman, E.A.; Karas, V.; Fortier, L.A. Platelet-rich plasma: A milieu of bioactive factors. Arthroscopy 2012, 28, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Madurantakam, P.; Yoganarasimha, S.; Hasan, F.K. Characterization of Leukocyte-platelet Rich Fibrin, A Novel Biomaterial. J. Vis. Exp. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, G.; Lingampalli, N.; Koltsov, J.C.B.; Leung, L.L.; Bhutani, N.; Robinson, W.H.; Chu, C.R. Men and Women Differ in the Biochemical Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2018, 46, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Engeland, C.G.; Sabzehei, B.; Marucha, P.T. Sex hormones and mucosal wound healing. Brain Behav. Immun. 2009, 23, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Horng, H.C.; Chang, W.H.; Yeh, C.C.; Huang, B.S.; Chang, C.P.; Chen, Y.J.; Tsui, K.H.; Wang, P.H. Estrogen Effects on Wound Healing. Int. J. Mol. Sci. 2017, 18, 2325. [Google Scholar] [CrossRef] [PubMed]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef] [PubMed]



| Hemocomponent/Experimental Groups | PRF Preparation Protocol | Characterization Parameters | Major Findings | Reference |
|---|---|---|---|---|
| - Human PRF exudates incorporated into Biodegradable Fibrin (FB) scaffolds Controls: - Bovine Biodegradable Fibrin scaffolds - Agarose scaffolds | Preparation according to Choukroun et al., 2001 [3]: - Blood collection without anticoagulant - Centrifugation (400 X g, 10 min) - Formation of a fibrin clot rich with platelets (PRF) in the middle of the tube, between the red blood cells and the acellular plasma | - Quantification of PDGF-BB, TGF-β1, IGF-1 and BMP-2 into PRF exudates - 2D and 3D cultures of human primary chondrocytes and a human chondrosarcoma cell line (SW-1353) - Proliferation studies - mRNA expression of type-II collagen and GAGs - Synthesis of GAGs and proteoglycans | When chondrocytes were cultured on FB scaffolds added with PRF exudates: - cell growth rate was significantly increased - mRNA expression of type-II collagen and GAGs was up-regulated - Synthesis of GAGs and proteoglycans was enhanced | Chien et al., 2012 [65] |
| - Rabbit i-PRF Control: - Rabbit PRP | - Blood collection without anticoagulant - Centrifugation (60 X g, 3 min) with Choukroun PRF Duo Centrifuge (Process for PRF, Nice, France) - Collection of the upper plasma layer designated as i-PRF | - i-PRF- and PRP-conditioned cultures of rabbit chondrocytes in normal conditions or in the presence of IL-1β - mRNA expression of chondrogenesis-related genes (SOX9, COL2A1 and ACAN) and osteoarthritis-related markers (ADAMTS4, PTGS2 and MMP13) | i-PRF was found to be superior to PRP in: - up-regulating chondrogenesis-related genes in normal conditions - counteracting IL-1β inflammatory effects in osteoarthritis-like environment | Abd El Raouf et al., 2017 [61] |
| - Rabbit PRF | Preparation according to Choukroun et al., 2001 [3] | - Quantification of PDGF, IGF-1 and TGF-β1 release - Mechanical tests - Ultrastructural morphology by SEM - In vitro and ex vivo evaluations of PRF chemotactic effect on rabbit chondrocytes - Proliferation of chondrocyte cultures - mRNA expression of cartilage markers (type-I and type-II collagen and Aggrecan) - GAG deposition | - PRF improved the chemotaxis, proliferation, and viability of the cultured chondrocytes - Chondrogenic markers were up-regulated in cell populations cultured with PRF-conditioned media - PRF increased the formation and deposition of the cartilaginous matrix produced by cultured chondrocytes | Wong et al., 2017 [66] |
| - Rabbit PRF | Preparation according to Choukroun et al., 2001 [3] | - PRF chemotactic effect on rabbit meniscocytes (scratch migration and transwell migration assays) - Cell proliferation - Histological evaluation of type-I and type-II collagen, Aggrecan and GAG deposition | - PRF stimulated cellular migration and proliferation of meniscocytes - Extracellular matrix synthesis by cultured meniscocytes was enhanced by treatment with PRF releseates | Wong et al., 2017 [67] |
| - Human FRP membrane | - Blood collection without anticoagulant but with a clot activator - Centrifugation (770× g, 12 min) - Pression of the fibrin clot with stainless steel plate (Box PRF BmdCon®) for exudate extraction - FRP membrane formation | - Proliferation of human ASCs - Differentiation of ASC micromass cultures towards the chondrogenic lineage | - FRP membrane eluates stimulated the proliferation of ASCs - Treatment with eluates induced mucopolysaccharide and aggrecan synthesis by differentiated ASCs | Souza et al., 2017 [68] |
| End Use Destination | Hemocomponent/Experimental Groups | PRF Preparation Protocol | Characterization Parameters | Major Findings | Reference |
|---|---|---|---|---|---|
