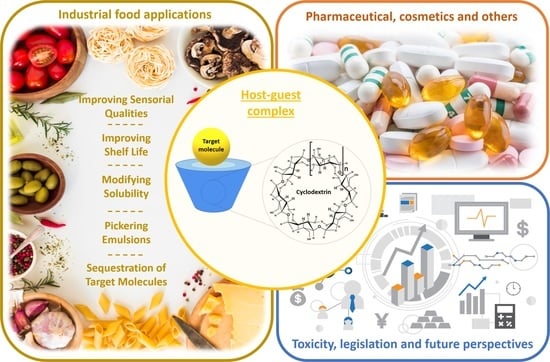
Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes
Abstract
:1. Introduction
2. Cyclodextrins
2.1. Definition
2.2. Mechanism
3. Applications in the Food Industry
3.1. Improving Sensorial Qualities
3.1.1. Color
3.1.2. Flavor
3.1.3. Taste
3.2. Improving Shelf Life
3.2.1. Against Oxidation
3.2.2. Against Light-Induced Decomposition
3.2.3. Against Heat-Induced Changes
3.3. Modifying Solubility
3.4. Sequestration of Selected Components
3.5. Pickering Emulsions
3.6. Other Food Applications
4. Other Applications
4.1. Pharmaceutical Applications
4.2. Cosmetics and Personal Care
4.3. Packing and Textile Industry
4.4. Bioconversion and Fermentation
4.5. Environment
4.6. Catalytic
4.7. Analytical
5. Advantages and Disadvantages of Their Use
6. Toxicity and Legislation
7. Future Perspectives and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wenz, G. An overview of host-guest chemistry and its application to nonsteroidal anti-inflammatory drugs. Clin. Drug Investig. 2000, 19, 21–25. [Google Scholar] [CrossRef]
- Cram, D.J.; Cram, J.M. Host-Guest Chemistry. Science 1974, 183, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. Supramolecular chemistry: Receptors, catalysts, and carriers. Science 1985, 227, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Eastburn, S.D.; Tao, B.Y. Applications of modified cyclodextrins. Biotechnol. Adv. 1994, 12, 325–339. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Buschmann, H.J.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar]
- Shibatani, T. Industrial Application of Immobilized Biocatalysts in Japan. In Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 11, pp. 585–591. [Google Scholar]
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Supramolecular cyclodextrin complex: Diversity, safety, and applications in ocular therapeutics. Exp. Eye Res. 2019, 189, 107829. [Google Scholar] [CrossRef]
- Bortolus, P.; Grabner, G.; Köhler, G.; Monti, S. Photochemistry of cyclodextrin host-guest complexes. Coord. Chem. Rev. 1993, 125, 261–268. [Google Scholar] [CrossRef]
- Da Hu, Q.; Tang, G.P.; Chu, P.K. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef]
- Wenz, G. Cyclodextrins as Building Blocks for Supramolecular Structures and Functional Units. Angew. Chem. Int. Ed. Engl. 1994, 33, 803–822. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Latest developments in the application of cyclodextrin host-guest complexes in beverage technology processes. Food Hydrocoll. 2020, 106, 105882. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging medicines of the new millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almagro, L.; Pedreño, M.Á. Use of cyclodextrins to improve the production of plant bioactive compounds. Phytochem. Rev. 2020, 19, 1061–1080. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- De Menezes, P.P.; de Andrade, T.A.; Frank, L.A.; de Souza, E.P.B.S.S.; das Trindade, G.G.G.; Trindade, I.A.S.; Serafini, M.R.; Guterres, S.S.; de Araújo, A.A.S. Advances of nanosystems containing cyclodextrins and their applications in pharmaceuticals. Int. J. Pharm. 2019, 559, 312–328. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Bruns, C.J. Exploring and exploiting the symmetry-breaking effect of cyclodextrins in mechanomolecules. Symmetry 2019, 11, 1249. [Google Scholar] [CrossRef] [Green Version]
- Linde, G.A.; Laverde, A.; Colauto, N.B. Changes to Taste Perception in the Food Industry: Use of Cyclodextrins. In Handbook of Behavior, Food and Nutrition; Preedy, V.R., Watson, R.R., Martin, C.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 99–118. [Google Scholar]
- Radu, C.-D.; Parteni, O.; Ochiuz, L. Applications of cyclodextrins in medical textiles. J. Control. Release 2016, 224, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.; Buera, P.; Mazzobre, F. Novel trends in cyclodextrins encapsulation. Applications in food science. Curr. Opin. Food Sci. 2017, 16, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Modern extraction techniques optimized to extract betacyanins from Gomphrena globosa L. Ind. Crops Prod. 2017, 105, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Blanch, G.P.; Ruiz del Castillo, M.L.; del Mar Caja, M.; Pérez-Méndez, M.; Sánchez-Cortés, S. Stabilization of all-trans-lycopene from tomato by encapsulation using cyclodextrins. Food Chem. 2007, 105, 1335–1341. [Google Scholar] [CrossRef] [Green Version]
- Andreu-Sevilla, A.J.; López-Nicolás, J.M.; Carbonell-Barrachina, Á.A.; García-Carmona, F. Comparative Effect of the Addition of α-, β-, or γ-Cyclodextrin on Main Sensory and Physico-Chemical Parameters. J. Food Sci. 2011, 76, S347–S353. [Google Scholar] [CrossRef] [PubMed]
