Biomaterials to Prevent Post-Operative Adhesion
Abstract
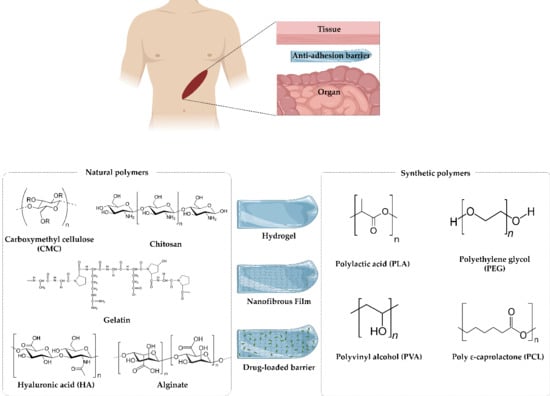
:1. Introduction
2. Polymers as Materials for Anti-Adhesion Barriers
2.1. Natural Polymers
2.1.1. Carboxymethyl Cellulose (CMC)
2.1.2. Hyaluronic Acid (HA)
2.1.3. Chitosan
2.1.4. Gelatin
2.1.5. Alginate
2.2. Synthetic Polymers
2.2.1. Polylactic Acid (PLA)
2.2.2. Polyvinyl Alcohol (PVA)
2.2.3. Poly ε-Caprolactone (PCL)
2.2.4. Polyethylene Glycol (PEG)
3. Various Strategies of Anti-Adhesion
3.1. Physical Barriers
3.1.1. Hydrogels
3.1.2. Films
3.2. Chemical Barriers
3.2.1. Anti-Inflammatory Agents
3.2.2. Anticoagulants
3.2.3. Fibrinolytic Agents
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| Ala | Alanine |
| CMC | Carboxymethyl cellulose |
| Gly | Glycine |
| GTA | Glutaraldehyde |
| IL | Interleukin |
| HA | Hyaluronic acid |
| Hyp | Hydroxyprolyl |
| NO | Nitric oxide |
| NSAID | Nonsteroidal anti-inflammatory drug |
| PCL | Poly ε-caprolactone |
| PEG | Polyethylene glycol |
| PEO | Polyethylene oxide |
| PGA | Polyglycolic acid |
| PGs | Prostaglandin |
| PLA | Polylactic acid |
| PVA | Polyvinyl alcohol |
| POE | Polyoxymethylene |
| PPG | polypropylene glycol |
| Ser | Sericine |
| TNF | Tumor necrosis factor |
| tPA | Tissue plasminogen activator |
References
- Cheong, Y.; Laird, S.; Li, T.C.; Shelton, J.B.; Ledger, W.L.; Cooke, I.D. Peritoneal healing and adhesion formation/reformation. Hum. Reprod. Update 2001, 7, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P. Reduction of postoperative adhesion development. Fertil Steril. 2016, 106, 994–997. [Google Scholar] [CrossRef] [Green Version]
- Attard, J.-A.P.; MacLean, A.R. Adhesive small bowel obstruction: Epidemiology, biology and prevention. Can. J. Surg. 2007, 50, 291–300. [Google Scholar] [PubMed]
- Dizerega, G.S. Biochemical events in peritoneal tissue repair. Eur. J. Surg. Suppl. 1997, 577, 10. [Google Scholar]
- Chegini, N.; Rong, H.; Bennett, B.; Stone, I.K. Peritoneal fluid cytokine and eicosanoid levels and their relation to the incidence of peritoneal adhesion. J. Soc. Gynecol. Investig. 1999, 6, 153–157. [Google Scholar] [CrossRef]
- Zeyneloglu, H.B.; Senturk, L.M.; Seli, E.; Oral, E.; Olive, D.L.; Arici, A. The role of monocyte chemotactic protein-1 in intraperitoneal adhesion formation. Hum. Reprod. 1998, 13, 1194–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.-L.; Singh, S.; Chen, H.-W.; Chen, H.-Y.; Chen, J.J.W.; Chen, W.-J.; Chen, H.-S.; Chen, S.-C. Intra-abdominal adhesion formation induces anti-oxidative injury, enhances cell proliferation, and prevents complement-mediated lysis. Wound Repair Regen. 2008, 16, 388–398. [Google Scholar] [CrossRef]
- Ellis, H. The causes and prevention of intestinal adhesions. BJS 1982, 69, 241–243. [Google Scholar] [CrossRef]
- Menzies, D.; Ellis, H. Intestinal obstruction from adhesions—How big is the problem? Ann. R. Coll. Surg. Engl. 1990, 72, 60–63. [Google Scholar]
- Robertson, D.; Lefebvre, G.; Leyland, N.; Wolfman, W.; Allaire, C.; Awadalla, A.; Best, C.; Contestabile, E.; Dunn, S.; Heywood, M.; et al. Adhesion prevention in gynaecological surgery. Int. J. Gynecol. Obstet. 2010, 111, 193–197. [Google Scholar] [CrossRef]
- Duffy, D.M.; Dizerega, G.S. Adhesion controversies: Pelvic pain as a cause of adhesions, crystalloids in preventing them. J. Reprod. Med. 1996, 41, 19–26. [Google Scholar] [PubMed]
- Howard, F.M. The Role of Laparoscopy in Chronic Pelvic Pain: Promise and Pitfalls. Obstet. Gynecol. Surv. 1993, 48, 357–387. [Google Scholar] [CrossRef]
- Thompson, J.S.; DiBaise, J.K.; Iyer, K.R.; Yeats, M.; Sudan, D.L. Post-operative short bowel syndrome. J. Am. Coll. Surg. 2005, 201, 85–89. [Google Scholar] [CrossRef]
- Van Goor, H. Consequences and complications of peritoneal adhesions. Color. Dis. 2007, 9, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ouaissi, M.; Gaujoux, S.; Veyrie, N.; Denève, E.; Brigand, C.; Castel, B.; Duron, J.; Rault, A.; Slim, K.; Nocca, D. Post-operative adhesions after digestive surgery: Their incidence and prevention: Review of the literature. J. Visc. Surg. 2012, 149, e104–e114. [Google Scholar] [CrossRef] [PubMed]
- Broek, R.P.T.; Schreinemacher, M.H.F.; Jilesen, A.P.J.; Bouvy, N.; Bleichrodt, R.P.; Van Goor, H. Enterotomy Risk in Abdominal Wall Repair. Ann. Surg. 2012, 256, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Broek, R.P.T.; Strik, C.; Issa, Y.; Bleichrodt, R.P.; Van Goor, H. Adhesiolysis-Related Morbidity in Abdominal Surgery. Ann. Surg. 2013, 258, 98–106. [Google Scholar] [CrossRef]
- Mais, V. Peritoneal adhesions after laparoscopic gastrointestinal surgery. World J. Gastroenterol. 2014, 20, 4917–4925. [Google Scholar] [CrossRef] [Green Version]