| Rabbits Chondral defect in the femoral condyle (diameter: 3 mm; depth: 0.5 mm) | Rabbit PRF combined with cartilage granules derived from the created defect Control: - cartilage defect with no implantation | Preparation according to Choukroun et al., 2001 [3]: - Blood collection without anticoagulant - Centrifugation (400 X g, 10 min) - Formation of a fibrin clot rich with platelets (PRF) in the middle of the tube, between the red blood cells and the acellular plasma | - 3-month implantation - MRI - ICRS Visual Histological Assessment Scale (distribution of cells, mineralization of cartilage, tissue surface and matrix, cell population viability, subchondral bone abnormalities) | - Less cartilage degradation in the PRF-treated group according to the MRI T2 values - Better histological scores in the PRF group, presenting normal cell distribution and cartilage mineralization, smooth and continuous tissue surface, hyaline cartilage-like formation and no subchondral abnormalities | Kuo et al., 2011 [69] |
| Dogs Full thickness articular cartilage defect in the femoral condyle (diameter: 6 mm; depth: 5 mm) | Dog PRF Control: - cartilage defect with no implantation | Preparation according to Choukroun et al., 2001 [3] | - 4-, 16- and 24-week implantation - ICRS evaluation score for macroscopic assessment of the repaired tissue - O’Driscoll histological grading scale for microscopic investigation | - Formation of cartilage-like reparative tissue in both experimental groups, with higher number of chondrocyte-like cells and better ECM deposition in the PRF groups - Macroscopic and histological grading scores were found to be higher in the PRF-treated groups, indicating a better quality of cartilage repair | Kazemi et al., 2014 [70] |
| - Dog L-PRF - Dog L-PRP Control: - cartilage defect with no implantation | Preparation according to Choukroun et al., 2001 [3] | - 4-, 16- and 24-week implantation - ICRS evaluation score for macroscopic assessment of the repaired tissue - O’Driscoll histological grading scale for microscopic investigation | - No significant difference in macroscopic scores between L-PRP and L-PRF treated defects, but lower scores in the untreated control group - High quality repair tissue in both L-PRF and L-PRP treated groups according to histological evaluations | Kazemi and Fakhrjou, 2015 [71] | |
| Rabbits Subcutaneous implant to test graft viability for rhinoplasty | - Diced rabbit cartilage wrapped with rabbit PRFM - Diced rabbit cartilage wrapped with acellular dermal tissue - Diced rabbit cartilage wrapped with oxidized methylcellulose - Diced rabbit cartilage alone | Preparation according to Choukroun et al., 2001 [3] | - 10-week implantation - Histological stainings - Graft evaluated for chondrocyte viability, collagen content, ECM fibrillar structure and changes in peripheral tissues | - Better preservation of cartilage graft viability in the PRFM group - Less fibrosis, higher chondrocyte viability, better ECM deposition and less inflammation in the PRFM group | Güler et al., 2015 [78] |
| Rabbits Subcutaneous implant to test graft viability for rhinoplasty | - Diced rabbit cartilage wrapped with rabbit PRF - Diced rabbit cartilage wrapped with oxidized regeneratedcellulose - Diced rabbit cartilage wrapped with fascia - Diced rabbit cartilage alone | Preparation according to Choukroun et al., 2001 [3] | - 2 month-implant - Macroscopic evaluation - Histological staining - Explants evaluated for graft viability, fibrosis, inflammation and vascularization | - Superior viability of the cartilage graft wrapped with PRF in comparison with the cartilage graft wrapped with oxidized regenerated cellulose - No significant differences among the other groups - The 4 groups were not significantly different in terms of inflammation rate, fibrosis and vascularization | Göral et al., 2016 [79] |
| Rabbits Full thickness articular cartilage defect in the patellar groove (diameter: 4 mm; depth: 3 mm) | - Rabbit PRF - Rabbit PRP - Rabbit PRF + rhSDF1 - Rabbit PRP + rhSDF1 - Gelatin + rhSDF1 Control: - Untreated cartilage defect | Preparation according to Choukroun et al., 2001 [3] | - 4-week implantation - ICRS scores for macroscopic evaluations - ICRS Visual Histological Assessment Scale - Immunofluorescence analysis of type-II collagen expression - Gene expression study of cartilage markers (Aggrecan, SOX9) | - Higher ICRS macroscopic scores in the PRF + rhSDF1 group, with complete repair and good integration with the surrounding cartilage - ICRS histological scores of treated groups, except for the PRP group, were significantly higher than the untreated control - Neo-cartilages highly positive to type-II collagen in the PRF + rhSDF1, PRP + rhSDF1 and Gelatin + rhSDF1 groups - Higher expression of SOX9 in the regenerated tissue of all treated groups than the control group - Higher expression of Aggrecan in the treated groups, except for PRP group | Bahmanpour et al., 2016 [62] |
| Horses Full thickness articular cartilage defect of the knee (diameter: 15 mm) | Horse APEF (Autologous Platelet-enriched Fibrin) +/− horse BMDMSCs | - Blood collection into an acid citrate dextrose bag - Isolation of fibrinogen from plasma by use of an ethanol precipitation technique - Obtainment of a fibrinogen/platelet mixture (1:1) with the thrombin solution | 1-year implantation Repair tissues were evaluated by: - Arthroscopy (ICRS scores) - Histological examination - MRI - Micro-CT - Indentation tests | - No significant differences between the two groups according to arthroscopic ICRS scores - Fair-to-good fill of chondral defects and integration with the surrounding cartilage in both groups according to histological scores - Less thick cartilaginous tissue in the repair site after the addition of BMDMSCs - No variations in the stiffness of the cartilaginous tissue between the two treatments | Goodrich et al., 2016 [72] |