- Reineccius, T.A.; Reineccius, G.A.; Peppard, T.L. Flavor release from cyclodextrin complexes: Comparison of alpha, beta, and gamma types. J. Food Sci. 2003, 68, 1234–1239. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Cravotto, G.; Binello, A.; Baranelli, E.; Carraro, P.; Trotta, F. Cyclodextrins as food additives and in food processing. Curr. Nutr. Sci. 2006, 2, 343–350. [Google Scholar] [CrossRef]
- Yuliani, S.; Bhandari, B.; Rutgers, R.; D’Arcy, B. Application of microencapsulated flavor to extrusion product. Food Rev. Int. 2004, 20, 163–185. [Google Scholar] [CrossRef]
- Reineccius, T.A.; Reineccius, G.A.; Peppard, T.L. Encapsulation of flavors using cyclodextrins: Comparison of flavor retention in alpha, beta, and gamma types. J. Food Sci. 2002, 67, 3271–3279. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Morales, J.; Rial-Otero, R.; Simal-Gándara, J. Factors controlling flavors binding constants to cyclodextrins and their applications in foods. Food Res. Int. 2010, 43, 1212–1218. [Google Scholar] [CrossRef]
- Yoshii, H.; Yasuda, M.; Furuta, T.; Kuwahara, H.; Ohkawara, M.; Linko, P. Retention of cyclodextrin complexed shiitake (Lentinus edodes) flavors with spray drying. Dry. Technol. 2005, 23, 1205–1215. [Google Scholar] [CrossRef]
- Tobitsuka, K.; Miura, M.; Kobayashi, S. Retention of a European pear aroma model mixture using different types of saccharides. J. Agric. Food Chem. 2006, 54, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Pagington, J.S. β-cyclodextrin and its uses in the flavour industry. In Developments in Food Flavours; Birch, G.G., Lindley, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Hedges, A.R.; Shieh, W.J.; Sikorski, C.T. Use of Cyclodextrins for Encapsulation in the Use and Treatment of Food Products. In Encapsulation and Controlled Release of Food Ingredients; ACS Publications: Washington, DC, USA, 1995; Volume 590, pp. 60–71. [Google Scholar]
- Bhandari, B.; D’Arcy, B.; Young, G. Flavour retention during high temperature short time extrusion cooking process: A review. Int. J. Food Sci. Technol. 2001, 36, 453–461. [Google Scholar] [CrossRef]
- Jouquand, C.; Ducruet, V.; Giampaoli, P. Partition coefficients of aroma compounds in polysaccharide solutions by the phase ratio variation method. Food Chem. 2004, 85, 467–474. [Google Scholar] [CrossRef]
- Kollengode, A.N.R.; Hanna, M.A. Cyclodextrin complexed flavors retention in extruded starches. J. Food Sci. 1997, 62, 1057–1060. [Google Scholar] [CrossRef]
- Young, O.A.; Gupta, R.B.; Sadooghy-Saraby, S. Effect of cyclodextrins on the flavour of goat milk and its yoghurt. J. Food Sci. 2012, 77, 122–127. [Google Scholar] [CrossRef]
- Reineccius, T.A.; Reineccius, G.A.; Peppard, T.L. Utilization of β-Cyclodextrin for Improved Flavor Retention in Thermally Processed Foods. J. Food Sci. 2004, 69, FCT58–FCT62. [Google Scholar] [CrossRef]
- Linde, G.A.; Junior, A.L.; de Faria, E.V.; Colauto, N.B.; de Moraes, F.F.; Zanin, G.M. Taste modification of amino acids and protein hydrolysate by α-cyclodextrin. Food Res. Int. 2009, 42, 814–818. [Google Scholar] [CrossRef]
- Binello, A.; Robaldo, B.; Barge, A.; Cavalli, R.; Cravotto, G. Synthesis of cyclodextrin-based polymers and their use as debittering agents. J. Appl. Polym. Sci. 2008, 107, 2549–2557. [Google Scholar] [CrossRef]
- Choi, M.J.; Ruktanonchai, U.; Soottitantawat, A.; Min, S.G. Morphological characterization of encapsulated fish oil with β-cyclodextrin and polycaprolactone. Food Res. Int. 2009, 42, 989–997. [Google Scholar] [CrossRef]
- Lee, S.H.; Yu, H.J.; Cho, N.S.; Park, J.H.; Kim, T.H.; Kim, K.H.; Lee, S.K. A method for preparing the inclusion complex of ginseng extract with gamma-cyclodextrin, and the composition comprising the same. In International Patent Classification IPC A23L; IPC Publication: Geneva, Switzerland, 2006; pp. 1–212. [Google Scholar]
- Shaw, P.E.; Tatum, J.H.; Wilson, C.W. Improved Flavor of Navel Orange and Grapefruit Juices by Removal of Bitter Components with β-Cyclodextrin Polymer. J. Agric. Food Chem. 1984, 32, 832–836. [Google Scholar] [CrossRef]
- Binello, A.; Cravotto, G.; Nano, G.M.; Spagliardi, P. Synthesis of chitosan-cyclodextrin adducts and evaluation of their bitter-masking properties. Flavour Fragr. J. 2004, 19, 394–400. [Google Scholar] [CrossRef]
- Szejtli, J.; Szente, L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Saldanha do Carmo, C.; Pais, R.; Simplício, A.L.; Mateus, M.; Duarte, C.M.M. Improvement of Aroma and Shelf-Life of Non-alcoholic Beverages Through Cyclodextrins-Limonene Inclusion Complexes. Food Bioprocess Technol. 2017, 10, 1297–1309. [Google Scholar] [CrossRef]
- Hedges, A.R. Cyclodextrins: Properties and Applications. In Starch; Academic Press: Cambridge, MA, USA, 2009; pp. 833–851. ISBN 9780127462752. [Google Scholar]
