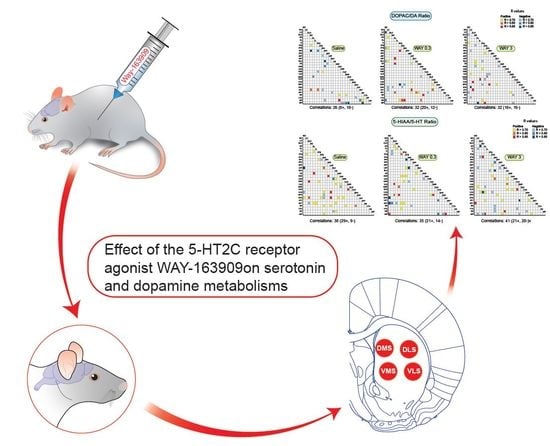
Effect of the 5-HT2C Receptor Agonist WAY-163909 on Serotonin and Dopamine Metabolism across the Rat Brain: A Quantitative and Qualitative Neurochemical Study
Abstract
:1. Introduction
2. Results
2.1. Quantitative Analysis of DA and 5-HT Metabolism after WAY-163909 Treatment
2.1.1. Pattern of Correlations of the 5-HIAA/5-HT Ratio across the Brain
2.1.2. Pattern of Correlations of the DOPAC/DA Ratio across the Brain
2.1.3. Pattern of Correlations between DOPAC/DA and 5-HIAA/5-HT Ratio across the Brain
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Sampling and Conditioning
4.3. HPLC Analysis and Electrochemical Detection
4.4. Pharmacological Treatment and Experimental Design
4.5. Statistical Analysis of the Data
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aCg | anterior cingulate cortex |
| ains | anterior insular cortex |
| BLA | basolateral nucleus of the amygdala |
| CE | central nucleus of the amygdala |
| CNS | central nervous system |
| core | core of the nucleus accumbens |
| DA | dopamine |
| dHP | dorsal hippocampus |
| DLO | dorsolateral orbitofrontal cortex |
| DLS | dorsolateral striatum |
| DMS | dorsomedial striatum |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| DR | dorsal raphe nucleus |
| dHY | dorsal hypothalamus |
| EPN | entodepuncular nucleus |
| HPLC | high pressure liquid chromatography |
| IL | infralimbic cortex |
| LO | lateral orbitofrontal cortex |
| M2 | motor cortex M2 |
| MO | medial orbitofrontal cortex |
| MR | median raphe nucleus |
| pCg | posterior cingulate cortex |
| pins | posterior insular cortex |
| PL | prelimbic cortex |
| 5-HIAA | 5-hydroxyindole-3-acetic acid |
| 5-HT | 5-hydroxytryptamine; serotonin |
| 5-HT2C receptors | Serotonin 2C receptors |
| shell | shell of the nucleus accumbens |
| SN | substantia nigra |
| STN | subthalamic nucleus |
| vHP | ventral hippocampus |
| vHY | ventral hypothalamus |
| VCS | ventrocaudal striatum |
| VLS | ventrolateral striatum |
| VMS | ventromedial striatum |
| VTA | ventral tegmental area |
| WAY-163909 | (7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7, 1hi]indole] |
References
- Di Giovanni, G.; De Deurwaerdere, P. New therapeutic opportunities for 5-ht2c receptor ligands in neuropsychiatric disorders. Pharmacol. Ther. 2016, 157, 125–162. [Google Scholar] [CrossRef] [PubMed]
- Anastasio, N.C.; Stutz, S.J.; Fox, R.G.; Sears, R.M.; Emeson, R.B.; DiLeone, R.J.; O’Neil, R.T.; Fink, L.H.; Li, D.; Green, T.A.; et al. Functional status of the serotonin 5-ht2c receptor (5-ht2cr) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology 2014, 39, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Tecott, L.H.; Sun, L.M.; Akana, S.F.; Strack, A.M.; Lowenstein, D.H.; Dallman, M.F.; Julius, D. Eating disorder and epilepsy in mice lacking 5-ht2c serotonin receptors. Nature 1995, 374, 542–546. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.; Lyons, D.; Cristiano, C.; Lettieri, M.; Olarte-Sanchez, C.; Burke, L.K.; Greenwald-Yarnell, M.; Cansell, C.; Doslikova, B.; Georgescu, T.; et al. Nucleus of the solitary tract serotonin 5-ht2c receptors modulate food intake. Cell Metab. 