| Rabbits Chondral defect in the femoral condyle (diameter: 3 mm) | - Rabbit PRF + cartilage granules (PRFCG) Controls: - Rabbit PRF - Untreated cartilage defect | Preparation according to Choukroun et al., 2001 [3] | - 3-month implantation - Gross anatomy evaluation - ICRS histological scores | - Repair tissue with an intact, smooth, and hyaline-like surface resembling normal cartilage in the PRFCG group - Integration of the PRFCG implant with adjacent normal tissue, with no signs of inflammation - Histologically, better repair of the cartilage defect in the PRFCG group versus the PRF and untreated groups | Wong et al., 2017 [66] |
| Rabbits 2 mm wedge shape full-thickness defect in the medial meniscus | - Rabbit PRF fragments + defect sutured with 5–0 prolene (PRF-augmented suture group) Controls: - Not sutured defects (non-suture group) - Defects sutured with 5–0 prolene (suture group) | Preparation according to Choukroun et al., 2001 [3] | - 3-month implantation - Semi-quantitative histological scores | - Better morphological integrity of the meniscus in the PRF-augmented suture group than the control groups - No signs of high-grade degeneration in the PRF-augmented suture group, but mucoid changes with clear signs of degeneration in the control groups - Better healing of the meniscal defect via PRF-augmentation according to histological scores - Better congruity of articular cartilage in the PRF treated group | Wong et al., 2017 [67] |
| Rabbits osteochondral defect in the patellar groove (diameter: 5 mm; depth: 2 mm) | - Rabbit PRF + osteochondral autograft - Rabbit PRP + osteochondral autograft Control: - Osteochondral autograft | Preparation according to Choukroun et al., 2001 [3] | - 3- and 12-week implantation - ICRS macroscopic scoring system for repair evaluation - Histological examination - Immunohistochemical analysis of type-I and type-II collagen | - Macroscopical healing of the defect in the PRF group versus PRP and control groups at 3 weeks - Macroscopical healing of the defect with normal or nearly normal cartilage in all the 3 groups at 12 weeks - In the nongrafted portion of the defect, formation of hyaline-like cartilage in the PRF group and fibrocartilage in the other 2 groups | Maruyama et al., 2017 [73] |
| Rabbits Full thickness osteochondral defect in the knee joint (diameter: 5 mm; depth: 5 mm) | - Rabbit i-PRF - Rabbit PRP Control: - Untreated defect | - Blood collection without anticoagulant - Centrifugation (60 X g, 3 min) with Choukroun PRF Duo Centrifuge (Process for PRF, Nice, France) - Collection of the upper plasma layer designated as i-PRF | - 4- and 12-week treatment - ICRS macroscopic scoring system - ICRS histological scoring - Safranin O/fast green staining of to assess GAG content | - At 4 weeks, higher macroscopic IRCS scores in the i-PRF group in comparison with PRP and control groups, with formation of white opaque tissue well integrated with the surrounding healthy cartilage - At 12 weeks, no significant macroscopic differences among all groups - Higher ICRS histological scores in the i-PRF group, revealing complete regeneration of the cartilage and subchondral bone, with complete integration to normal tissues and identification of normal chondrocytes | Abd El Raouf et al., 2017 [61] |
| Dogs Osteochondral defect in the femoral condyle (diameter: 6 mm; depth: 5 mm) | - Dog PRF seeded with dog BM-MSCs Control: - Untreated defect | Preparation according to Choukroun et al., 2001 [3] | - 4-, 16- and 24-week implantation - ICRS evaluation score for macroscopic analysis - O’Driscoll histological grading scale for microscopic studies | - Consistently better integration of the repair tissue in the treated group versus the untreated control according to macroscopic scoring results - Formation of fibrous tissue in both experimental groups at 4 weeks - Histological detection of chondrocyte-like cells and cartilaginous ECM in the treated group at 16 and 24 weeks - Significantly higher histological scores in the treated group | Kazemi et al., 2017 [74] |
| Pigs Osteochondral defect in the femoral condyle (diameter: 8 mm; depth: 5 mm) | - Pig PRF +/- autologous cartilage fragments - Autologous cartilage fragments Control: - Untreated defect | - Blood collection with clot activator and gel - Centrifugation (1,066 X g, 10 min) - Separation of the jelly-like PRF from the gel-clot without the red blood cells sinking to the bottom of the tube | - 6-month implantation - Gross appearance of coverage, tissue color, defect margins, and surface - ICRS histological grading score | - Significantly better healing and repair tissue integration in the PRF+cartilage group in comparison with other 3 groups - Significantly greater histological scores in the PRF+cartilage group, with smooth repaired hyaline-like cartilage containing columnar arrangements of chondrocytes and integration of the regenerated tissue with the normal hyaline cartilage as well as the underlying subchondral bone | Sheu et al., 2017 [75] |