- Capelezzo, A.P.; Mohr, L.C.; Dalcanton, F.; de Mello, J.M.M.; Fiori, M.A. β-Cyclodextrins as Encapsulating Agents of Essential Oils. In Cyclodextrin—A Versatile Ingredient; IntechOpen: London, UK, 2018. [Google Scholar]
- Furuta, T.; Yoshii, H.; Fujimoto, T.; Yasunishi, A.; Linko, Y.-Y.; Linko, P. A Short-Cut Method for Estimating l-Menthol Retention Included in Cyclodextrin During Drying a Single Drop. In Proceedings of the Eighth International Symposium on Cyclodextrins; Springer: Berlin/Heidelberg, Germany, 1996; pp. 583–586. [Google Scholar]
- Vilanova, N.; Solans, C. Vitamin A Palmitate-β-cyclodextrin inclusion complexes: Characterization, protection and emulsification properties. Food Chem. 2015, 175, 529–535. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef]
- Fernandes, A.; Rocha, M.A.A.; Santos, L.M.N.B.F.; Brás, J.; Oliveira, J.; Mateus, N.; de Freitas, V. Blackberry anthocyanins: β-Cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. 2018, 245, 426–431. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, X.; Dou, W.; Chou, G.; Wang, Z. Preparation and characterization of inclusion complexes formed between baicalein and cyclodextrins. Carbohydr. Polym. 2013, 95, 733–739. [Google Scholar] [CrossRef]
- Hǎdǎruga, D.I.; Ünlüsayin, M.; Gruia, A.T.; Birǎu, C.; Rusu, G.; Hǎdǎruga, N.G. Thermal and oxidative stability of Atlantic salmon oil (Salmo salar L.) and complexation with β-cyclodextrin. Beilstein J. Org. Chem. 2016, 12, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, X.; Yang, Q.; Zhang, N.; Du, Y.; Zhu, H. Preparation and characterization of inclusion complex of benzyl isothiocyanate extracted from papaya seed with β-cyclodextrin. Food Chem. 2015, 184, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hădărugă, D.; Hădărugă, N.; Costescu, C.; David, I.; Gruia, A. Thermal and oxidative stability of the Ocimum basilicum L. essential oil/β-cyclodextrin supramolecular system. Beilstein J. Org. Chem. 2014, 10, 2809–2820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalogeropoulos, N.; Yannakopoulou, K.; Gioxari, A.; Chiou, A.; Makris, D.P. Polyphenol characterization and encapsulation in β-cyclodextrin of a flavonoid-rich Hypericum perforatum (St John’s wort) extract. LWT—Food Sci. Technol. 2010, 43, 882–889. [Google Scholar] [CrossRef]
- Hadaruga, D.I.; Hadaruga, N.G.; Rivis, A.; Gruia, A.; Pinzaru, I.A. Thermal and oxidative stability of the Allium sativum L. bioactive compounds/α- and β-cyclodextrin nanoparticles. Rev. Chim. 2007, 58, 1009–1015. [Google Scholar]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and release characterisation of garlic oil-β- cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT—Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Cetin Babaoglu, H.; Bayrak, A.; Ozdemir, N.; Ozgun, N. Encapsulation of clove essential oil in hydroxypropyl beta-cyclodextrin for characterization, controlled release, and antioxidant activity. J. Food Process. Preserv. 2017, 41, e13202. [Google Scholar] [CrossRef]
- Kayaci, F.; Sen, H.S.; Durgun, E.; Uyar, T. Functional electrospun polymeric nanofibers incorporating geraniol-cyclodextrin inclusion complexes: High thermal stability and enhanced durability of geraniol. Food Res. Int. 2014, 62, 424–431. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Cid, A.; Torrado-Agrasar, A.; Mejuto, J.C.; Simal-Gandara, J. Encapsulation of Essential Oils by Cyclodextrins: Characterization and Evaluation. In Cyclodextrin—A Versatile Ingredient; IntechOpen: London, UK, 2018. [Google Scholar]
- Sharma, N.; Baldi, A. Exploring versatile applications of cyclodextrins: An overview. Drug Deliv. 2016, 23, 739–757. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, Z.H.; Sun, E.; Jia, X. Bin Effect of β-cyclodextrin complexation on solubility and enzymatic conversion of naringin. Int. J. Mol. Sci. 2012, 13, 14251–14261. [Google Scholar] [CrossRef] [Green Version]
- Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Simal-Gándara, J.; Torrado-Agrasar, A. Antioxidant and antimicrobial properties of encapsulated guava leaf oil in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2018, 111, 219–225. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Torrado-Agrasar, A.; Simal-Gándara, J. Physico-chemical characterization and evaluation of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Food Hydrocoll. 2017, 65, 157–164. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Torrado-Agrasar, A.; Simal-Gándara, J.; Mejuto, J.C. Encapsulation of yarrow essential oil in hydroxypropyl-beta-cyclodextrin: Physiochemical characterization and evaluation of bio-efficacies. CyTA—J. Food 2017, 15, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Alpha-Cyclodextrin as a Novel Food. Available online: https://www.foodstandards.gov.au/code/applications/documents/A494_Alpha_cylclodextrin_FAR_FINAL.pdf (accessed on 26 January 2021).