2018, 28, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.G.; Lee, M.D.; Dourish, C.T. Similarities in the action of ro 60-0175, a 5-ht2c receptor agonist and d-fenfluramine on feeding patterns in the rat. Psychopharmacology 2000, 152, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.A.; Fletcher, P.J. Serotonin and drug reward: Focus on 5-ht2c receptors. Eur. J. Pharmacol. 2003, 480, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.A.; Fletcher, P.J. Therapeutic potential of 5-ht2c receptor agonists for addictive disorders. ACS Chem. Neurosci. 2015, 6, 1071–1088. [Google Scholar] [CrossRef] [PubMed]
- Howell, L.L.; Cunningham, K.A. Serotonin 5-ht2 receptor interactions with dopamine function: Implications for therapeutics in cocaine use disorder. Pharmacol. Rev. 2015, 67, 176–197. [Google Scholar] [CrossRef]
- Fletcher, P.J.; Soko, A.D.; Higgins, G.A. Impulsive action in the 5-choice serial reaction time test in 5-ht(2)c receptor null mutant mice. Psychopharmacology 2013, 226, 561–570. [Google Scholar] [CrossRef]
- Anastasio, N.C.; Stutz, S.J.; Fink, L.H.; Swinford-Jackson, S.E.; Sears, R.M.; DiLeone, R.J.; Rice, K.C.; Moeller, F.G.; Cunningham, K.A. Serotonin (5-ht) 5-ht2a receptor (5-ht2ar):5-ht2cr imbalance in medial prefrontal cortex associates with motor impulsivity. ACS Chem. Neurosci. 2015, 6, 1248–1258. [Google Scholar] [CrossRef]
- Siuciak, J.A.; Chapin, D.S.; McCarthy, S.A.; Guanowsky, V.; Brown, J.; Chiang, P.; Marala, R.; Patterson, T.; Seymour, P.A.; Swick, A.; et al. Cp-809,101, a selective 5-ht2c agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 2007, 52, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kennett, G.A.; Whitton, P.; Shah, K.; Curzon, G. Anxiogenic-like effects of mcpp and tfmpp in animal models are opposed by 5-ht1c receptor antagonists. Eur. J. Pharmacol. 1989, 164, 445–454. [Google Scholar] [CrossRef]
- Millan, M.J. The neurobiology and control of anxious states. Prog. Neurobiol. 2003, 70, 83–244. [Google Scholar] [CrossRef]
- Millan, M.J. Serotonin 5-ht2c receptors as a target for the treatment of depressive and anxious states: Focus on novel therapeutic strategies. Therapie 2005, 60, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Graf, M. 5-ht2c receptor activation induces grooming behaviour in rats: Possible correlations with obsessive-compulsive disorder. Off. J. Hung. Assoc. Psychopharmacol. 2006, 8, 23–28. [Google Scholar]
- Kreiss, D.S.; De Deurwaerdere, P. Purposeless oral activity induced by meta-chlorophenylpiperazine (m-cpp): Undefined tic-like behaviors? J. Neurosci. Methods 2017, 292, 30–36. [Google Scholar] [CrossRef]
- De Gregorio, D.; Enns, J.P.; Nunez, N.A.; Posa, L.; Gobbi, G. D-lysergic acid diethylamide, psilocybin, and other classic hallucinogens: Mechanism of action and potential therapeutic applications in mood disorders. Prog. Brain Res. 2018, 242, 69–96. [Google Scholar]
- Chagraoui, A.; Thibaut, F.; Skiba, M.; Thuillez, C.; Bourin, M. 5-ht2c receptors in psychiatric disorders: A review. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 2016, 66, 120–135. [Google Scholar] [CrossRef]
- Abramowski, D.; Rigo, M.; Duc, D.; Hoyer, D.; Staufenbiel, M. Localization of the 5-hydroxytryptamine2c receptor protein in human and rat brain using specific antisera. Neuropharmacology 1995, 34, 1635–1645. [Google Scholar] [CrossRef]