| Rabbits Osteochondral defect in the femoral condyle (diameter: 3 mm; length: 2 mm) | - Rabbit PRF releasates (PRFr) +/− autologous bone marrow-derived MSCs - Autologous bone marrow-derived MSCs Control: - Untreated defect | - Blood collection into a serum separation tube - Centrifugation (3,000 rpm, 10 min) - Obtainment of a fibrin clot (PRF) between a clear yellow serum layer and a coagulated red blood cell layer | - 12-week treatment - Gross assessment of shape, color, contour, and uniformity of the cartilage - Histological scoring system | - Decrease of the defect size and increase of the regenerated cartilage volume in the PRFr+MSCs group - Better histological indices (i.e., matrix deposition, cell distribution, and tissue surface) in the PRFr+MSCs group - Thicker hyaline-like cartilaginous tissue with normal GAG production in the PRFr+MSCs group in comparison with other 3 groups | Wu et al., 2017 [53] |
| Rabbits Osteochondral defect in the femoral condyle (diameter: 3 mm; length: 2 mm) | - Rabbit PRF releasates (PRFr) +/− autologous ADSCs - Rabbit PRFr + chondrocytes - Autologous ADSCs Control: - Untreated defect | Preparation according to Wu et al., 2017 [53] | - 14-week treatment - Gross investigation of defect filling, integration to border zone and macroscopic appearance of the implant - ICRS histological grading score | - Decrease of the defect size and increase of the repaired cartilage volume in the PRFr+ADMSCs group - Better matrix, cell distribution, and surface indices in the PRFr+ADSCs group than other groups according to histological grading scores - Thicker hyaline cartilage-specific ECM in the PRFr+ADMSCs group - Similar histological scores for ADSCs and PRFr groups | Hsu et al., 2018 [76] |
| Rabbits Full thickness cartilage defect of the ear (5 × 5 × 1 mm) | - Rabbit PRF +/- allogenic ADSCs - Allogenic ADSCs Control: - Untreated defect | Preparation according to Choukroun et al., 2001 [3] | - 1-, 2-and 3-month implantation - Macroscopic evaluation - Histological analysis - Gene/protein expression study of type-II collagen - Immune response evaluation by determining blood levels of CD4/CD8, IL-2 and IL-4 | - Best rate of repair at all observation points in the PRF+ADSCs group, with 90% greater repair rate than other groups at 3 months - More efficient repair of the cartilage defect in the PRF+ADSCs group, with the treated area almost completely filled by naïve chondrocytes. - Higher type-II collagen expression, both at the gene and protein levels, in the PRF and PRF+ADSCs groups - No significant immune response induced by allogenic ADSC transplantation | Xu et al., 2018 [77] |
| End Use Destination | Hemocomponent/Experimental Groups | PRFPreparation Protocol | Characterization Parameters | Major Findings | Reference |
|---|---|---|---|---|---|
| Hemophilic ankle arthropathy (focal lesions) | n = 5 patients (mean age = 33 ± 6.78 years): collagen membrane loaded with BMDCs and PRF | Preparation according to the Vivostat® system | Mean follow up: 2 years The postoperative outcome was evaluated by: - AOFAS scores - radiographs - MRI and Mocart scores | - All patients showed complete filling of the talar defect - The implant borders were completely/partially integrated with the adjacent cartilage - In all patients presented inhomogeneous, hyperintense repair tissue was detected - Three patients had subchondral bone edema or cyst - Overall, the data showed good osteochondral regeneration and no progression of joint degeneration | Buda et al., 2015 [82] |
| Knee cartilage focal lesions | n = 15 patients: microfractures and PRF; n = 16 patients: microfractures and PRP; n = 17 patients: microfractures alone | - | Follow up: 2, 5 years Postoperative evaluation of patients was performed by: - clinical scores (i.e., IKDC, VAS pain) - MRI and Mocart scores | - Platelet concentrates allowed to achieved better clinical results compared to microfracture alone - The PRF was more effective than the PRP at 2 years, with loss of significance at 5 years - According to Mocart score, PRF gave better results earlier than the other two treatments | Papalia et al., 2016 [84] |
| Knee cartilage focal lesions | n = 25 patients (mean age = 29 ± 7.3 years): single-step AMIC procedure based on microfracture and application of autologous PRF called CLP-MB membrane, combined with an injectable collagen scaffold (Cartifill) | - Blood collection by apheresis - Separation of CLP and plasma - Cryoprecipitate formation from freeze/thawed plasma - Mixing of CLP and cryoprecipitate (CLP mix) - Activation of the CLP mix with calcium gluconate - Incubation at 37 °C for 10 min - Centrifugation (7333× g, 25 min) | Pre-implant characterization: - assessment of blood cell composition, CD34+/CD133+/VEGFR2+ cell content, fibrinogen concentration during each preparation phase - release of PDGF-AB, TGF-β1 and VEGF -mechanical tests Clinical trial: Follow-up: 1, 6 and 12 months Patients were evaluated by: - NMR and/or radiographic scans - VAS pain - IKDC scores | - Quality control tests during each phase of CLP-MB preparation assured for the obtainment of a standardized, traceable and safe product - The treatment with the hemocomponent provided short-term pain relief and functional improvement | D’Antimo et al., 2017 [85] |