- Braithwaite, M.C.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Tomar, L.K.; Tyagi, C.; Pillay, V. A novel multi-tiered experimental approach unfolding the mechanisms behind cyclodextrin-vitamin inclusion complexes for enhanced vitamin solubility and stability. Int. J. Pharm. 2017, 532, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Kuttiyawong, K.; Saehu, S.; Ito, K.; Pongsawasdi, P. Synthesis of large-ring cyclodextrin from tapioca starch by amylomaltase and complex formation with Vitamin E acetate for solubility enhancement. Process Biochem. 2015, 50, 2168–2176. [Google Scholar] [CrossRef]
- Xu, X.; Peng, S.; Bao, G.; Zhang, H.; Yin, C. β-cyclodextrin inclusion complexes with vitamin A and its esters: A comparative experimental and molecular modeling study. J. Mol. Struct. 2021, 1223, 129001. [Google Scholar] [CrossRef]
- Decock, G.; Fourmentin, S.; Surpateanu, G.G.; Landy, D.; Decock, P.; Surpateanu, G. Experimental and theoretical study on the inclusion compounds of aroma components with β-cyclodextrins. Supramol. Chem. 2006, 18, 477–482. [Google Scholar] [CrossRef]
- Fenyvesi, E.; Vikmon, M.; Szente, L. Cyclodextrins in Food Technology and Human Nutrition: Benefits and Limitations. Crit. Rev. Food Sci. Nutr. 2016, 56, 1981–2004. [Google Scholar] [CrossRef]
- Verrone, R.; Catucci, L.; Cosma, P.; Fini, P.; Agostiano, A.; Lippolis, V.; Pascale, M. Effect of β-cyclodextrin on spectroscopic properties of ochratoxin a in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 475–479. [Google Scholar] [CrossRef]
- Essa, H.A.; Ayesh, A.M. Mycotoxins Reduction and Inhibition of Enzymatic Browning during Apple Juice Processing. Available online: https://www.kau.edu.sa/Files/857/Researches/58183_28330.pdf (accessed on 26 January 2021).
- Dos Santos, C.; Buera, M.P.; Mazzobre, M.F. Phase solubility studies and stability of cholesterol/β-cyclodextrin inclusion complexes. J. Sci. Food Agric. 2011, 91, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Awad, A.C.; Bennink, M.R.; Gill, J.L. Cholesterol Reduction in Liquid Egg Yolk using β-Cyclodextrin. J. Food Sci. 1995, 60, 691–694. [Google Scholar] [CrossRef]
- Plank, D.W.; Novak, D.J. Method for Reducing Acrylamide Levels in Food Products and Food Products Produced Thereby. U.S. Patent No. 7,264,838, 4 September 2007. [Google Scholar]
- Murray, B.S. Pickering emulsions for food and drinks. Curr. Opin. Food Sci. 2019, 27, 57–63. [Google Scholar] [CrossRef]
- Diaz-Salmeron, R.; Chaab, I.; Carn, F.; Djabourov, M.; Bouchemal, K. Pickering emulsions with α-cyclodextrin inclusions: Structure and thermal stability. J. Colloid Interface Sci. 2016, 482, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, S.; Kida, T.; Akashi, M.; Sato, H.; Shizuma, M.; Ono, D. Preparation of Pickering emulsions through interfacial adsorption by soft cyclodextrin nanogels. Beilstein J. Org. Chem. 2015, 11, 2355–2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, Y.; Luo, Z.; Lu, X.; Peng, X. Modulation of Cyclodextrin Particle Amphiphilic Properties to Stabilize Pickering Emulsion. J. Agric. Food Chem. 2018, 66, 228–237. [Google Scholar] [CrossRef]
- Singh, N.; Sahu, O. Sustainable cyclodextrin in textile applications. In The Impact and Prospects of Green Chemistry for Textile Technology; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 83–105. ISBN 9780081024911. [Google Scholar]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Sing, M.; Sharma, R.; Banerjee, U.C. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef]
- Cid, A.; Astray, G.; Morales, J.; Mejuto, J.C.; Simal-Gándara, J. Influence of b-Cyclodextrins upon the Degradation of Carbofuran Derivatives. J. Pestic. Biofertil. 2018, 1, 1–4. [Google Scholar] [CrossRef]
- Shu, H.J.; Zeng, C.M.; Wang, C.; Covey, D.F.; Zorumski, C.F.; Mennerick, S. Cyclodextrins sequester neuroactive steroids and differentiate mechanisms that rate limit steroid actions. Br. J. Pharmacol. 2007, 150, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, L.; Nardello-Rataj, V. Pickering emulsions based on cyclodextrins: A smart solution for antifungal azole derivatives topical delivery. Eur. J. Pharm. Sci. 2016, 82, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Vikas, Y.; Sandeep, K.; Braham, D.; Manjusha, C.; Budhwar, V. Cyclodextrin Complexes: An Approach to Improve the Physicochemical Properties of Drugs and Applications of Cyclodextrin Complexes. Asian J. Pharm. 2018, 12, 394. [Google Scholar]
- Palem, C.R.; Siva Chaitanya Chopparapu, K.; Subrahmanyam, P.V.R.S.; Yamsani, M.R. Cyclodextrins and their derivatives in drug delivery: A review. Curr. Trends Biotechnol. Pharm. 2012, 6, 255–279. [Google Scholar]