- Clemett, D.A.; Punhani, T.; Duxon, M.S.; Blackburn, T.P.; Fone, K.C. Immunohistochemical localisation of the 5-ht2c receptor protein in the rat cns. Neuropharmacology 2000, 39, 123–132. [Google Scholar] [CrossRef]
- Pasqualetti, M.; Ori, M.; Castagna, M.; Marazziti, D.; Cassano, G.B.; Nardi, I. Distribution and cellular localization of the serotonin type 2c receptor messenger rna in human brain. Neuroscience 1999, 92, 601–611. [Google Scholar] [CrossRef]
- Pazos, A.; Palacios, J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985, 346, 205–230. [Google Scholar] [CrossRef]
- De Deurwaerdere, P.; Lagiere, M.; Bosc, M.; Navailles, S. Multiple controls exerted by 5-ht2c receptors upon basal ganglia function: From physiology to pathophysiology. Exp. Brain Res. 2013, 230, 477–511. [Google Scholar] [CrossRef] [PubMed]
- Lagiere, M.; Bosc, M.; Whitestone, S.; Manem, J.; Elboukhari, H.; Benazzouz, A.; Di Giovanni, G.; De Deurwaerdere, P. Does the serotonin2c receptor segregate circuits of the basal ganglia responding to cingulate cortex stimulation? CNS Neurosci. Ther. 2018, 24, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Navailles, S.; Lagiere, M.; Le Moine, C.; De Deurwaerdere, P. Role of 5-ht2c receptors in the enhancement of c-fos expression induced by a 5-ht2b/2c inverse agonist and 5-ht 2 agonists in the rat basal ganglia. Exp. Brain Res. 2013, 230, 525–535. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdere, P.; Di Giovanni, G. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: Therapeutic implications. Prog. Neurobiol. 2017, 151, 175–236. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Chesselet, M.F. Presynaptic regulation of neurotransmitter release in the brain: Facts and hypothesis. Neuroscience 1984, 12, 347–375. [Google Scholar] [CrossRef]
- Chagraoui, A.; Whitestone, S.; Baassiri, L.; Manem, J.; Di Giovanni, G.; De Deurwaerdere, P. Neurochemical impact of the 5-ht2c receptor agonist way-163909 on monoamine tissue content in the rat brain. Neurochem. Int. 2019, 124, 245–255. [Google Scholar] [CrossRef]
- Commissiong, J.W. Monoamine metabolites: Their relationship and lack of relationship to monoaminergic neuronal activity. Biochem. Pharmacol. 1985, 34, 1127–1131. [Google Scholar] [CrossRef]
- Sharp, T.; Zetterstrom, T.; Ungerstedt, U. An in vivo study of dopamine release and metabolism in rat brain regions using intracerebral dialysis. J. Neurochem. 1986, 47, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Fitoussi, A.; Dellu-Hagedorn, F.; De Deurwaerdere, P. Monoamines tissue content analysis reveals restricted and site-specific correlations in brain regions involved in cognition. Neuroscience 2013, 255, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Navailles, S.; Bioulac, B.; Gross, C.; De Deurwaerdere, P. Chronic l-dopa therapy alters central serotonergic function and l-dopa-induced dopamine release in a region-dependent manner in a rat model of parkinson’s disease. Neurobiol. Dis. 2011, 41, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.R.; Bloom, F.E.; Roth, R.H. The Biochemical Basis of Neuropharmacology, 8th ed.; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- De Deurwaerdere, P.; Binda, C.; Corne, R.; Leone, C.; Valeri, A.; Valoti, M.; Ramsay, R.R.; Fall, Y.; Marco-Contelles, J. Comparative analysis of the neurochemical profile and mao inhibition properties of n-(furan-2-ylmethyl)-n-methylprop-2-yn-1-amine. ACS Chem. Neurosci. 