- Rocca, A.; Aprea, G.; Surfaro, G.; Amato, M.; Giuliani, A.; Paccone, M.; Salzano, A.; Russo, A.; Tafuri, D.; Amato, B. Prevention and treatment of peritoneal adhesions in patients affected by vascular diseases following surgery: A review of the literature. Open Med. 2016, 11, 106–114. [Google Scholar] [CrossRef]
- Beck, D.E.; Ferguson, M.A.; Opelka, F.G.; Fleshman, J.W.; Gervaz, P.; Wexner, S.D. Effect of previous surgery on abdominal opening time. Dis. Colon Rectum 2000, 43, 1749–1753. [Google Scholar] [CrossRef]
- Coleman, M.G.; McLain, A.D.; Moran, B.J. Impact of previous surgery on time taken for incision and division of adhesions during laparotomy. Dis. Colon Rectum 2000, 43, 1297–1299. [Google Scholar] [CrossRef]
- Kusuki, I.; Suganuma, I.; Ito, F.; Akiyama, M.; Sasaki, A.; Yamanaka, K.; Tatsumi, H.; Kitawaki, J. Usefulness of Moistening Seprafilm Before Use in Laparoscopic Surgery. Surg. Laparosc. Endosc. Percutan. Tech. 2014, 24, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Liakakos, T.; Thomakos, N.; Fine, P.M.; Dervenis, C.; Young, R.L. Peritoneal Adhesions: Etiology, Pathophysiology, and Clinical Significance. Dig. Surg. 2001, 18, 260–273. [Google Scholar] [CrossRef]
- Choi, G.J.; Park, H.K.; Kim, D.S.; Lee, D.; Kang, H. Effect of statins on experimental postoperative adhesion: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 14754. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.C.; Panitch, A. Abdominal Adhesions: Current and Novel Therapies. J. Surg. Res. 2011, 165, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Park, H.; Park, Y.; Kang, H.; Lee, D. Development of a Lidocaine-Loaded Alginate/CMC/PEO Electrospun Nanofiber Film and Application as an Anti-Adhesion Barrier. Polymers 2020, 12, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, T.; Van Hai, H.; Park, M.; Lee, S.-Y.; Lee, B.T. Controlled release of Mitomycin C from modified cellulose based thermo-gel prevents post-operative de novo peritoneal adhesion. Carbohydr. Polym. 2019, 229, 115552. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Abuzar, S.; Seo, Y.; Han, H.; Jeon, Y.; Park, E.J.; Baik, S.H.; Hwang, S.-J. Oxaliplatin-loaded chemically cross-linked hydrogels for prevention of postoperative abdominal adhesion and colorectal cancer therapy. Int. J. Pharm. 2019, 565, 50–58. [Google Scholar] [CrossRef]
- Abuzar, S.M.; Ahn, J.-H.; Park, K.S.; Park, E.J.; Baik, S.H.; Hwang, S.-J. Pharmacokinetic Profile and Anti-Adhesive Effect of Oxaliplatin-PLGA Microparticle-Loaded Hydrogels in Rats for Colorectal Cancer Treatment. Pharmaceutics 2019, 11, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.-D.; Kim, J.-W.; Noh, S.-H.; Kim, S.-W.; Jang, E.-C.; Nah, J.-W.; Lee, Y.-G.; Kim, M.-K.; Ito, Y.; Son, T.-I. Potent anti-adhesion agent using a drug-eluting visible-light curable hyaluronic acid derivative. J. Ind. Eng. Chem. 2019, 70, 204–210. [Google Scholar] [CrossRef]
- Cai, X.; Hu, S.; Yu, B.; Cai, Y.; Yang, J.; Li, F.; Zheng, Y.; Shi, X. Transglutaminase-catalyzed preparation of crosslinked carboxymethyl chitosan/carboxymethyl cellulose/collagen composite membrane for postsurgical peritoneal adhesion prevention. Carbohydr. Polym. 2018, 201, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, X.; Luo, J.; Wen, Q.; Wu, Z.; Ding, Q.; Zhao, L.; Yang, L.; Wang, B.; Fu, S. Naproxen Nanoparticle-Loaded Thermosensitive Chitosan Hydrogel for Prevention of Postoperative Adhesions. ACS Biomater. Sci. Eng. 2019, 5, 1580–1588. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, Y.; Li, H.; Yan, T.; Wei, X.; Wu, G.; He, J.; Huang, Y. Biodegradable N, O-carboxymethyl chitosan/oxidized regenerated cellulose composite gauze as a barrier for preventing postoperative adhesion. Carbohydr. Polym. 2018, 207, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Allègre, L.; Le Teuff, I.; Leprince, S.; Warembourg, S.; Taillades, H.; Garric, X.; Letouzey, V.; Huberlant, S. A new bioabsorbable polymer film to prevent peritoneal adhesions validated in a post-surgical animal model. PLoS ONE 2018, 13, e0202285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-C.; Bai, M.-Y.; Hsu, W.-Y.; Yu, M.-H. Evaluation of a series of silk fibroin protein-based nonwoven mats for use as an anti-adhesion patch for wound management in robotic surgery. J. Biomed. Mater. Res. Part A 2017, 106, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.S.; Cao, Y.; Uhrich, K.E. Extrudable salicylic acid-based poly(anhydride-esters) for injectable drug releasing applications. J. Bioact. Compat. Polym. 2019, 34, 178–189. [Google Scholar] [CrossRef]
- Wang, J.; Peng, C.; Chen, Z.; Sun, S.; Shi, Z.; Jin, L.; Zhao, W.; Zhao, C. Engineering antimicrobial and biocompatible electrospun PLGA fibrous membranes by irradiation grafting polyvinylpyrrolidone and periodate. Colloids Surf. B Biointerfaces 2019, 181, 918–926. [Google Scholar] [CrossRef]