- Lakkakula, J.R.; Maçedo Krause, R.W. A vision for cyclodextrin nanoparticles in drug delivery systems and pharmaceutical applications. Nanomedicine 2014, 9, 877–894. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Rai, A. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied Sci. 2010, 2, 72. [Google Scholar] [CrossRef]
- Centini, M.; Maggiore, M.; Casolaro, M.; Andreassi, M.; Maffei Facino, R.; Anselmi, C. Cyclodextrins as cosmetic delivery systems. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 109–112. [Google Scholar] [CrossRef]
- Voncina, B. Application of Cyclodextrins in Textile Dyeing. In Textile Dyeing; IntechOpen: London, UK, 2011. [Google Scholar]
- Grechin, A.G.; Buschmann, H.J.; Schollmeyer, E. Quantification of Cyclodextrins Fixed onto Cellulose Fibers. Text. Res. J. 2007, 77, 161–164. [Google Scholar] [CrossRef]
- Yong, G.P.; Zhang, B.; Zhang, Y.M.; Li, G.S. Excitation-light-responsive phosphorescent color changes in a β-cyclodextrin inclusion complex. J. Mater. Chem. 2012, 22, 13481–13483. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Abdalla, W.A.; El-Zairy, E.M.R.; Khalil, H.M. Utilization of monochloro-triazine β-cyclodextrin for enhancing printability and functionality of wool. Carbohydr. Polym. 2013, 92, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Ursache, M.; Loghin, C.; Mureşan, R.; Cerempei, A.; Mureşan, A. Investigation on the effects of antibacterial finishes on dyed cotton knitted fabrics. Tekst. Konfeksiyon 2011, 21, 249–256. [Google Scholar]
- Wang, J.H.; Cai, Z. Incorporation of the antibacterial agent, miconazole nitrate into a cellulosic fabric grafted with β-cyclodextrin. Carbohydr. Polym. 2008, 72, 695–700. [Google Scholar] [CrossRef]
- Popescu, O.; Dunca, S.; Grigoriu, A. Antibacterial action of silver applied on cellulose fibers grafted with monochlorotriazinyl-β-cyclodextrin. Cellul. Chem. Technol. 2013, 47, 247–255. [Google Scholar]
- Abdel-Halim, E.S.; Al-Deyab, S.S.; Alfaifi, A.Y.A. Cotton fabric finished with β-cyclodextrin: Inclusion ability toward antimicrobial agent. Carbohydr. Polym. 2014, 102, 550–556. [Google Scholar] [CrossRef]
- Bajpai, M.; Gupta, P.; Bajpai, S.K. Silver(I) ions loaded cyclodextrin-grafted-cotton fabric with excellent antimicrobial property. Fibers Polym. 2010, 11, 8–13. [Google Scholar] [CrossRef]
- Racu, C.; Cogeanu, A.M.; Diaconescu, R.M.; Grigoriu, A. Antimicrobial treatments of hemp fibers grafted with β-cyclodextrin derivatives. Text. Res. J. 2012, 82, 1317–1328. [Google Scholar] [CrossRef]
- Uekama, K. Recent aspects of pharmaceutical application of cyclodextrins. J. Incl. Phenom. 2002, 44, 3–7. [Google Scholar] [CrossRef]
- Masajtis, J.; Dutkiewicz, J.; Członka, R.; Broniarczyk-Dyła, G.; Arkuszewska, C.; Skwarczyńska-Banyś, E.; Omulecki, A.; Kot, P. Correlations between the properties and effects of clinical therapy using textile material with antipsoriatic action. Polim. Med. 1992, 22, 26–41. [Google Scholar]
- Nichifor, M.; Constantin, M.; Mocanu, G.; Fundueanu, G.; Branisteanu, D.; Costuleanu, M.; Radu, C.D. New multifunctional textile biomaterials for the treatment of leg venous insufficiency. J. Mater. Sci. Mater. Med. 2009, 20, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Z.; Williams, G.R.; Hou, X.X.; Zhu, L.M. Electrospun curcumin-loaded fibers with potential biomedical applications. Carbohydr. Polym. 2013, 94, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohdy, F.A.; Fouda, M.M.G.; Rehan, M.F.; Aly, A.S. Repellency of controlled-release treated cotton fabrics based on cypermethrin and prallethrin. Carbohydr. Polym. 2008, 73, 92–97. [Google Scholar] [CrossRef]
- Romi, R.; Lo Nostro, P.; Bocci, E.; Ridi, F.; Baglioni, P. Bioengineering of a cellulosic fabric for insecticide delivery via grafted cyclodextrin. Biotechnol. Prog. 2005, 21, 1724–1730. [Google Scholar] [CrossRef]
- Hebeish, A.; Fouda, M.M.G.; Hamdy, I.A.; EL-Sawy, S.M.; Abdel-Mohdy, F.A. Preparation of durable insect repellent cotton fabric: Limonene as insecticide. Carbohydr. Polym. 2008, 74, 268–273. [Google Scholar] [CrossRef]
- Bar, R. Cyclodextrin-aided bioconversions and fermentations. Trends Biotechnol. 1989, 7, 2–4. [Google Scholar] [CrossRef]
- Calcagnile, M.; Bettini, S.; Damiano, F.; Talà, A.; Tredici, S.M.; Pagano, R.; Di Salvo, M.; Siculella, L.; Fico, D.; De Benedetto, G.E.; et al. Stimulatory Effects of Methyl-β-cyclodextrin on Spiramycin Production and Physical-Chemical Characterization of Nonhost@Guest Complexes. ACS Omega 2018, 3, 2470–2478. [Google Scholar] [CrossRef] [Green Version]
- Jarho, P.; Vander Velde, D.; Stella, V.J. Cyclodextrin-catalyzed deacetylation of spironolactone is pH and cyclodextrin dependent. J. Pharm. Sci. 2000, 89, 241–249. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, W.; Shen, H.; Liu, Q.; Yu, W.; Ji, H.; She, Y. Progress in the immobilization of β-cyclodextrin and their application in adsorption of environmental pollutants. Chin. J. Org. Chem. 2016, 36, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Taka, A.L.; Pillay, K.; Yangkou Mbianda, X. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef]