2017, 8, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Godar, S.C.; Alzghoul, L.; Zhang, J.; Darling, R.D.; Simpson, K.L.; Bini, V.; Chen, K.; Wellman, C.L.; Lin, R.C.; et al. Monoamine oxidase a and a/b knockout mice display autistic-like features. Int. J. Neuropsychopharmacol. 2013, 16, 869–888. [Google Scholar] [CrossRef]
- Shih, J.C.; Chen, K. Mao-a and -b gene knock-out mice exhibit distinctly different behavior. Neurobiology 1999, 7, 235–246. [Google Scholar]
- De Deurwaerdere, P.; Di Giovanni, G.; Millan, M.J. Expanding the repertoire of l-dopa’s actions: A comprehensive review of its functional neurochemistry. Prog. Neurobiol. 2017, 151, 57–100. [Google Scholar] [CrossRef]
- Dellu-Hagedorn, F.; Fitoussi, A.; De Deurwaerdere, P. Correlative analysis of dopaminergic and serotonergic metabolism across the brain to study monoaminergic function and interaction. J. Neurosci. Methods 2017, 280, 54–63. [Google Scholar] [CrossRef]
- Kaenmaki, M.; Tammimaki, A.; Myohanen, T.; Pakarinen, K.; Amberg, C.; Karayiorgou, M.; Gogos, J.A.; Mannisto, P.T. Quantitative role of comt in dopamine clearance in the prefrontal cortex of freely moving mice. J. Neurochem. 2010, 114, 1745–1755. [Google Scholar] [CrossRef]
- Mongeau, R.; Martin, C.B.; Chevarin, C.; Maldonado, R.; Hamon, M.; Robledo, P.; Lanfumey, L. 5-ht2c receptor activation prevents stress-induced enhancement of brain 5-ht turnover and extracellular levels in the mouse brain: Modulation by chronic paroxetine treatment. J. Neurochem. 2010, 115, 438–449. [Google Scholar] [CrossRef]
- Di Giovanni, G.; Di Matteo, V.; Di Mascio, M.; Esposito, E. Preferential modulation of mesolimbic vs. Nigrostriatal dopaminergic function by serotonin(2c/2b) receptor agonists: A combined in vivo electrophysiological and microdialysis study. Synapse 2000, 35, 53–61. [Google Scholar] [CrossRef]
- Di Matteo, V.; Di Giovanni, G.; Di Mascio, M.; Esposito, E. Biochemical and electrophysiological evidence that ro 60-0175 inhibits mesolimbic dopaminergic function through serotonin(2c) receptors. Brain Res. 2000, 865, 85–90. [Google Scholar] [CrossRef]
- De Deurwaerdere, P.; Navailles, S.; Berg, K.A.; Clarke, W.P.; Spampinato, U. Constitutive activity of the serotonin2c receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J. Neurosci. 2004, 24, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Rivet, J.M.; Lejeune, F.; Newman-Tancredi, A.; Adhumeau-Auclair, A.; Nicolas, J.P.; Cistarelli, L.; Melon, C.; Millan, M.J. Serotonin(2c) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: A combined dialysis and electrophysiological analysis in the rat. Synapse 2000, 36, 205–221. [Google Scholar] [CrossRef]