- Niu, L.; Feng, C.; Shen, C.; Wang, B.; Zhang, X.-M. PLGA/PLCA casting and PLGA/PDPA electrospinning bilayer film for prevention of postoperative adhesion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 2030–2039. [Google Scholar] [CrossRef]
- Sezer, U.A.; Sanko, V.; Gulmez, M.; Aru, B.; Sayman, E.; Aktekin, A.; Aker, F.V.; Demirel, G.Y.; Sezer, S. Polypropylene composite hernia mesh with anti-adhesion layer composed of polycaprolactone and oxidized regenerated cellulose. Mater. Sci. Eng. C 2019, 99, 1141–1152. [Google Scholar] [CrossRef]
- Li, X.; Zou, B.; Zhao, N.; Wang, C.; Du, Y.; Mei, L.; Wang, Y.; Ma, S.; Tian, X.; He, J.; et al. Potent Anti-adhesion Barrier Combined Biodegradable Hydrogel with Multifunctional Turkish Galls Extract. ACS Appl. Mater. Interfaces 2018, 10, 24469–24479. [Google Scholar] [CrossRef]
- El-Salamouni, N.S.; Gowayed, M.A.; Labib, G.S. Controlled release Ibu-cryobarriers for the prevention of post-operative adhesions: In-vitro/in-vivo comparative study. Int. J. Pharm. 2019, 565, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Feng, C.; Dang, J.; Niu, L.; Shen, C.; Yang, X.; Zhang, T.; Zhang, X. Anti-Adhesive, Platelet Gathering Effects of c-RGD Modified Poly(p-dioxanone-co-l-Phe) Electrospun Membrane and Its Comprehensive Application in Intestinal Anastomosis. Macromol. Biosci. 2019, 20, e1900344. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Fan, C.-N.; Cho, F.-N.; Kan, Y.-Y.; Chang, Y.-H.; Kang, H.-Y. A novel technique to apply a Seprafilm (hyaluronate–carboxymethylcellulose) barrier following laparoscopic surgeries. Fertil. Steril. 2008, 90, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Kohane, D.S. Polymers in the prevention of peritoneal adhesions. Eur. J. Pharm. Biopharm. 2008, 68, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, B.; Nisell, H.; Granberg, I. Surgicel—An absorbable hemostatic material—In prevention of peritoneal adhesions in rats. Acta Chir. Scand. 1978, 144, 375–378. [Google Scholar] [PubMed]
- Reddy, S.; Santanam, N.; Reddy, P.; Rock, J.A.; Murphy, A.A.; Parthasarathy, S. Interaction of Interceed oxidized regenerated cellulose with macrophages: A potential mechanism by which Interceed may prevent adhesions. Am. J. Obstet. Gynecol. 1997, 177, 1315–1321. [Google Scholar] [CrossRef]
- Mohri, Y.; Uchida, K.; Araki, T.; Inoue, Y.; Tonouchi, H.; Miki, C.; Kusunoki, M. Hyaluronic acid-carboxycellulose membrane (Seprafilm) reduces early post-operative small bowel obstruction in gastrointestinal surgery. Am. Surg. 2005, 71, 861–863. [Google Scholar]
- Kimmelman, C.P.; Edelstein, D.R.; Cheng, H.J. Sepragel Sinus (Hylan B) as a Postsurgical Dressing for Endoscopic Sinus Surgery. Otolaryngol. Neck Surg. 2001, 125, 603–608. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Chung, P.K.; Yoo, J.C. Effect of sodium hyaluronate/carboxymethyl cellulose (Guardix-sol) on retear rate and postoperative stiffness in arthroscopic rotator cuff repair patients: A prospective cohort study. J. Orthop. Surg. 2017, 25, 2309499017718908. [Google Scholar] [CrossRef]
- Sheldon, H.K.; Gainsbury, M.L.; Cassidy, M.R.; Chu, D.I.; Stucchi, A.; Becker, J.T. A Sprayable Hyaluronate/Carboxymethylcellulose Adhesion Barrier Exhibits Regional Adhesion Reduction Efficacy and Does Not Impair Intestinal Healing. J. Gastrointest. Surg. 2011, 16, 325–333. [Google Scholar] [CrossRef]
- Haney, A.; Doty, E. A barrier composed of chemically cross-linked hyaluronic acid (Incert) reduces postoperative adhesion formation 11Incert is a registered trademark of Anika Therapeutics, Inc., Woburn, Massachusetts. Fertil. Steril. 1998, 70, 145–151. [Google Scholar] [CrossRef]
- Wallwiener, M.; Brucker, S.; Hierlemann, H.; Brochhausen, C.; Solomayer, E.; Wallwiener, C. Innovative barriers for peritoneal adhesion prevention: Liquid or solid? A rat uterine horn model. Fertil. Steril. 2006, 86, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P. Reduction of De Novo Postsurgical Adhesions by Intraoperative Precoating with Sepracoat (HAL-C) Solution: A Prospective, Randomized, Blinded, Placebo-Controlled Multicenter Study**Sepracoat and HAL-C are trademarks; they are the property of Genzyme Corporation, Cambridge, Massachusetts. Fertil. Steril. 1998, 69, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Belluco, C.; Meggiolaro, F.; Pressato, D.; Pavesio, A.; Bigon, E.; Donà, M.; Forlin, M.; Nitti, N.; Lise, M. Prevention of Postsurgical Adhesions with an Autocrosslinked Hyaluronan Derivative Gel. J. Surg. Res. 2001, 100, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.; Thornton, M.; Campeau, J.; Hoehler, F.; Dizerega, G. R-242. Clinical Lubricoat® 0.5% ferric hyaluronate gel for the reduction of adhesions following peritoneal cavity surgery: An open-label pilot study. Hum. Reprod. 1997, 12, 339–340. [Google Scholar] [CrossRef] [Green Version]