- Breslow, R.; Dong, S.D. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 1998, 98, 1997–2011. [Google Scholar] [CrossRef] [PubMed]
- Hapiot, F.; Menuel, S.; Ferreira, M.; Léger, B.; Bricout, H.; Tilloy, S.; Monflier, E. Catalysis in Cyclodextrin-Based Unconventional Reaction Media: Recent Developments and Future Opportunities. ACS Sustain. Chem. Eng. 2017, 5, 3598–3606. [Google Scholar] [CrossRef]
- Bai, C.C.; Tian, B.R.; Zhao, T.; Huang, Q.; Wang, Z.Z. Cyclodextrin-catalyzed organic synthesis: Reactions, mechanisms, and applications. Molecules 2017, 22, 1475. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, L.; Douyère, G.; Nardello-Rataj, V. Supramolecular chemistry and self-organization: A veritable playground for catalysis. Catalysts 2019, 9, 163. [Google Scholar] [CrossRef] [Green Version]
- Zare Asadabadi, A.; Hoseini, S.J.; Bahrami, M.; Nabavizadeh, S.M. Catalytic applications of β-cyclodextrin/palladium nanoparticle thin film obtained from oil/water interface in the reduction of toxic nitrophenol compounds and the degradation of azo dyes. New J. Chem. 2019, 43, 6513–6522. [Google Scholar] [CrossRef]
- Crini, G.; Morcellet, M. Synthesis and applications of adsorbents containing cyclodextrins. J. Sep. Sci. 2002, 25, 789–813. [Google Scholar] [CrossRef]
- Zhu, Q.; Scriba, G.K.E. Advances in the Use of Cyclodextrins as Chiral Selectors in Capillary Electrokinetic Chromatography: Fundamentals and Applications. Chromatographia 2016, 79, 1403–1435. [Google Scholar] [CrossRef]
- Martin, J.; Díaz-Montaña, E.J.; Asuero, A.G. Cyclodextrins: Past and Present. In Cyclodextrin—A Versatile Ingredient; Arora, P., Dhingra, N., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Harangi, J.; Béke, G.; Harangi, M.; Mótyán, J.A. The digestable parent cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 335–339. [Google Scholar] [CrossRef]
- Munro, I.C.; Newberne, P.M.; Young, V.R.; Bär, A. Safety assessment of γ-cyclodextrin. Regul. Toxicol. Pharmacol. 2004, 39, 3–13. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [Green Version]
- Lina, B.A.R.; Bär, A. Subchronic oral toxicity studies with α-cyclodextrin in rats. Regul. Toxicol. Pharmacol. 2004, 39, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.; et al. Re-evaluation of β-cyclodextrin (E 459) as a food additive. EFSA J. 2016, 14, e04628. [Google Scholar] [CrossRef]
- European Medicines Agency. Cyclodextrins Used as Excipients; European Medicines Agency: Amsterdam, The Netherlands, 2017; Volume 44. [Google Scholar]


| Properties | Unit | α-CDs | β-CDs | γ-CDs |
|---|---|---|---|---|
| Formula | C36H60O30 | C42H70O35 | C48H80O40 | |
| Structure |  |  |  | |
| Mol wt | 972.84 | 1134.98 | 1297.12 | |
| Glucopyranose units | 6 | 7 | 8 | |
| Solubility (water, 25 °C) | % w/v | 14.5 | 1.85 | 23.2 |
| Outer diameter | Å | 14.6 | 15.4 | 17.5 |
| Cavity diameter | Å | 4.7–5.3 | 6.0–6.5 | 7.5–8.3 |
| Height of torus | Å | 7.9 | 7.9 | 7.9 |
| Cavity volume | Å3 | 174 | 262 | 427 |
| Crystal form | Hexagonal plates | Monoclinic parallelograms | Quadratic prisms | |
| Others | Most accessible, lowest-priced and generally the most useful |
| CDs | Extract | Compounds | Characteristic | Effect | Ref. |
|---|---|---|---|---|---|
| A. Food Flavor Improvement | |||||
| α | Shiitake | Cyclic sulphur compounds | Shiitake mushroom | Flavor retention | [34] |
| α | European pear | Five ester types | Pear | Heat protection at 120 °C for 60 min. | [35] |
| β | Food | - | - | Encapsulation the best protection against heat and evaporation | [36,37] |
| β | Food | Several volatile compounds | - | Protection during high temperature short time extrusion cooking process | [38] |
| β | Polysaccharide solutions | ketones, hexanal, t–2-hexenal, ethyl butanoate and 1-hexanol | - | Retain some aroma compounds during thermal processes (cooking, pasteurization) | [39] |
| β | Corn starch | Eugenol | Clove | 79% odor retention during extrusion | [40] |
| β | Goat milk and its yogurt | 4-methyloctanoic acid | Goat flavor | Reduce goat flavor | [41] |
| β | Thermally processed foods | l-menthol | Menthol | Improved flavor retention | [42] |
| α, β, γ | Aqueous ethanol | l-menthol, ethyl butyrate, ethyl hexanoate, benzaldehyde, citral, and methyl anthranilate | - | Temperature dependent | [28] |
| B. Food taste improvement | |||||
| α | Soy protein | Phenylalanine, tryptophane, tyrosine, isoleucine, proline and histidine | Taste modification | Reduce bitter taste | [43] |
| β | Milk casein hydrolysate | - | Bitter | Bitter taste eliminated by adding 10% β-CDs to the protein hydrolysate | [29] |
| β | β-polymers | Limonin, naringin | Bitter | Debittering agents | [44] |
