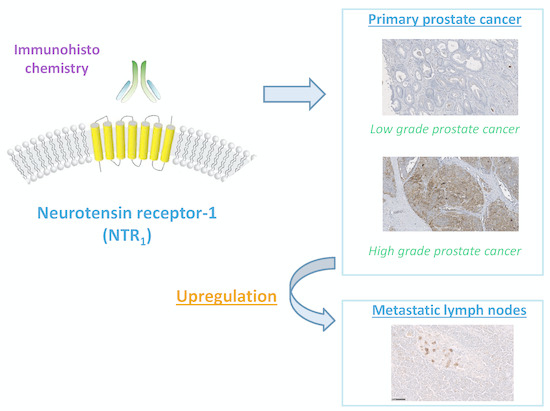
Neurotensin Receptor-1 Expression in Human Prostate Cancer: A Pilot Study on Primary Tumors and Lymph Node Metastases
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Antibody Used
4.2. Cell Lines
4.3. Xenograft
4.4. Western Blotting
4.5. Immunofluorescence
4.6. Immunochemistry Procedure
4.7. Human Samples
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PSMA | Prostate Specific Membrane Antigen |
| NTR1 | Neurotensin receptor-1 |
| NTR2 | Neurotensin receptor-2 |
| NTR3 | Neurotensin receptor-3 |
| FITC | Fluorescein isothiocyanate |
| DAPI | 4′,6-Diamidino-2-phenylindol |
| IHC | Immunohistochemistry |
| PET | Positron Emission Tomography |
| CK | Cytokeratine |
| BPH | Benign Prostatic Hyperplasia |
| CRPC | Castration Resistant Prostate Cancer |
References
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Castoria, G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front. Oncol. 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Di Santi, A.; Cernera, G.; Rossi, V.; Abbondanza, C.; Moncharmont, B.; Sinisi, A.A.; et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget 2015, 7, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, I.; Powers, S.; Huntley, B.; Powis, G.; Pittelkow, M.; Maihle, N.J. Neurotensin is an autocrine trophic factor stimulated by androgen withdrawal in human prostate cancer. Proc. Natl. Acad. Sci. USA 1994, 91, 4673–4677. [Google Scholar] [CrossRef] [PubMed]
- Morgat, C.; Mishra, A.K.; Varshney, R.; Allard, M.; Fernandez, P.; Hindié, E. Targeting Neuropeptide Receptors for Cancer Imaging and Therapy: Perspectives with Bombesin, Neurotensin, and Neuropeptide-Y Receptors. J. Nucl. Med. 2014, 55, 1650–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorino, G.P.; Deeble, P.D.; Parsons, S.J. Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway. Oncogene 2007, 26, 745–756. [Google Scholar] [CrossRef]
- DaSilva, J.O.; Amorino, G.P.; Casarez, E.V.; Pemberton, B.; Parsons, S.J. Neuroendocrine-derived peptides promote prostate cancer cell survival through activation of IGF-1R signaling. Prostate 2013, 73, 801–812. [Google Scholar] [CrossRef]
- Hassan, S.; Dobner, P.R.; Carraway, R.E. Involvement of MAP-kinase, PI3-kinase and EGF-receptor in the stimulatory effect of Neurotensin on DNA synthesis in PC3 cells. Regul. Pept. 2004, 120, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Tian, H.; Niu, X.; Wang, J.; Li, X.; Jiang, N.; Wen, S.; Chen, X.; Ren, S.; Xu, C.; et al. Neurotensin and its receptors mediate neuroendocrine transdifferentiation in prostate cancer. Oncogene 2019, in press. [Google Scholar] [CrossRef]
- Vias, M.; Burtt, G.; Culig, Z.; Veerakumarasivam, A.; Neal, D.E.; Mills, I.G. A role for neurotensin in bicalutamide resistant prostate cancer cells. Prostate 2007, 67, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Kyoda, Y.; Tanaka, T.; Maeda, T.; Kobayashi, K.; Uchida, K.; Kitamura, H.; Hirata, K.; Tsukamoto, T.; Masumori, N. The potential of neurotensin secreted from neuroendocrine tumor cells to promote gelsolin-mediated invasiveness of prostate adenocarcinoma cells. Lab. Investig. 2015, 95, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Swift, S.L.; Burns, J.E.; Maitland, N.J. Altered Expression of Neurotensin Receptors Is Associated with the Differentiation State of Prostate Cancer. Cancer Res. 2010, 70, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Valerie, N.C.K.; Casarez, E.V.; DaSilva, J.O.; Dunlap-Brown, M.E.; Parsons, S.J.; Amorino, G.P.; Dziegielewski, J. Inhibition of Neurotensin Receptor 1 Selectively Sensitizes Prostate Cancer to Ionizing Radiation. Cancer Res. 2011, 71, 6817–6826. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.M.; Severns, V.; Brown, D.C.; Bisoffi, M.; Sillerud, L.O. Prostate Cancer Targeting Motifs: Expression of ανβ3, Neurotensin Receptor 1, Prostate Specific Membrane Antigen, and Prostate Stem Cell Antigen in Human Prostate Cancer Cell Lines and Xenografts. Prostate 2012, 72, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wang, H.; Zhang, H.; Wang, M.; Giglio, B.; Ma, X.; Jiang, G.; Yuan, H.; Wu, Z.; Li, Z. Imaging Neurotensin Receptor in Prostate Cancer With 64Cu-Labeled Neurotensin Analogs. Mol. Imaging 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Souazé, F.; Dupouy, S.; Viardot-Foucault, V.; Bruyneel, E.; Attoub, S.; Gespach, C.; Gompel, A.; Forgez, P. Expression of Neurotensin and NT1 Receptor in Human Breast Cancer: A Potential Role in Tumor Progression. Cancer Res. 2006, 66, 6243–6249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alifano, M.; Souazé, F.; Dupouy, S.; Camilleri-Broët, S.; Younes, M.; Ahmed-Zaïd, S.-M.; Takahashi, T.; Cancellieri, A.; Damiani, S.; Boaron, M.; et al. Neurotensin Receptor 1 Determines the Outcome of Non–Small Cell Lung Cancer. Clin. Cancer Res. 2010, 16, 4401–4410. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Strict rules are needed for validation of G-protein-coupled receptor immunohistochemical studies in human tissues. Endocrine 2014, 47, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.R.; Baek, M.W.; Sim, J.; Choi, H.-S.; Han, J.M.; Kim, Y.L.; Hwang, J.-I.; Kwon, H.B.; Beaudet, N.; Sarret, P.; et al. Intermolecular cross-talk between NTR1 and NTR2 neurotensin receptor promotes intracellular sequestration and functional inhibition of NTR1 receptors. Biochem. Biophys. Res. Commun. 2010, 391, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Maschauer, S.; Greff, C.; Einsiedel, J.; Ott, J.; Tripal, P.; Hübner, H.; Gmeiner, P.; Prante, O. Improved radiosynthesis and preliminary in vivo evaluation of a 18F-labeled glycopeptide–peptoid hybrid for PET imaging of neurotensin receptor 2. Bioorg. Med. Chem. 2015, 23, 4026–4033. [Google Scholar] [CrossRef]
- Souazé, F.; Viardot-Foucault, V.; Roullet, N.; Toy-Miou-Leong, M.; Gompel, A.; Bruyneel, E.; Comperat, E.; Faux, M.C.; Mareel, M.; Rostène, W.; et al. Neurotensin receptor 1 gene activation by the Tcf/β-catenin pathway is an early event in human colonic adenomas. Carcinogenesis 2006, 27, 708–716. [Google Scholar] [CrossRef]
- Toy-Miou-Leong, M.; Cortes, C.L.; Beaudet, A.; Rostène, W.; Forgez, P. Receptor Trafficking via the Perinuclear Recycling Compartment Accompanied by Cell Division Is Necessary for Permanent Neurotensin Cell Sensitization and Leads to Chronic Mitogen-activated Protein Kinase Activation. J. Biol. Chem. 2004, 279, 12636–12646. [Google Scholar] [CrossRef]
- Bakht, M.K.; Derecichei, I.; Li, Y.; Ferraiuolo, R.-M.; Dunning, M.; Oh, S.W.; Hussein, A.; Youn, H.; Stringer, K.F.; Jeong, C.W.; et al. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr. Relat. Cancer 2019, 26, 131–146. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Körner, M.; Waser, B.; Strobel, O.; Büchler, M.; Reubi, J.C. Neurotensin receptors in pancreatic ductal carcinomas. EJNMMI Res. 2015, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Long, X.; Zhang, L.; Chen, J.; Liu, P.; Li, H.; Wei, F.; Yu, W.; Ren, X.; Yu, J. NTS/NTR1 co-expression enhances epithelial-to-mesenchymal transition and promotes tumor metastasis by activating the Wnt/β-catenin signaling pathway in hepatocellular carcinoma. Oncotarget 2016, 7, 70303–70322. [Google Scholar] [CrossRef] [PubMed]