- Johns, D.B.; Keyport, G.M.; Hoehler, F.; Dizerega, G.S. Reduction of postsurgical adhesions with intergel® adhesion prevention solution: A multicenter study of safety and efficacy after conservative gynecologic surgery11Intergel® Adhesion Prevention Solution, Trademark Ethicon, Inc., Somerville, New Jersey. Fertil. Steril. 2001, 76, 595–604. [Google Scholar] [CrossRef]
- Connors, R.C.; Muir, J.J.; Liu, Y.; Reiss, G.R.; Kouretas, P.C.; Whitten, M.G.; Sorenson, T.K.; Prestwich, G.D.; Bull, D.A. Postoperative Pericardial Adhesion Prevention Using Carbylan-SX in a Rabbit Model. J. Surg. Res. 2007, 140, 237–242. [Google Scholar] [CrossRef]
- Brown, C.B.; Luciano, A.A.; Martin, D.; Peers, E.; Scrimgeour, A.; Dizerega, G.S.; Martin, D. Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: A double-blind, randomized, controlled study. Fertil. Steril. 2007, 88, 1413–1426. [Google Scholar] [CrossRef]
- Rein, M.S.; Hill, J.A. 32-Percent Dextran-70 (Hyskon) Inhibits Lymphocyte and Macrophage Function-Invitro—A Potential New Mechanism for Adhesion Prevention. Fertil. Steril. 1989, 52, 953–957. [Google Scholar] [CrossRef]
- Dabrowski, A.; Lepère, M.; Zaranis, C.; Coelio, C.; Hauters, P. Efficacy and safety of a resorbable collagen membrane COVA+™ for the prevention of postoperative adhesions in abdominal surgery. Surg. Endosc. 2015, 30, 2358–2366. [Google Scholar] [CrossRef]
- Tchartchian, G.; Hackethal, A.; Herrmann, A.; Bojahr, B.; Wallwiener, C.; Ohlinger, R.; Ebert, A.D.; De Wilde, R.L. Evaluation of SprayShield™ Adhesion Barrier in a single center: Randomized controlled study in 15 women undergoing reconstructive surgery after laparoscopic myomectomy. Arch. Gynecol. Obstet. 2014, 290, 697–704. [Google Scholar] [CrossRef]
- Dunn, R.; Lyman, M.D.; Edelman, P.G.; Campbell, P.K. Evaluation of the SprayGel™ adhesion barrier in the rat cecum abrasion and rabbit uterine horn adhesion models. Fertil. Steril. 2001, 75, 411–416. [Google Scholar] [CrossRef]
- e Quinino, R.M.; Araujo-Filho, I.; Lima, F.P.; Barbosa, A.L.C.; Maia, T.D.C.; Goldenberg, A. Adhesion prevention in reabsorbable polyethylene glycol hydrogel (Coseal®) coated polypropylene mesh in rabbits. Acta Cir. Bras. 2013, 28, 807–814. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, K.E.; Schwartz, H.E.; Roda, N.; Thornton, M.; Kobak, W.; Dizerega, G.S. Effect of oxiplex* films (PEO/CMC) on adhesion formation and reformation in rabbit models and on peritoneal infection in a rat model**Oxiplex, FzioMed, Inc., San Luis Obispo, California. Fertil. Steril. 2000, 73, 831–838. [Google Scholar] [CrossRef]
- Okuyama, N.; Rodgers, K.E.; Wang, C.Y.; Girgis, W.; Oz, M.; Amand, K.S.; Pines, E.; DeCherney, A.H.; Rose, E.A.; Cohn, D.; et al. Prevention of Retrosternal Adhesion Formation in a Rabbit Model Using Bioresorbable Films of Polyethylene Glycol and Polylactic Acid. J. Surg. Res. 1998, 78, 118–122. [Google Scholar] [CrossRef]
- Avital, S.; Bollinger, T.J.; Wilkinson, J.D.; Marchetti, F.; Hellinger, M.D.; Sands, L.R. Preventing Intra-Abdominal Adhesions With Polylactic Acid Film: An Animal Study. Dis. Colon Rectum 2005, 48, 153–157. [Google Scholar] [CrossRef]
- Weis, C.; Odermatt, E.K. A-part gel-An efficient adhesion prevention barrier. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82, 174–182. [Google Scholar] [CrossRef]
- Sohn, E.J.; Ahn, H.B.; Roh, M.S.; Ryu, W.Y.; Kwon, Y.H. Efficacy of Temperature-Sensitive Guardix-SG for Adhesiolysis in Experimentally Induced Eyelid Adhesion in Rabbits. Ophthalmic Plast. Reconstr. Surg. 2013, 29, 458–463. [Google Scholar] [CrossRef]
- Aunoble, S.; Alsawad, Y.; Meyrat, R.; Rigal, J.; Le Huec, J.-C. Polytetrafluoroethylene Membrane (Gore Preclude Vessel Guard) for Vessel Protection during Anterior Lumbar Surgery. J. Spinal Disord. Tech. 2011, 24, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.J.; Lee, D.W.; Song, S.Y.; Park, Y.; Kim, J.; Kim, H.G.; Nam, K.T.; Lee, W.J.; Nam, K.-H.; Lee, J.H.; et al. Development of novel biocompatible thermosensitive anti-adhesive agents using human-derived acellular dermal matrix. PLoS ONE 2019, 14, e0212583. [Google Scholar] [CrossRef] [PubMed]
- Van Steensel, S.; Liu, H.; Mommers, E.H.; Lenaerts, K.; Bouvy, N. Comparing Five New Polymer Barriers for the Prevention of Intra-abdominal Adhesions in a Rat Model. J. Surg. Res. 2019, 243, 453–459. [Google Scholar] [CrossRef]
- Saif, A.; Shami, N.; Anwar, S.; Asif, S. Role of Hyaluronic Acid Barrier Gel in Adhesion Prevention. Pak. J. Med. Health Sci. 2019, 13, 13–16. [Google Scholar]