| Rhinoplasty (dorsal nasal augmentation) | n = 19 patients: cartilage scales-cartilage pâté compound graft with PRGF n = 21 patients: cartilage scales-cartilage pâté compound graft with i-PRF n = 8 patients: cartilage pâté graft with a-PRF | Preparation according to Choukroun et al., 2001 [3] | Follow-up controls every 3 months Medical records to assess the surgical outcome included: - follow-up notes - pre- and post-operative photographic documentation | - Satisfactory dorsal nasal augmentation in 47 patients - 1 mm-horizontal displacement of the graft in one patient 3 months after surgery, with no tendency for further displacement - No dorsal irregularities, nor signs of resorption, erythema, inflammation | Kovacevic et al., 2017 [86] |
| Hemocomponent/Experimental Groups | PRF Preparation Protocol | Characterization Parameters | Major Findings | Reference |
|---|---|---|---|---|
| - Human PR-matrix - Human PP-matrix - Human purified fibrin | - Blood collection into 3.8% (wt/vol) sodium citrate - Centrifugation at 4 °C: (a) PR-plasma → 460× g, 8 min (b) PP-plasma → 4500× g, 12 min - Platelet counts before clotting - Addition of calcium chloride at a final concentration of 22.8 mM | - Proliferation of human tenocytes - Secretion of TGF-β1, VEGF and HGF (+/− cells) - Synthesis of type-I collagen (Coll-I) | - Significantly increased platelets cells proliferation - Increase in Coll-I synthesis with any difference between PR- and PP-matrices - Higher levels of TGF-β1 in PR-matrix samples (i.e., +/− tenocytes) than PP-matrices - Increased synthesis of VEGF and HGF by tenocytes on fibrin matrices - Significantly higher levels of VEGF, but not HGF, in presence of platelets | Anitua et al., 2006 [101] |
| - Dog PRF matrix - Dog PRF membrane - Dog whole blood clot | a) PRF matrix - Blood collection in tube with trisodium citrate - 1st centrifugation (1100× g, 6 min) - Supernatant transfer in a tube with calcium chloride - 2nd centrifugation (1450× g, 15 min) b) PRF membran e- Blood collection in tube with trisodium citrate and the proprietary separator gel. - 1st centrifugation (1100× g, 6 min) - Supernatant transfer in a glass vial with calcium chloride - 2nd centrifugation (4500× g, 25 min) - Suspension of the resulting membrane in serum | - Quantification of eluted TGF-β1 - Evaluation of the mitogenic effect on canine tenocytes | - Both PRF constructs release significantly higher levels of TGF-β1 than blood clot, significantly increasing cell proliferation - Significantly higher levels of TGF-β1 were released from PRF membrane than PRF matrix, significantly increasing cell proliferation | Visser et al., 2010 [102] |
| - Human standard/gelatinous L-PRF - Human dry/compressed L-PRF | - Blood collection (at 8.30 am.) incitrate tubes - Centrifugation for 12 min with different G-forces: (1) 200× g, (2) 400× g, (3) 1000× g - Count of platelets, leukocytes and red blood cells in extracted supernatant and “buffy coat” versus normal blood | - Leukocyte content - Release of GFs (i.e., TGF-β1, VEGF, MPO, IGF1, PDGF-AB, CXCL4) - Relationship between matrix preparation methods and GFs concentrations | - Highest concentration of platelets and leukocytes with 400× g centrifugation - L-PRF clots showed in vitro a continuous release of GFs which were significantly higher than levels expressed by normal blood at each culture time point - Higher release of GFs (i.e., CXCL4, IGF-1, PDGF-AB, and VEGF) by the standard/gelatinous- compared to the dry/compressed group | Zumstein et al., 2012 [111] |
| - Human PRF-matrix - Fibrin matrix based on PRP (ViscoGel; Arthrex, Naples, FL) Controls: - Human highly cross-linked collagen membrane (Arthroflex; LifeNet Health, Virginia Beach, VA) - Porcine non-cross-linked collagen membrane (Mucograft; Geistlich Pharma, Lucerne, Switzerland) - Human fresh-frozen rotator cuff tendon (allograft) | - Blood collection - Centrifugation (3000 rpm, 10 min) | - Differentiation, proliferation of human MSCs | - MSCs successfully differentiated into all cell lines - A significantly greater number of cells adhered to both the non-cross-linked porcine collagen scaffold and PRF-matrix - Significantly higher proliferation in the non-cross-linked porcine collagen scaffold vs PRF-matrix and fibrin matrix based on platelet-rich plasma - No significant differences at the live/dead assay | Beitzel et al., 2014 [103] |
| End Use Destination | Hemocomponent/Experimental Groups | PRF Preparation Protocol | Characterization Parameters | Major Findings | Reference |
|---|---|---|---|---|---|