- Browne, C.J.; Ji, X.; Higgins, G.A.; Fletcher, P.J.; Harvey-Lewis, C. Pharmacological modulation of 5-ht2c receptor activity produces bidirectional changes in locomotor activity, responding for a conditioned reinforcer, and mesolimbic da release in c57bl/6 mice. Neuropsychopharmacology 2017, 42, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, L.; Acconcia, S.; Ceglia, I.; Invernizzi, R.W.; Samanin, R. Stimulation of 5-hydroxytryptamine (5-ht(2c) ) receptors in the ventrotegmental area inhibits stress-induced but not basal dopamine release in the rat prefrontal cortex. J. Neurochem. 2002, 82, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Marquis, K.L.; Sabb, A.L.; Logue, S.F.; Brennan, J.A.; Piesla, M.J.; Comery, T.A.; Grauer, S.M.; Ashby, C.R., Jr.; Nguyen, H.Q.; Dawson, L.A.; et al. Way-163909 [(7br,10ar)-1,2,3,4,8,9,10,10a-octahydro-7bh-cyclopenta-[b][1,4]diazepino[6,7,1hi ]indole]: A novel 5-hydroxytryptamine 2c receptor-selective agonist with preclinical antipsychotic-like activity. J. Pharmacol. Exp. Ther. 2007, 320, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Lucas, G.; Spampinato, U. Role of striatal serotonin2a and serotonin2c receptor subtypes in the control of in vivo dopamine outflow in the rat striatum. J. Neurochem. 2000, 74, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Leggio, G.M.; Cathala, A.; Neny, M.; Rouge-Pont, F.; Drago, F.; Piazza, P.V.; Spampinato, U. In vivo evidence that constitutive activity of serotonin2c receptors in the medial prefrontal cortex participates in the control of dopamine release in the rat nucleus accumbens: Differential effects of inverse agonist versus antagonist. J. Neurochem. 2009, 111, 614–623. [Google Scholar] [CrossRef]
- Dunlop, J.; Marquis, K.L.; Lim, H.K.; Leung, L.; Kao, J.; Cheesman, C.; Rosenzweig-Lipson, S. Pharmacological profile of the 5-ht(2c) receptor agonist way-163909; therapeutic potential in multiple indications. CNS Drug Rev. 2006, 12, 167–177. [Google Scholar] [CrossRef]
- Dunlop, J.; Sabb, A.L.; Mazandarani, H.; Zhang, J.; Kalgaonker, S.; Shukhina, E.; Sukoff, S.; Vogel, R.L.; Stack, G.; Schechter, L.; et al. Way-163909 [(7br, 10ar)-1,2,3,4,8,9,10,10a-octahydro-7bh-cyclopenta-[b][1,4]diazepino[6,7,1hi]indol e], a novel 5-hydroxytryptamine 2c receptor-selective agonist with anorectic activity. J. Pharmacol. Exp. Ther. 2005, 313, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.J.; Murnane, K.S. The serotonin 2c receptor agonist way-163909 attenuates ketamine-induced hypothermia in mice. Eur. J. Pharmacol. 2019, 842, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.U.; Dewan, S.; Nilsson, S.R.O.; Robbins, T.W.; Heath, C.J.; Saksida, L.M.; Bussey, T.J.; Alsio, J. Selective effects of 5-ht2c receptor modulation on performance of a novel valence-probe visual discrimination task and probabilistic reversal learning in mice. Psychopharmacology 2018, 235, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Berro, L.F.; Perez Diaz, M.; Maltbie, E.; Howell, L.L. Effects of the serotonin 2c receptor agonist way163909 on the abuse-related effects and mesolimbic dopamine neurochemistry induced by abused stimulants in rhesus monkeys. Psychopharmacology 2017, 234, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, W.; Furuta, T.; Nakamura, K.C.; Hioki, H.; Fujiyama, F.; Arai, R.; Kaneko, T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 2009, 29, 444–453. [Google Scholar] [CrossRef]