- Siswomihardjo, W. Biocompatibility Issues of Biomaterials. In Biomaterials and Medical Devices: A Perspective from an Emerging Country; Mahyudin, F., Hermawan, H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 41–65. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Kulkarni, V.; Butte, K.; Rathod, S. Natural Polymers—A comprehensive Review. Int. J. Res. Pharma. Biomed. Sci. 2012, 3, 1597–1613. [Google Scholar]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Zheng, X.-J.; Tang, K.-Y. Natural Polymer Composites with Collagen and Gelatin as the Matrices: A Review. Polym. Bull. 2010, 2, 58–68. [Google Scholar]
- Davis, W.B. Unique Bacterial Polysaccharide Polymer Gel in Cosmetics, Pharmaceuticals and Foods. U.S. Patent 5,158,772, 27 October 1991. [Google Scholar]
- Manoukian, O.S.; Sardashti, N.; Stedman, T.; Gailiunas, K.; Ojha, A.; Penalosa, A.; Mancuso, C.; Hobert, M.; Kumbar, S.G. Biomaterials for Tissue Engineering and Regenerative Medicine; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 462–482. [Google Scholar]
- Kommareddy, S.; Shenoy, D.B.; Amiji, M. Gelatin Nanoparticles and Their Biofunctionalization. In Nanotechnologies for the Life Sciences; Kumar, C.S.S.R., Ed.; Wiley: Weinheim, Germany, 2007. [Google Scholar]
- Hollabaugh, C.B.; Burt, L.H.; Walsh, A.P. Carboxymethylcellulose. Uses and Applications. Ind. Eng. Chem. 1945, 37, 943–947. [Google Scholar] [CrossRef]
- Ugwoke, M.I.; Agu, R.U.; Vanbilloen, H.; Baetens, J.; Augustijns, P.; Verbeke, N.; Mortelmans, L.; Verbruggen, A.; Kinget, R.; Bormans, G. Scintigraphic evaluation in rabbits of nasal drug delivery systems based on carbopol 971p® and carboxymethylcellulose. J. Control. Release 2000, 68, 207–214. [Google Scholar] [CrossRef]
- Chen, R.-N.; Ho, H.-O.; Yu, C.-Y.; Sheu, M.-T. Development of swelling/floating gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose for Losartan and its clinical relevance in healthy volunteers with CYP2C9 polymorphism. Eur. J. Pharm. Sci. 2010, 39, 82–89. [Google Scholar] [CrossRef]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 2000, 1, 15–30. [Google Scholar]
- Gaihre, B.; Jayasuriya, A.C. Fabrication and characterization of carboxymethyl cellulose novel microparticles for bone tissue engineering. Mater. Sci. Eng. C 2016, 69, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Urano, H.; Iwatsuki, K.; Yamamoto, M.; Ohnisi, T.; Kurimoto, S.; Endo, N.; Hirata, H. Novel Anti-Adhesive CMC-PE Hydrogel Significantly Enhanced Morphological and Physiological Recovery after Surgical Decompression in an Animal Model of Entrapment Neuropathy. PLoS ONE 2016, 11, e0164572. [Google Scholar] [CrossRef]
- Cipe, G.; Koksal, H.M.; Yildirim, S.; Celayir, M.F.; Baykan, A. Efficacy of hyaluronic acid - carboxymethyl cellulose membrane (Seprafilm (R)) and polylactic acid barrier film (Surgiwrap (TM)) for the prevention of adhesions after thyroid surgery: An experimental model. Turk. J. Med. Sci. 2011, 41, 73–79. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R.E. Hyaluronan 1. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef]
- Saettone, M.F.; Chetoni, P.; Torracca, M.T.; Burgalassi, S.; Giannaccini, B. Evaluation of muco-adhesive properties and in vivo activity of ophthalmic vehicles based on hyaluronic acid. Int. J. Pharm. 1989, 51, 203–212. [Google Scholar] [CrossRef]
- Eriksson, S.; Fraser, J.E.; Laurent, T.C.; Pertoft, H.; Smedsrød, B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp. Cell Res. 1983, 144, 223–228. [Google Scholar] [CrossRef]
- Tammi, R.; Rilla, K.; Pienimäki, J.-P.; Maccallum, D.K.; Hogg, M.; Luukkonen, M.; Hascall, V.C.; Tammi, M. Hyaluronan Enters Keratinocytes by a Novel Endocytic Route for Catabolism. J. Biol. Chem. 2001, 276, 35111–35122. [Google Scholar] [CrossRef] [Green Version]
- Mais, V.; Bracco, G.; Litta, P.; Gargiulo, T.; Melis, G. Reduction of postoperative adhesions with an auto-crosslinked hyaluronan gel in gynaecological laparoscopic surgery: A blinded, controlled, randomized, multicentre study. Hum. Reprod. 2006, 21, 1248–1254. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.-Z.; Hou, C.-L.; Gu, Q.-S.; Jiang, L.-X.; Zhu, B.; Sheng, A.-L. A thermosensitive chitosan-based hydrogel barrier for post-operative adhesions’ prevention. Biomaterials 2009, 30, 5534–5540. [Google Scholar] [CrossRef]
- Kim, E.H.; Lim, S.; Kim, T.E.; Jeon, I.O.; Choi, Y.S. Preparation of in situ Injectable Chitosan/Gelatin Hydrogel Using an Acid-tolerant Tyrosinase. Biotechnol. Bioprocess Eng. 2018, 23, 500–506. [Google Scholar] [CrossRef]
- Chang, J.-J.; Lee, Y.-H.; Wu, M.-H.; Yang, M.-C.; Chien, C.-T. Electrospun anti-adhesion barrier made of chitosan alginate for reducing peritoneal adhesions. Carbohydr. Polym. 2012, 88, 1304–1312. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chen, C.-H.; Fong, Y.T.; Chen, J.-P. Prevention of peritendinous adhesions with electrospun chitosan-grafted polycaprolactone nanofibrous membranes. Acta Biomater. 2014, 10, 4971–4982. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, S.-H.; Mao, S.-H.; Tsai, M.-J.; Chou, P.-Y.; Liao, C.-H.; Chen, J.-P. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr. Polym. 2017, 173, 721–731. [Google Scholar] [CrossRef]
- Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A Valuable Protein for Food and Pharmaceutical Industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef]
- Hellio, D.; Djabourov, M. Physically and Chemically Crosslinked Gelatin Gels. Macromol. Symp. 2006, 241, 23–27. [Google Scholar] [CrossRef]
- Baek, S.; Park, H.; Kim, M.; Lee, D. Preparation of PCL/(+)-catechin/gelatin film for wound healing using air-jet spinning. Appl. Surf. Sci. 2020, 509, 145033. [Google Scholar] [CrossRef]
- Horii, T.; Tsujimoto, H.; Miyamoto, H.; Yamanaka, K.; Tanaka, S.; Torii, H.; Ozamoto, Y.; Takamori, H.; Nakamachi, E.; Ikada, Y.; et al. Physical and biological properties of a novel anti-adhesion material made of thermally cross-linked gelatin film: Investigation of the usefulness as anti-adhesion material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 689–696. [Google Scholar] [CrossRef]
- Torii, H.; Takagi, T.; Urabe, M.; Tsujimoto, H.; Ozamoto, Y.; Miyamoto, H.; Ikada, Y.; Hagiwara, A. Anti-adhesive effects of a newly developed two-layered gelatin sheet in dogs. J. Obstet. Gynaecol. Res. 2017, 43, 1317–1325. [Google Scholar] [CrossRef] [Green Version]
- Shahram, E.; Sadraie, S.H.; Kaka, G.; Khoshmohabat, H.; Hosseinalipour, M.; Panahi, F.; Naimi-Jamal, M.R. Evaluation of chitosan–gelatin films for use as postoperative adhesion barrier in rat cecum model. Int. J. Surg. 2013, 11, 1097–1102. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, M.; Shukla, P.; Bajpai, S. Ca(II)+Ba(II) ions crosslinked alginate gels prepared by a novel diffusion through dialysis tube (DTDT) approach and preliminary BSA release study. Polym. Degrad. Stab. 2016, 134, 22–29. [Google Scholar] [CrossRef]
- Baek, S.; Park, H.; Chen, K.; Park, H.; Lee, D. Development of an implantable PCL/alginate bilayer scaffold to prevent secondary infections. Korean J. Chem. Eng. 2020, 37, 677–687. [Google Scholar] [CrossRef]
- Kim, D.; Choi, G.J.; Baek, S.; Abdullah, A.; Jang, S.; Hong, S.A.; Kim, B.G.; Lee, J.; Kang, H.; Lee, D. Characterization of Anti-Adhesion Properties of Alginate/Polyethylene Oxide Film to Reduce Postsurgical Peritoneal Adhesions. Sci. Adv. Mater. 2017, 9, 1669–1677. [Google Scholar] [CrossRef]
- Gunatillake, P. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Way, T.-D.; Hsieh, S.-R.; Chang, C.-J.; Hung, T.-W.; Chiu, C.-H. Preparation and characterization of branched polymers as post-operative anti-adhesion barriers. Appl. Surf. Sci. 2010, 256, 3330–3336. [Google Scholar] [CrossRef]
- Peña, J.; Corrales, T.; Izquierdo-Barba, I.; Doadrio, J.C.; Vallet-Regí, M. Long term degradation of poly(ɛ-caprolactone) films in biologically related fluids. Polym. Degrad. Stab. 2006, 91, 1424–1432. [Google Scholar] [CrossRef]
- Ni, C.; Lu, R.; Tao, L.; Shi, G.; Li, X.; Qin, C. Synthesis of poly(vinyl alcohol-graft-lactic acid) copolymer and its application as medical anti-tissue adhesion thin film. Polym. Bull. 2015, 72, 1515–1529. [Google Scholar] [CrossRef]
- Fu, S.Z.; Li, Z.; Fan, J.M.; Meng, X.H.; Shi, K.; Qu, Y.; Yang, L.L.; Wu, J.B.; Fan, J.; Luo, F.; et al. Biodegradable and Thermosensitive Monomethoxy Poly(ethylene glycol)–Poly(lactic acid) Hydrogel as a Barrier for Prevention of Post-Operative Abdominal Adhesion. J. Biomed. Nanotechnol. 2014, 10, 427–435. [Google Scholar] [CrossRef]
- Renz, B.W.; Leitner, K.; Odermatt, E.; Worthley, D.L.; Angele, M.K.; Jauch, K.-W.; Lang, R.A. PVA gel as a potential adhesion barrier: A safety study in a large animal model of intestinal surgery. Langenbeck’s Arch. Surg. 2014, 399, 349–357. [Google Scholar] [CrossRef]
- Freytag, C.; Odermatt, E.K. Standard Biocompatibility Studies Do Not Predict All Effects of PVA/CMC Anti-Adhesive Gel in vivo. Eur. Surg. Res. 2016, 56, 109–122. [Google Scholar] [CrossRef]
- Li, J.; Feng, X.; Liu, B.; Yu, Y.; Sun, L.; Liu, T.; Wang, Y.; Ding, J.; Chen, X. Polymer materials for prevention of postoperative adhesion. Acta Biomater. 2017, 61, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-H.; Son, S.-R.; Sakar, S.K.; Nguyen, T.-H.; Kim, S.-W.; Min, Y.-K.; Lee, B.-T. Evaluation of the potential anti-adhesion effect of the PVA/Gelatin membrane. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 102, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Li, J.; Sun, D.; Li, H.; Qian, F.; Wang, L. 20(S)-Ginsenoside Rg3-loaded electrospun membranes to prevent postoperative peritoneal adhesion. Biomed. Microdevices 2019, 21, 78. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Rutkowska, M.; Jastrzębska, M.; Janik, H. Biodegradation of polycaprolactone in sea water. React. Funct. Polym. 1998, 38, 27–30. [Google Scholar] [CrossRef]