| Sheeps Achilles tendon injected at 2.5 cm proximal to the bone insertion | - Injection of autologous sheep calcified PR-plasma - Injection of autologous sheep PP-plasma - Injection of saline | - Blood collection into 3.8% (wt/vol) sodium citrate - Centrifugation at 4 °C: (a) PR-plasma → 460× g, 8 min (b) PP-plasma → 4500× g, 12 min - Platelet counts before clotting - Addition of calcium chloride at a final concentration of 22.8 mM | - Cell density, morphology and distribution - Vascularization - Inflammation | - Higher increase in cell density in the fascicles treated with PR- and PP-plasma - Ovoid but aligned cells in PR- and PP- treated tendons - Neovascularization is promoted with both PR-and PP-plasma - No inflammatory cells in both PR-and PP-plasma treatment | Anitua et al., 2006 [101] |
| Sheeps acute model of Achilles tendon rupture | - Re-approximation of the tendon ends with suture only - Re-approximation with suture augmented with ADP wrapped around the repair and sutured to the tendon - ADP wrapped around the proximal and distal margins of the tendon, bridging a 1.5 cm gap, with autologous PRPFM sutured in place within the gap | - PRPFM—Cascade Autologous Platelet System-4, Musculoskeletal Transplant Foundation | - Mechanical tests - Cell and tissue morphology - Vascularization - Scaffold incorporation- Inflammation | - Significant difference in elongation between the operated limb vs unoperated limb in suture only group and ADP + PRPFM group but not in suture + ADP group - No apparent fibrosis in all groups - Increased tendon thickness in suture only group - New tendon fibers without increasing tendon thickness (2/6 animals) in suture + ADP group - Complete bridging of the gap, with no change in tendon thickness in ADP + PRPFM (2/6 animals) - Peripheral integration of the APD to tendon fibers - APD +/− PRPFM augments Achilles tendon repair | Sarrafian et al., 2010 [114] |
| Dogs patellar tendon; sharp incision of the central third | - Autologous dog PRF membrane to fill the injury site - Surgical closure following resection of the central third of the patellar tendon | - Blood collection in tubes with trisodium citrate and a separator gel. - 1st centrifugation (1100× g, 6 min) - Transfer of PRP supernatant in a vial containing 1.0 M calcium chloride. - 2nd centrifugation (4500× g, 25 min) while fibrin polymerization ensued | - Gross healing assessment and cross-sectional area - Cell density - Vascularization - Collagen and GAG | - Repair tissue in both groups - No histological significant difference (i.e., cellularity, vascularity, collagen organization, or GAG content) - Hypercellular fibrovascular repair tissue in defect site of both groups - Significantly greater cross-sectional area of PRF membrane–treated tendons vs the control group - PRF membrane did not enhance the rate/quality of tendon healing but it increases repair tissue surrounding the defect. | Visser et al., 2011 [117] |
| Rabbits Toe flexor tendon; sharp transection between the A1 and A2 pulley and immediate surgical repair | - Allogenic PRP - Allogenic PRP-F matrix - Commercial fibrin (Beriplast P Combi Set; CSL Behing K.K., Tokyo, Japan) Control: Natural healing of the repair site | - Blood collection in syringe with acid citrate dextrose-A - 1st centrifugation (2400 rpm, 10 min at 4 °C) - 2nd centrifugation of plasma (3600 rpm, 10 min at 4 °C) - Platelets count - Addition of fibrin matrix (Beriplast P Combi-Set; CSL Behring K.K., Tokyo, Japan): liquid A (0.25 µL) + liquid B (0.25 µL) | - Edema of the toes - Adhesions extent - Mechanical tests - Histological analysis | - No significant difference in edema/adhesion scores - Significantly increased healing strength by PRP-F matrix | Sato et al., 2012 [115] |
| Rabbits Experiment 1 Bone-patellar tendon-bone. Removal of the central half of each patellar tendon Experiment 2 Removal of medial collateral ligament | Experiment 1 - Allogenic rabbit CPFS; Control: untreated defect of the controlateral patella Experiment 2 Allogenic CPFS sheet Control: Insertion of rivets without reconstruction of the controlatelar medial collateral ligament | - Blood collection in tubes with a sodium citrate solution (5% wt/vol) - 1st centrifugation (3000 rpm, 15 min at 4 °C) - 2nd centrifugation of platelet poor plasma (3000 rpm, 15 min at 4 °C) - Freezing of buffy coat layer and platelet poor plasma (−80 °C) - Defrosting and enriching by ultrafiltration twice of platelet poor plasma; defrosting of buffy coat. - Blending of the two fractions and addition of calcium gluconate (final concentration 23 mM) - Incubation at 37 °C for 3 h - Pressure treatment in aqueous solution of 10 mM calcium chloride at 4 °C | - Repair tissue thickness - Mechanical tests - Inflammation | Experiment 1 - the ultimate failure load and stiffness were higher for the CPFS-treated group than untreated knee - Presence of dense and longitudinally aligned collagen bundles - No signs of immunological rejection of allogenic scaffold Experiment 2 - CPFS promoted ligament repair tissue vs the untreated side - The ultimate failure load of the CPFS repair tissue at 20 weeks was 78% of that in healthy controls of the same age CPFS enhanced/accelerated healing of tendons and ligaments | Matsunaga et al., 2013 [118] |