- de Lange, R.; Dimoudis, N.; Weidle, U.H. Identification of genes associated with enhanced metastasis of a large cell lung carcinoma cell line. Anticancer Res. 2003, 23, 187–194. [Google Scholar]
- Muteganya, R.; Goldman, S.; Aoun, F.; Roumeguère, T.; Albisinni, S. Current Imaging Techniques for Lymph Node Staging in Prostate Cancer: A Review. Front. Surg. 2018, 5, 74. [Google Scholar] [CrossRef]
- Maschauer, S.; Einsiedel, J.; Hübner, H.; Gmeiner, P.; Prante, O. 18F- and 68Ga-Labeled Neurotensin Peptides for PET Imaging of Neurotensin Receptor 1. J. Med. Chem. 2016, 59, 6480–6492. [Google Scholar] [CrossRef]
- Schulz, J.; Rohracker, M.; Stiebler, M.; Goldschmidt, J.; Stöber, F.; Noriega, M.; Pethe, A.; Lukas, M.; Osterkamp, F.; Reineke, U.; et al. Proof of Therapeutic Efficacy of a 177Lu-Labeled Neurotensin Receptor 1 Antagonist in a Colon Carcinoma Xenograft Model. J. Nucl. Med. 2017, 58, 936–941. [Google Scholar] [CrossRef]
- Baum, R.P.; Singh, A.; Schuchardt, C.; Kulkarni, H.R.; Klette, I.; Wiessalla, S.; Osterkamp, F.; Reineke, U.; Smerling, C. 177Lu-3BP-227 for Neurotensin Receptor 1–Targeted Therapy of Metastatic Pancreatic Adenocarcinoma: First Clinical Results. J. Nucl. Med. 2018, 59, 809–814. [Google Scholar] [CrossRef]
- Woythal, N.; Arsenic, R.; Kempkensteffen, C.; Miller, K.; Janssen, J.-C.; Huang, K.; Makowski, M.R.; Brenner, W.; Prasad, V. Immunohistochemical Validation of PSMA Expression Measured by 68Ga-PSMA PET/CT in Primary Prostate Cancer. J. Nucl. Med. 2018, 59, 238–243. [Google Scholar] [CrossRef] [PubMed]



| Variable | NTR1 Overexpression | p |
|---|---|---|
| Age | 0.622 | |
| ≤64 years (n = 19) | 1/19 | |
| >64 years (n = 25) | 3/25 | |
| PSA value | 1.000 | |
| <10 ng/mL (n = 22) | 2/22 | |
| ≥10 ng/mL (n = 22) | 2/22 | |
| pT-status | 1.000 | |
| pT2 (n = 15) | 1/15 | |
| pT3–T4 (n = 29) | 3/29 | |
| N-status | 0.282 | |
| N0–X (n = 27) | 1/27 | |
| N+ (n = 17) | 3/17 | |
| Gleason score | 0.358 | |
| 5–6 (n = 12) | 1/12 | |
| 7 (n = 12) | 0/12 | |
| 8–9 (n = 20) | 3/20 | |
| Gleason score grouped | 0.316 | |
| 5–7 (n = 24) | 1/24 | |
| 8–9 (n = 20) | 3/20 | |
| Margin status | 0.558 | |
| R0 (n = 33) | 4/33 | |
| R1 (n = 11) | 0/11 |
| NTR1 Overexpression | p | |
|---|---|---|
| Tumor sample | 0.038 | |
| Primary tumor (n = 44) | 4/44 | |
| Metastatic lymph nodes (n = 15 †) | 5/15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgat, C.; Chastel, A.; Molinie, V.; Schollhammer, R.; Macgrogan, G.; Vélasco, V.; Malavaud, B.; Fernandez, P.; Hindié, E. Neurotensin Receptor-1 Expression in Human Prostate Cancer: A Pilot Study on Primary Tumors and Lymph Node Metastases. Int. J. Mol. Sci. 2019, 20, 1721. https://doi.org/10.3390/ijms20071721
Morgat C, Chastel A, Molinie V, Schollhammer R, Macgrogan G, Vélasco V, Malavaud B, Fernandez P, Hindié E. Neurotensin Receptor-1 Expression in Human Prostate Cancer: A Pilot Study on Primary Tumors and Lymph Node Metastases. International Journal of Molecular Sciences. 2019; 20(7):1721. https://doi.org/10.3390/ijms20071721
Chicago/Turabian StyleMorgat, Clément, Adrien Chastel, Vincent Molinie, Romain Schollhammer, Gaétan Macgrogan, Valérie Vélasco, Bernard Malavaud, Philippe Fernandez, and Elif Hindié. 2019. "Neurotensin Receptor-1 Expression in Human Prostate Cancer: A Pilot Study on Primary Tumors and Lymph Node Metastases" International Journal of Molecular Sciences 20, no. 7: 1721. https://doi.org/10.3390/ijms20071721