- Lo, H.-Y.; Kuo, H.-T.; Huang, Y.-Y. Application of Polycaprolactone as an Anti-Adhesion Biomaterial Film. Artif. Organs 2010, 34, 648–653. [Google Scholar] [CrossRef]
- Shi, R.; Xue, J.; Wang, H.; Wang, R.; Gong, M.; Chen, D.; Zhang, L.; Tian, W. Fabrication and evaluation of a homogeneous electrospun PCL-gelatin hybrid membrane as an anti-adhesion barrier for craniectomy. J. Mater. Chem. B 2015, 3, 4063–4073. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, S.-H.; Shalumon, K.; Chen, J.-P. Dual functional core–sheath electrospun hyaluronic acid/polycaprolactone nanofibrous membranes embedded with silver nanoparticles for prevention of peritendinous adhesion. Acta Biomater. 2015, 26, 225–235. [Google Scholar] [CrossRef]
- Kahovec, J.; Fox, R.B.; Hatada, K. Nomenclature of regular single-strand organic polymers (IUPAC Recommendations 2002). Pure Appl. Chem. 2002, 74, 1921–1956. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef] [Green Version]
- Arantes, V.; Toyonaga, T.; Piñeros, E.A.F. Polyethylene glycol submucosal irrigation: A novel approach to improve visibility during endoscopic submucosal dissection. Endosc. Int. Open 2014, 2, E193–E195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Song, B.R.; Lee, J.W.; Park, S.H.; Kang, T.W.; Yun, H.-W.; Park, S.-H.; Min, B.; Kim, M.S. Preparation of a Cross-Linked Cartilage Acellular-Matrix Film and Its In Vivo Evaluation as an Antiadhesive Barrier. Polymers 2019, 11, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.-X.; Yuan, F.; Zhang, H.-H.; Liao, N.-N.; Luo, J.-W.; Sun, Y.-L. Evaluation of surgical anti-adhesion products to reduce postsurgical intra-abdominal adhesion formation in a rat model. PLoS ONE 2017, 12, e0172088. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.A.; Hernandez-Vaquero, D. Preventing peridural fibrosis with nonsteroidal anti-inflammatory drugs. Eur. Spine J. 2008, 17, 451–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, E.S.; Scheib, S.; Patzkowsky, K.E.; Simpson, K.; Wang, K.C. The sticky business of adhesion prevention in minimally invasive gynecologic surgery. Curr. Opin. Obstet. Gynecol. 2017, 29, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Z.; Lu, S.; Zhang, T.; Zhou, N.; Ren, P.; Wang, F.; Yang, Y.; Ji, Z. Assembled anti-adhesion polypropylene mesh with self-fixable and degradable in situ mussel-inspired hydrogel coating for abdominal wall defect repair. Biomater. Sci. 2018, 6, 3030–3041. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Zhang, Z.; Zhu, L.; Wen, Y.; Zhang, T.; Ren, P.; Wang, F.; Ji, Z. Combination of Polypropylene Mesh and in Situ Injectable Mussel-Inspired Hydrogel in Laparoscopic Hernia Repair for Preventing Post-Surgical Adhesions in the Piglet Model. ACS Biomater. Sci. Eng. 2020, 6, 1735–1743. [Google Scholar] [CrossRef]
- Ahmed, E. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2013, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Pei, Y.-Y.; Guo, D.-M.; An, Q.; Xiao, Z.-Y.; Zhai, S.-R.; Zhai, B. Hydrogels with diffusion-facilitated porous network for improved adsorption performance. Korean J. Chem. Eng. 2018, 35, 2384–2393. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Miao, Y.; Zhang, X.; Fan, Z.; Singh, G.; Zhang, X.; Xu, K.; Li, B.; Hu, Z.; et al. Hydrogen bonds autonomously powered gelatin methacrylate hydrogels with super-elasticity, self-heal and underwater self-adhesion for sutureless skin and stomach surgery and E-skin. Biomaterials 2018, 171, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Picaud, L.; Thibault, B.; Mery, E.; Ouali, M.; Martinez, A.; Delord, J.-P.; Couderc, B.; Ferron, G. Evaluation of the effects of hyaluronic acid-carboxymethyl cellulose barrier on ovarian tumor progression. J. Ovarian Res. 2014, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.-W.; Fang, J.-F.; Yang, C.-L.; Chen, J.-H.; Su, L.-T.; Jan, S.-H. Preparation and Evaluation of a Hyaluronate-Collagen Film for Preventing Post-Surgical Adhesion. J. Int. Med. Res. 2005, 33, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2018, 96, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Zhou, C.; Fan, H.; Zhang, B.; Pei, X.; Fan, Y.; Jiang, Q.; Bao, R.; Yang, Q.; Dong, Z.; et al. Novel 3D porous biocomposite scaffolds fabricated by fused deposition modeling and gas foaming combined technology. Compos. Part B Eng. 2018, 152, 151–159. [Google Scholar] [CrossRef]
- Guo, S.; Kang, G.; Phan, D.-T.; Hsu, M.N.; Por, Y.C.; Chen, C.-H. Polymerization-Induced Phase Separation Formation of Structured Hydrogel Particles via Microfluidics for Scar Therapeutics. Sci. Rep. 2018, 8, 2245. [Google Scholar] [CrossRef] [Green Version]