- Gardiner, K.; Du, Y. A-to-i editing of the 5ht2c receptor and behaviour. Brief. Funct. Genom. Proteom. 2006, 5, 37–42. [Google Scholar] [CrossRef]
- Berg, K.A.; Cropper, J.D.; Niswender, C.M.; Sanders-Bush, E.; Emeson, R.B.; Clarke, W.P. Rna-editing of the 5-ht(2c) receptor alters agonist-receptor-effector coupling specificity. Br. J. Pharmacol. 2001, 134, 386–392. [Google Scholar] [CrossRef]
- Martin, C.B.; Ramond, F.; Farrington, D.T.; Aguiar, A.S., Jr.; Chevarin, C.; Berthiau, A.S.; Caussanel, S.; Lanfumey, L.; Herrick-Davis, K.; Hamon, M.; et al. Rna splicing and editing modulation of 5-ht(2c) receptor function: Relevance to anxiety and aggression in vgv mice. Mol. Psychiatry 2013, 18, 656–665. [Google Scholar] [CrossRef]
- Niswender, C.M.; Sanders-Bush, E.; Emeson, R.B. Identification and characterization of rna editing events within the 5-ht2c receptor. Ann. N. Y. Acad. Sci. 1998, 861, 38–48. [Google Scholar] [CrossRef]
- Burns, C.M.; Chu, H.; Rueter, S.M.; Hutchinson, L.K.; Canton, H.; Sanders-Bush, E.; Emeson, R.B. Regulation of serotonin-2c receptor g-protein coupling by rna editing. Nature 1997, 387, 303–308. [Google Scholar] [CrossRef]
- Dellu-Hagedorn, F.; Rivalan, M.; Fitoussi, A.; De Deurwaerdere, P. Inter-individual differences in the impulsive/compulsive dimension: Deciphering related dopaminergic and serotonergic metabolisms at rest. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef] [PubMed]
- Puginier, E.; Bharatiya, R.; Chagraoui, A.; Manem, J.; Cho, Y.H.; Garret, M.; De Deurwaerdere, P. Early neurochemical modifications of monoaminergic systems in the r6/1 mouse model of huntington’s disease. Neurochem. Int. 2019, 128, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Beyeler, A.; Kadiri, N.; Navailles, S.; Boujema, M.B.; Gonon, F.; Moine, C.L.; Gross, C.; De Deurwaerdere, P. Stimulation of serotonin2c receptors elicits abnormal oral movements by acting on pathways other than the sensorimotor one in the rat basal ganglia. Neuroscience 2010, 169, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Alsio, J.; Nilsson, S.R.; Gastambide, F.; Wang, R.A.; Dam, S.A.; Mar, A.C.; Tricklebank, M.; Robbins, T.W. The role of 5-ht2c receptors in touchscreen visual reversal learning in the rat: A cross-site study. Psychopharmacology 2015, 232, 4017–4031. [Google Scholar] [CrossRef] [PubMed]
- Flaisher-Grinberg, S.; Klavir, O.; Joel, D. The role of 5-ht2a and 5-ht2c receptors in the signal attenuation rat model of obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 2008, 11, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.J.; Rizos, Z.; Noble, K.; Higgins, G.A. Impulsive action induced by amphetamine, cocaine and mk801 is reduced by 5-ht(2c) receptor stimulation and 5-ht(2a) receptor blockade. Neuropharmacology 2011, 61, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Bagdy, G.; Graf, M.; Anheuer, Z.E.; Modos, E.A.; Kantor, S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-cpp treatment are reversed by pretreatment with the 5-ht2c receptor antagonist sb-242084 but not the 5-ht1a receptor antagonist way-100635. Int. J. Neuropsychopharmacol. 2001, 4, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.M.; Merchant, K.M. Serotonin 2c receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003, 993, 1–9. [Google Scholar] [CrossRef]