| Rats Tendon-bone insertion site, rotator cuff. Transection and transosseous suture repair of the supraspinatus tendon | - Surgical repair + allogenic PRFM Control: - Controlateral shoulder, only surgical repair | - Blood collection in syringe with 0.5 cc of acid citrate dextrose anticoagulant solution and thixotropic polyester separator gel. - 1st centrifugation (1500 rpm, 15 min) - 2nd centrifugation of the platelet-rich layer (3000 rpm, 6 min) | - Mechanical tests - Histological analysis (i.e., collagen tissue organization/maturation; cartilage formation | - Higher ultimate load to failure, stress, and stiffness values for experimental group repairs - No differences in biomechanical testing between the groups - Less collagen organization and cartilage formation at the insertion site in the experimental group - PRF-membrane does not recapitulate the native enthesis with exuberant/disordered healing response with fibrovascular scar tissue | Hasan et al., 2016 [108] |
| Rabbits Flexor digitorum profundus tendon | Part I - Autologous rabbit PRF, wrapped around the repair site, tagged with suture Part II - Autologous rabbit PRF interposed between the tendon repair ends by a 2-strand repair Control: Control tendons | - Blood collection without anticoagulant - Centrifugation (2700 rpm, 12 min at room temperature) - Compression of the PRF clot | - Range of motions analysis - Cross-sectional area - Mechanical tests | - No significant increase in range of motion - Significant increase in cross-sectional area of the tendons in the PRF group - The control had a higher load and stress to failure but similar stiffness and modulus to the PRF groups - The PRF did not have a major influence on cellular organization - Undesirable effect on the biomechanical properties of repaired flexor tendons | Liao et al., 2017 [116] |
| End Use Destination | Hemocomponent/Experimental Groups | PRF Preparation Protocol | Characterization Parameters | Major Findings | Reference | |
|---|---|---|---|---|---|---|
| Full-thickness rotator cuff tear | n = 20 patients (mean age = 57.6 years): Arthroscopic single-row rotator cuff repair + 2 autologous PRP, sutured into the repair site n = 20 patients (mean age = 57.8 years; range = 44–69 years): Arthroscopic single-row rotator cuff repair | - Cascade autologous platelet system | Mean follow up and range: PRP, 28.3 (24–44) months; no PRP, 33 (24–44) months - MRI - Clinical outcome measures by ASES, Rowe, SANE, SST and Constant scores | - Retears: with PRP: 6 of 20 (30%); no PRP: 12 of 20 (60%) - Cuff tear size (no. healed): <3 cm, 7 of 14 (50%) no PRP; 12 of 14 (86%) with PRP. ≥3 cm, 1 of 6 (17%) no PRP; 2 of 6 (33%) with PRP - Significant clinical differences showing lower re-tear rates by MRI only with Rowe score | Barber et al., 2011 [104] | |
| Arthroscopic rotator cuff repair | n = 43 patients (mean age = 55.5 years): Tear size (cm): small (<1); medium (1–3), PRFM n = 45 patients (mean age = 55.2 years): Tear size (cm): small (<1), medium (1–3), no PRFM | - Blood collection in a tube with trisodium citrate and a thixotropic polyester separator gel - 1st centrifugation (1100 rpm, 6 min) - Transfer of the supernatant in a bottle containing calcium chloride (1.0 M) - 2nd centrifugation (4500 RCF, 25 min) | Follow up: 16 months - Clinical outcome by Constant scores - MRI | - Statistically significant improvement in both groups but any among groups - Difference in alterations of MRI signal intensity - Re-rupture in 10.5% patients of control group and 2.5% in PRFM group but any additional treatment occurred - No difference in tendon thickness or in size of the tendon footprint tendon thickness | Castricini et al., 2011 [107] | |
| Arthroscopic rotator cuff repair | n = 16 patients (mean age = 65 ± 7 years): Tear size: 3.8 ± 1.1 cm, PRFM n = 21 patients (mean age = 65 ± 9 years): Tear size: 3.9 ± 1.1 cm, no PRFM | - Blood collection in a tube with trisodium citrate - 1st centrifugation (1100 rpm, 6 min) - Transfer of supernatant into a second tube containing calcium chloride, which initiates the fibrin-clotting cascade - 2nd centrifugation (1450 rpm, 15 min) The Cascade Autologous Platelet System was used to prepare the PRFM | Mean follow-up: PRFM group, 13 ± 4 months; Untreated group, 27 ± 8 months - Operative time - MRI - Clinical outcome scores by Constant, WORC, SANE, ASES, UCL | - Retear rates: statistically significantly higher in the PRFM group (56.2%) vs. controls (38.1%) - Functional outcome scores postoperatively: not significantly improved in PRFM vs. controls - Operative time (min): 152 ± 31 in PRFM group vs 161 ± 40 in control group - 2 infections in the PRFM group - The augmentation of at-risk rotator cuff tears with PRFM did not result in improved retear rates or functional outcome scores compared with controls | Bergeson et al., 2012 [106] | |
| Full-thickness rotator cuff tear | n = 40 patients (mean age = 58.90 ± 9.86 years): Tear size (nr. of patients): small: 10; medium: 20; large: 10, PRFM treatment n = 39 patients (mean age = 7.21 ± 9.42 years): Tear size (nr. of patients): small: 10; medium: 19; large: 10, No PRFM | - Cascade Membrane (Musculoskeletal Tissue Foundation, Edison, NJ, USA) | Follow-up: 6 weeks, 3 and 12 months - Power doppler ultrasound - Manual muscle testing ratio - Clinical outcome scores by ASES and l’Insalata - Strength measurements using a handheld dynamometer | - Intact repair in 24 of 36 (67%) in the PRFM group and 25 of 31 (81%) in the control group - No differences in tendon-to-bone healing - No demonstrable effect on tendon healing vascularity, manual muscle strength, or clinical rating scales by PRFM - Negative effect of PRFM on healing according to regression analysis | Rodeo et al., 2012 [109] | |