| β | Canned citrus and citrus juice | Naringin, limonin, hesperidin | Bitter, precipitation | Reduce bitter taste of naringin, limonin, and hesperidin and prevent precipitation | [22] |
| β | Fish oil | - | Taste, oxidation | Eliminate unpleasant taste, smell, and stabilization against oxidation | [45] |
| γ | Ginseng | - | Bitter | Debittering agent | [46] |
| α, β | Navel orange and grapefruit juices | Limonin, naringin | Bitter | Improve flavor | [47] |
| β, γ | Caffeine and bitter natural extracts (artichoke leaves, aloe, and gentian) | β- and γ-CDs linked to chitosan through succinyl or maleyl bridges | Bitter | Bitter-masking properties | [48] |
| CDs | Subtract | Properties | Study | Effect | Info | Ref. |
|---|---|---|---|---|---|---|
| β | 2-nonanone | Aromatic, antifungal | TGA, DSC, against B. cinereal | Improve antifungal, thermal stability | complex 1:0.5 (80% growth inhibition). | [55] |
| β | cyanidin-3-O-glucoside | Several | DSC | Improve bioavailability, thermal protection | - | [56] |
| β | S. baicalensis BA | Anti-inflammatory, antioxidant, antitumor | Increase solubility, stability | 13672.67 L/mol | [57] | |
| β | S. salar EO | DSC, KFT | Thermal and oxidative stability | Complex 1:1 and 3:1 | [58] | |
| β | Benzyl isothiocyanate (papaya) | Antimicrobial | DSC, TGA | Improve stability, controlled release | 600.8 L/mol | [59] |
| β | O. basilicum EO | Aromatic, medicinal | GC-MS | Improve stability against air/oxygen and temperature | - | [60] |
| β | Methanolic extract of H. perforatum | Antioxidant | DSC | Intact at temperatures at which the free extract was oxidized | Food supplement or a novel additive to enhance the antioxidant capacity of fresh or thermally processed food | [61] |
| β | Garlic | Antimicrobial, antioxidant | TGA, DSC, SEM | Thermal and oxidative stability | Nanoencapsulation yields >60% | [62] |
| β | Garlic oil | Antimicrobial, antioxidant | DSC | Improve protection against oxidation | Complex 1:1 | [63] |
| β | Oils | Antimicrobial | DSC, against S. enterica and L. innocua | Thermal protection | Masking the sensory effect of the attributes of antimicrobial agents and potentiate their activity. | [64] |
| HP | Clove EO | Antioxidant | DPPH | Prevent degradation and loss of active compounds. prolong shelf life | Complex 1:1 | [65] |
| γ | Geraniol | Aromatic, to treat infectious diseases, preserve food | SEM analysis | High thermal stability and enhanced durability of active agents and functional food ingredients | - | [66] |
| CDs | Drug | Trade Name | Admin. Route | Use | Market |
|---|---|---|---|---|---|
| α | Alprostadil | Prostavastin, Caverject, Edex | Intravenous | Erectile dysfunction; certain heart, lung, and blood vessel problems in infants; temporarily keep the arteriosus duct open before having a surgery | EU, Japan, USA |
| Cefotiam hexetil HCl | Pansporin T | Oral | Infections | Japan | |
| Limaprost | Opalmon, Prorenal | Oral | Vasodilator | ||
| β | Benexate | Ulgut, Lonmiel | Oral | Treatment of peptic ulcer | Japan |
| Albendazole | Zentel, Colidetol | Oral | Anti-microbial | EU | |
| Gliclazide | Diamicron | Oral | Anti-diabetic | EU | |
| Danazol | Danatrol | Oral | Endometriosis | EU | |
| Dexamethasone | Glymesason | Dermal | Anti-inflammatory, treat eczema/dermatitis | Japan | |
| Ibuproxam | Calmatel, Deflogon | Oral, topical | Anti-inflammatory | EU | |
| Iodine | Mena-Gargle | Topical | Infections | Japan | |
| Fenoprofen | Nalfon, Mylan, Naprofen | Oral | Anti-inflammatory | EU | |
| Chlordiazepoxide | Transilium | Oral | Reduces anxiety | Argentina | |
| Isradipine | Almodipino | Oral | Enhance solubility and photostability | - | |
| Cephalosporin | Meiact | Oral | Antibiotic | Japan | |
| Nicotine | Nicorette | Sublingual | Aid to smoking cessation | EU | |
| Nimesulide | Nimedex, Mesulid | Oral | Analgesic, antipyretic, and anti-inflammatory | EU | |
| Diphenhydramin | Stada-Travel | Oral | Neurological treatments | EU | |
| Glimepiride | Amaryl, glimepiride ALTER, glimepiride, Roname, Tandemacte | Oral | Increase dissolution rate, time of action and efficacy | - | |
| Sulindac | Clinoril | Oral | Anti-inflammatory | EU | |
| Nitroglycerin | Nitropen | Sublingual | Treat / prevent chest pain or pressure | Japan | |
| Omeprazole | Omebeta | Oral | Intestinal / esophagus ulcers, reflux disease, heartburn, syndromes of stomach acid | EU | |
| Dinoprostone | Prostarmon E | Sublingual | Oxytocic | Japan | |
| Piroxicam | Brexin | Oral | Analgesic, antipyretic, anti-inflammatory | EU | |
| Tiaprofenic acid | Surgamyl | Oral | Analgesic, antipyretic, anti-inflammatory | EU | |
| 2-HP-β | Cisapride | Propulsid | Rectal | Gastro-esophageal reflux | EU |
| Voriconazole | Voriconazole Teva, Vfenf | Oral. injection | Enhance solubility, dissolution rate, and chemical stability | EU | |