- Jenck, F.; Bos, M.; Wichmann, J.; Stadler, H.; Martin, J.R.; Moreau, J.L. The role of 5-ht2c receptors in affective disorders. Expert Opin. Investig. Drugs 1998, 7, 1587–1599. [Google Scholar] [CrossRef]
- Pockros-Burgess, L.A.; Pentkowski, N.S.; Der-Ghazarian, T.; Neisewander, J.L. Effects of the 5-ht2c receptor agonist cp809101 in the amygdala on reinstatement of cocaine-seeking behavior and anxiety-like behavior. Int. J. Neuropsychopharmacol. 2014, 17, 1751–1762. [Google Scholar] [CrossRef]
- Hayashi, A.; Sonoda, R.; Kimura, Y.; Takasu, T.; Suzuki, M.; Sasamata, M.; Miyata, K. Antiobesity effect of ym348, a novel 5-ht2c receptor agonist, in zucker rats. Brain Res. 2004, 1011, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig-Lipson, S.; Zhang, J.; Mazandarani, H.; Harrison, B.L.; Sabb, A.; Sabalski, J.; Stack, G.; Welmaker, G.; Barrett, J.E.; Dunlop, J. Antiobesity-like effects of the 5-ht2c receptor agonist way-161503. Brain Res. 2006, 1073–1074, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Navailles, S.; Lagiere, M.; Roumegous, A.; Polito, M.; Boujema, M.B.; Cador, M.; Dunlop, J.; Chesselet, M.F.; Millan, M.J.; De Deurwaerdere, P. Serotonin2c ligands exhibiting full negative and positive intrinsic activity elicit purposeless oral movements in rats: Distinct effects of agonists and inverse agonists in a rat model of parkinson’s disease. Int. J. Neuropsychopharmacol. 2013, 16, 593–606. [Google Scholar] [CrossRef] [PubMed]




| Brain Regions | 5-HIAA | DOPAC | HVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Saline | WAY 0.3 | WAY 3 | Saline | WAY 0.3 | WAY 3 | Saline | WAY 0.3 | WAY 3 | |
| MO | 206 ± 30 | 224 ± 20 | 252 ± 19 | 122 ± 22 | 121 ± 15 | 180 ± 22 | 23.9 ± 3.12 | 91.4 ± 20 * | 103 ± 24 ** |
| LO | 212 ± 34 | 188 ± 26 | 328 ± 54 | 35.8 ± 2.81 | 44.1 ± 15.3 | 66.1 ± 9.22 | nd | nd | nd |
| DLO | 73.6 ± 11.9 | 37.1 ± 2.95 * | 59.1 ± 11.3 | 13.2 ± 2.88 | 12.6 ± 1.8 | 20.3 ± 3.92 | nd | nd | nd |
| M2 | 92.2 ± 20.1 | 87.3 ± 12.6 | 104 ± 15.6 | 27.6 ± 3.09 | 20.9 ± 1.7 | 30.4 ± 5.76 | 1.82 ± 0.61 | 1.28 ± 0.34 | 1.99 ± 0.35 |
| PL | 137 ± 10.9 | 149 ± 24.7 | 116 ± 13.9 | 9.12 ± 1.30 | 12.5 ± 3.43 | 9.71 ± 1.95 | 36.2 ± 11.7 | 103 ± 20.3 | 62.9 ± 19.7 |
| IL | 147 ± 35.3 | 124 ± 19.1 | 121 ± 31.2 | 29.9 ± 5.16 | 19.4 ± 1.94 | 25.5 ± 6.39 | 18.5 ± 3.92 | 16.3 ± 3.21 | 24.6 ± 4.94 |
| aCg | 336 ± 16.0 | 312 ± 72.6 | 228 ± 45.1 | 180 ± 14.1 | 118 ± 34.6 | 115 ± 27.5 | nd | nd | nd |
| pCg | 386 ± 43.8 | 348 ± 34.5 | 308 ± 16.8 | 41.9 ± 3.28 | 38.0 ± 4.49 | 30.3 ± 1.46 | nd | nd | nd |
| ains | 136 ± 18.9 | 113 ± 20 | 106 ± 11.8 | 24.5 ± 5.13 | 27.9 ± 4.72 | 21.2 ± 2.4 | 8.86 ± 2.75 | 6.67 ± 1.22 | 6.15 ± 0.71 |
| pins | 99.6 ± 8.35 | 79 ± 11.7 | 102 ± 15.9 | 17.1 ± 4.1 | 15.2 ± 1.6 | 16.9 ± 3.63 | 3.98 ± 0.56 | 3.75 ± 0.76 | 4.22 ± 0.70 |
| dHP | 81.8 ± 9.15 | 60.3 ± 8.36 | 81.8 ± 4.75 | 12.3 ± 0.34 | 14.24 ± 0.90 | 12.43 ± 0.67 | nd | nd | nd |
| vHP | 159 ± 3.61 | 139 ± 17.6 | 132 ± 11.9 | 15.2 ± 2.2 | 15.5 ± 3.04 | 13.71 ± 4.2 | nd | nd | nd |