| Arthroscopic rotator cuff repair | n = 30 patients (mean age = 59.67 ± 8.16 years): Tear size: 1.77 ± 0.84 cm, PRFM treatment (commercially available) n = 30 patients (mean age = 64.50 ± 8.59 years): Tear size: 1.72 ± 1.18 cm, no PRFM | - Cascade Membrane (Musculoskeletal Tissue Foundation, Edison, NJ, USA) | Follow-up: 1 h, 3, 6, 9, and 12 weeks, 1 year - Operative time - VAS pain scores, ROM, SST, FF, ER, UCLA, ASES scores - Narcotic consumption - MRI | - No complications - Longer mean surgery time for the PRFM group than control group - No significant difference in VAS, ROM, SST, FF, ER or ASES scores or narcotic use - Similar UCLA scores in both groups at baseline but statistically significantly lower in the PRFM group at follow-up - No differences in MRI - No significant improvement in perioperative morbidity, structural integrity or clinical outcome in PRFM in early follow-up | Weber et al., 2013 [110] | |
| Full-thickness rotator cuff tears | n = 20 patients (mean age = 55 years): Tear size: ≤3 cm in anteroposterior length, suture-bridging double-row repair + PRPFM n = 20 patients (mean age = 57 years): Tear size: no greater than 3 cm in anteroposterior length, triple - loaded single row repair + PRPFM | - Cascade Membrane (Musculoskeletal Tissue Foundation, Edison, NJ, USA) | Mean follow-up and range: double-row group, 27 (12–46) months; single-row group, 28 (12–49) months - ASES, Rowe, SST, Constant, SANE - MRI | - No statistical difference on clinical outcome scores between groups - No MRI difference in rotator cuff tendon re-tear rate (i.e., 15% in both groups) | Barber et al., 2016 [105] | |
| Arthroscopic rotator cuff repair | n = 17 patients (mean age = 65 years): Tear size (area): 322 ± 180 mm2, L-PRF n = 18 patients (mean age = 66 years): Tear size (area): 445 ± 421 mm2, No L-PRF | - Blood collection (at 8.30 am.) incitrate tubes - Centrifugation for 12 min with different G-forces: (1) 200× g, (2) 400× g and (3) 1000× g. - Count of platelets, leukocytes and red blood cells in extracted supernatant and “buffy coat” vs normal blood | - Mean follow-up: L-PRF, 14 months; Untreated group, 15 months - SSV, VAS for pain, SST, Constant-Murley | - No complications in either group - No significant differences in clinical outcome, healing rate, mean postoperative defect size, and tendon quality at 12 mo follow-up | Zumstein et al., 2016 [112] | |
| Acute rupture of Achilles tendon | n = 11 patients (mean age = 32.5 ± 3.4 years): PRF augmentation n = 9 patients (mean age = 34.5 ± 3 years): No PRF n = 8 patients (mean age = 30 ± 4.4 years): Healthy | - Blood collection in a tube with sodium citrate - Centrifugation (3000 rpm, 10 min) The protocol included specific jellifying agents (i.e., calcium gluconate and batroxobin) | Follow-up: 6 months - Gait analysis | - % of the stance time of the operated leg, double-support time of the healthy leg, network of the ankle during the gait cycle showed statistically significant differences between the no-PRF and the healthy group - No differences between PRF and healthy groups - Suture + PRF augmentation shows significant functional improvements in motion efficiency | Alviti et al., 2017 [113] | |
| Gluteus medius tendons | n = 18 patients (mean age = 60.26 ± 8.8 years): Tear size: small or low-grade partial tear (33.3%); large or high-grade partial tear (50.0%); large or high-grade full tear (16.7%), PRFM n = 29 patients (mean age = 63.09 ± 12.0 years): Tear size: small or low-grade partial tear (31.0%); large or high-grade partial tear (58.6%); large or high-grade full tear (10.4%), no PRFM | - | Follow-up: 1 year - Demographic variables | - No effect of PRFM on repair in terms of pain or clinical evidence of retears - PRFM may have a role in improving subjective outcomes of overall and hip-specific physical functioning | Saltzman et al., 2018 [119] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbon, S.; Stocco, E.; Macchi, V.; Contran, M.; Grandi, F.; Borean, A.; Parnigotto, P.P.; Porzionato, A.; De Caro, R. Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1701. https://doi.org/10.3390/ijms20071701
Barbon S, Stocco E, Macchi V, Contran M, Grandi F, Borean A, Parnigotto PP, Porzionato A, De Caro R. Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside. International Journal of Molecular Sciences. 2019; 20(7):1701. https://doi.org/10.3390/ijms20071701
Chicago/Turabian StyleBarbon, Silvia, Elena Stocco, Veronica Macchi, Martina Contran, Francesca Grandi, Alessio Borean, Pier Paolo Parnigotto, Andrea Porzionato, and Raffaele De Caro. 2019. "Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside" International Journal of Molecular Sciences 20, no. 7: 1701. https://doi.org/10.3390/ijms20071701