| Hydrocortisone | Dexocort | Buccal | Relieve the soreness of mouth ulcers and speed up healing | EU | |
| Rhein | Rhein | Oral | Improvement in photostability | - | |
| Indomethacin | Indocid | Eye drops | Anti-inflammatory | EU | |
| Itraconazole | Sporanox | Oral, intravenous | Fungal infections | EU, USA | |
| Mitomycin | Mitozytrex | Intravenous | Cancer | USA | |
| ME-β | 17β-Oestradiol | Aerodiol | Nasal spray | Menopausal climacteric symptoms | EU |
| Chloramphenicol | Clorocil | Eye drops | Ear infections | EU | |
| SP-β | Voriconazole | Vfend | Intravenous | Fungal infections | EU, USA |
| Ziprasidone maleate | Geodon, Zeldox | Intramuscular | Acute agitation in adults with schizophrenia | EU, USA | |
| 2-HP-γ | Diclofenac sodium | Voltaren | Eye drops | Eye surgery, hay fever | EU |
| Improved Function | Mechanism | Type | Drugs |
|---|---|---|---|
| Increase in bioavailability | Increased solubility and stability | β, γ, natural | Thalidomide, nimuselide, prednisolone, oteprednol etabonate, tacrolimus, sulfhamethazole |
| Increased availability | Increase in solid stability | β | Quinapril |
| Increased solubility | Forming inclusion complexes with their nonpolar molecules or functional groups | β | Bromazepan, ibuprofen, naproxen, ofloxacin, ketoralac, nimesulide, omeprazole, tenoxicam |
| Increased stability | Obstruction of the reactants to diffuse into the cavity and react with the protected guest | β | Metoprolol, nifedipine, quinapril |
| Increased absorption | Oral delivery | Β, HP-β | Ketoconazole, testosterone |
| Rectal delivery | 2HP-β | Flurbiprofen, carmafur, biphenyl acetic acid | |
| Nasal delivery | 2HP-β | Morphine, antiviral drug, insulin | |
| Trans-dermal delivery | 6-O-(carboxymethyl)-O-ethylβ | Prostaglandin | |
| Ocular delivery | 2HP-β, β | Dexamethasone, carbonicanhydrase inhibitors | |
| Delivery | Protein and peptide delivery | Modified CDs | Growth hormone, interleukin-2, aspartame, albumin and MABs |
| Reduction of local irritancy and toxicity | Forming inclusion complexes with toxicity or irritant compounds | 2-HP-β (2,6-diOmethyl), β | Pilocarpine, phenothiazine euroleptics, all-transretenoic acid |
| Prevention of incompatibility | Prevent drug-drug or drug-additive interaction. | β, γ | Piroxicam, omeprazole |
| Activity | CDs | Compounds | Fabric | Study | Ref. |
|---|---|---|---|---|---|
| Antibacterial | MCT-β | Ag NPs, triclosan | Wool | Activity more than 75%, even after 15 washing cycles. | [106] |
| MCT-β | Aqueous/alcoholic extracts from plants | Cotton | Other effects associated | [107] | |
| MCT-β | Miconazole nitrate | Cotton | C. albicans, Aurococcus and Bacillus | [108] | |
| MCT-β | Ag NPs | Cotton | Staphylococcus aureus, E. coli | [109] | |
| TCA-β | Octenidine dihydrochloride | Cotton | Reasonable activity after 20 washing cycles | [110] | |
| CTR | Silver (I) | Cotton | E. coli | [111] | |
| MCT-β | Ferulic acid, caffeic acid, ethyl ferulate allantoin | Hemp | Sanogenetic properties of the hemp fibers are significantly modified by the chemical treatments | [112] | |
| Antiallergic, anti-psoriasis | 2,6-di-O-methyl | Tacrolimus | Cotton | Drug delivery | [113] |
| Anti-psoriasis | - | Dithranol | Cotton | Clinical test | [114] |
| Chronic venous insufficiency | β | Troxerutin | Pa-66/PU in stockings | In vivo tests on Wistar rats, clinical studies | [115] |
| Anti-inflammatory, antioxidant, antitumor | β | Curcumin | Nanofibre | Two sequential stages for drug release | [116] |
| Against mosquitos | β | Cypermethrin, prallethrin, permethrin, N,N-diethyl-m-toluamide | Cotton | Treated fabrics retain high number of insecticides | [117,118] |
| MCT-β | Limonene | Cotton | Effect of washing and storing | [119] |
| CDs | Food | Pharmacopoeia Monographs | ||||
|---|---|---|---|---|---|---|
| US | Europe | Japan | US | Europe | Japan | |
| α | GRAS | Novel food | Natural product | Yes | Yes | Yes |
| β | GRAS | Food additive | Natural product | Yes | Yes | Yes |
| γ | GRAS | Pending | Natural product | In progress | In progress | Yes |
| HP-β | - | - | - | Yes | Yes | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Pereira, A.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. https://doi.org/10.3390/ijms22031339
Gonzalez Pereira A, Carpena M, García Oliveira P, Mejuto JC, Prieto MA, Simal Gandara J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. International Journal of Molecular Sciences. 2021; 22(3):1339. https://doi.org/10.3390/ijms22031339
Chicago/Turabian StyleGonzalez Pereira, Antía, Maria Carpena, Paula García Oliveira, Juan Carlos Mejuto, Miguel Angel Prieto, and Jesus Simal Gandara. 2021. "Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes" International Journal of Molecular Sciences 22, no. 3: 1339. https://doi.org/10.3390/ijms22031339