| BLA | 151 ± 17.7 | 215 ± 25.9 | 229 ± 17.5 | 104 ± 53.9 | 116 ± 23.6 | 100 ± 32.7 | nd | nd | nd |
| CE | 132 ± 18.6 | 199 ± 21.4 * | 108 ± 19.9 | 59.0 ± 10.0 | 131 ± 40.3 | 45.8 ± 13.9 | nd | nd | nd |
| shell | 178 ± 17.5 | 123 ± 10.5 | 154 ± 16.1 | 728 ± 85.8 | 595 ± 73.0 | 726 ± 71.0 | 540 ± 83.6 | 449 ± 41.4 | 586 ± 52.8 |
| core | 76.0 ± 6.36 | 63.8 ± 7.52 | 73.0 ± 11.1 | 847 ± 78 | 682 ± 106 | 825 ± 145 | 150 ± 9.02 | 124 ± 16.1 | 166 ± 23.5 |
| VMS | 142 ± 23.8 | 137 ± 13.8 | 170 ± 23.5 | 480 ± 80.2 | 477 ± 72.6 | 569 ± 84.6 | 257 ± 56.7 | 272 ± 48.4 | 352 ± 107 |
| VLS | 208 ± 12.3 | 244 ± 18.2 | 274 ± 28.2 | 876 ± 123 | 661 ± 49.6 | 799 ± 58.6 | 407 ± 62.8 | 363 ± 15.2 | 445 ± 36.6 |
| DMS | 96.2 ± 15.9 | 85.6 ± 6.45 | 79.1 ± 7.23 | 709 ± 109 | 709 ± 98.6 | 720 ± 83.1 | 603 ± 95.8 | 667 ± 66.4 | 806 ± 59.0 |
| DLS | 190 ± 66.9 | 110 ± 17.6 | 144 ± 20.3 | 797 ± 129 | 727 ± 92.5 | 838 ± 110 | 982 ± 93.3 | 827 ± 97.0 | 1113 ± 167 |
| VCS | 172 ± 36.9 | 173 ± 38.4 | 250 ± 42.2 | 307 ± 65.4 | 272 ± 76.9 | 471 ± 82.6 | 359 ± 80.6 | 359 ± 96.8 | 521 ± 94.2 |
| EPN | 147 ± 17.2 | 174 ± 25.8 | 111 ± 15.4 | 16.7 ± 2.92 | 18.6 ± 3.81 | 11.40 ± 2.11 | 1.76 ± 0.27 | 2.38 ± 0.65 | 1.79 ± 0.23 |
| STN | 257 ± 43.4 | 260 ± 37.6 | 310 ± 34.9 | 132 ± 32.5 | 148 ± 22.6 | 181 ± 29.7 | 6.48 ± 2.15 | 7.57 ± 1.29 | 9.05 ± 1.40 |
| vHY | 74.9 ± 8.82 | 54.3 ± 6.15 | 51.8 ± 8.67 | 28.2 ± 7.14 | 22.8 ± 4.02 | 25.0 ± 3.40 | nd | nd | nd |
| dHY | 156 ± 23.6 | 101 ± 14.9 | 81 ± 21.6 | 3.16 ± 0.62 | 2.19 ± 0.52 | 2.41 ± 0.63 | 2.63 ± 0.77 | 1.86 ± 0.57 | 3.87 ± 1.29 |
| SN | 738 ± 55.9 | 766 ± 47.9 | 754 ± 37.8 | 474 ± 82.4 | 436 ± 70.1 | 642 ± 61.7 | 194 ± 59.8 | 133 ± 34.5 | 176 ± 50.9 |
| VTA | 428 ± 44.3 | 459 ± 41.4 | 385 ± 62.8 | 625 ± 64.3 | 650 ± 91.4 | 597 ± 67.6 | 83.0 ± 10.8 | 85.0 ± 8.17 | 75.4 ± 10.5 |
| DR | 396 ± 32.6 | 373 ± 19.8 | 407 ± 33.1 | 23.2 ± 5.9 | 19.2 ± 3.9 | 31.9 ± 7.3 | nd | nd | nd |
| MR | 318 ± 62 | 230 ± 35 | 265 ± 40.5 | 63.9 ± 18.8 | 107 ± 28.8 | 37.9 ± 19.2 | 15.4 ± 1.8 | 16.4 ± 2.9 | 9.13 ± 1.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitestone, S.; De Deurwaerdère, P.; Baassiri, L.; Manem, J.; Anouar, Y.; Di Giovanni, G.; Bharatiya, R.; Chagraoui, A. Effect of the 5-HT2C Receptor Agonist WAY-163909 on Serotonin and Dopamine Metabolism across the Rat Brain: A Quantitative and Qualitative Neurochemical Study. Int. J. Mol. Sci. 2019, 20, 2925. https://doi.org/10.3390/ijms20122925
Whitestone S, De Deurwaerdère P, Baassiri L, Manem J, Anouar Y, Di Giovanni G, Bharatiya R, Chagraoui A. Effect of the 5-HT2C Receptor Agonist WAY-163909 on Serotonin and Dopamine Metabolism across the Rat Brain: A Quantitative and Qualitative Neurochemical Study. International Journal of Molecular Sciences. 2019; 20(12):2925. https://doi.org/10.3390/ijms20122925
Chicago/Turabian StyleWhitestone, Sara, Philippe De Deurwaerdère, Lynn Baassiri, Julien Manem, Youssef Anouar, Giuseppe Di Giovanni, Rahul Bharatiya, and Abdeslam Chagraoui. 2019. "Effect of the 5-HT2C Receptor Agonist WAY-163909 on Serotonin and Dopamine Metabolism across the Rat Brain: A Quantitative and Qualitative Neurochemical Study" International Journal of Molecular Sciences 20, no. 12: 2925. https://doi.org/10.3390/ijms20122925