- Mao, D.; Li, Q.; Li, D.; Tan, Y.; Che, Q. 3D porous poly (ε-caprolactone)/58S bioactive glass–sodium alginate/gelatin hybrid scaffolds prepared by a modified melt molding method for bone tissue engineering. Mater. Des. 2018, 160, 1–8. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, X.; Gao, F.; Li, D.; Wu, M. Preparation and Characterization of High Amylose Corn Starch–Microcrystalline Cellulose Aerogel with High Absorption. Materials 2019, 12, 1420. [Google Scholar] [CrossRef] [Green Version]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Hennink, W.E.; Van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, W. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017, 71, 1–25. [Google Scholar] [CrossRef]
- Chou, P.-Y.; Chen, S.-H.; Chen, C.-H.; Fong, Y.T.; Chen, J.-P.; Chen, S.-H. Thermo-responsive in-situ forming hydrogels as barriers to prevent post-operative peritendinous adhesion. Acta Biomater. 2017, 63, 85–95. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kuo, C.-Y.; Chen, S.-H.; Mao, S.-H.; Chang, C.-Y.; Shalumon, K.T.; Chen, J.-P. Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion. Int. J. Mol. Sci. 2018, 19, 1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coburn, J.; Gibson, M.; Monagle, S.; Patterson, Z.; Elisseeff, J.H. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc. Natl. Acad. Sci. USA 2012, 109, 10012–10017. [Google Scholar] [CrossRef] [Green Version]
- Hauser, C.; Deng, R.; Mishra, A.; Loo, Y.; Khoe, U.; Zhuang, F.; Cheong, D.W.; Accardo, A.; Sullivan, M.B.; Riekel, C.; et al. Natural tri- to hexapeptides self-assemble in water to amyloid β-type fiber aggregates by unexpected α-helical intermediate structures. Proc. Natl. Acad. Sci. USA 2011, 108, 1361–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, V.C.F.; Mulyadi, A.; Dunn, C.K.; Deng, Y.; Qi, H.J. Direct Ink Write 3D Printed Cellulose Nanofiber Aerogel Structures with Highly Deformable, Shape Recoverable, and Functionalizable Properties. ACS Sustain. Chem. Eng. 2018, 6, 2011–2022. [Google Scholar] [CrossRef]
- Hu, C.; Liu, S.; Zhang, Y.; Li, B.; Yang, H.; Fan, C.-Y.; Cui, W. Long-term drug release from electrospun fibers for in vivo inflammation prevention in the prevention of peritendinous adhesions. Acta Biomater. 2013, 9, 7381–7388. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.; Ramakrishna, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofibers. Polymers 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Tan, A.R.; Ifkovits, J.L.; Baker, B.M.; Brey, D.M.; Mauck, R.L.; Burdick, J.A. Electrospinning of photocrosslinked and degradable fibrous scaffolds. J. Biomed. Mater. Res. Part A 2008, 87, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Arnoult, O.; Smith, M.; Wnek, G.E. Electrospinning of in situ crosslinked collagen nanofibers. J. Mater. Chem. 2012, 22, 19412. [Google Scholar] [CrossRef]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Zhao, M.L.; Sui, G.; Deng, X.L.; Lu, J.G.; Ryu, S.K.; Yang, X.P. PLLA/HA Electrospin Hybrid Nanofiber Scaffolds: Morphology, In Vitro Degradation and Cell Culture Potential. Adv. Mater. Res. 2006, 11, 243–246. [Google Scholar] [CrossRef]
- Tseng, Y.-Y.; Liu, S.-J. Nanofibers used for the delivery of analgesics. Nanomedicine 2015, 10, 1785–1800. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Krishnan, U.M.; Sethuraman, S. Fabrication and characterization of chitosan-gelatin blend nanofibers for skin tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 264–272. [Google Scholar] [CrossRef]
- Chen, F.; Li, X.; Mo, X.; He, C.; Wang, H.; Ikada, Y. Electrospun chitosan-P(LLA-CL) nanofibers for biomimetic extracellular matrix. J. Biomater. Sci. Polym. Ed. 2008, 19, 677–691. [Google Scholar] [CrossRef]
- Mirtič, J.; Balažic, H.; Zupančič, Š.; Kristl, J. Effect of Solution Composition Variables on Electrospun Alginate Nanofibers: Response Surface Analysis. Polymers 2019, 11, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudecki, A.; Gola, J.M.; Ghavami, S.; Skonieczna, M.; Markowski, J.; Likus, W.; Lewandowska, M.; Maziarz, W.; Łos, M.J. Structure and properties of slow-resorbing nanofibers obtained by (co-axial) electrospinning as tissue scaffolds in regenerative medicine. PeerJ 2017, 5, e4125. [Google Scholar] [CrossRef] [Green Version]
- Nam, C.; Lee, S.; Ryu, M.; Lee, J.; Lee, H. Electrospun nanofiber filters for highly efficient PM2.5 capture. Korean J. Chem. Eng. 2019, 36, 1565–1574. [Google Scholar] [CrossRef]
- Pijlman, B.; Dörr, P.; Brommer, E.; Vemer, H. Prevention of adhesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 53, 155–163. [Google Scholar] [CrossRef]
- Chu, A.J. Tissue factor upregulation drives a thrombosis–inflammation circuit in relation to cardiovascular complications. Cell Biochem. Funct. 2006, 24, 173–192. [Google Scholar] [CrossRef]
- Hindocha, A.; Beere, L.; Dias, S.; Watson, A.; Ahmad, G. Adhesion prevention agents for gynaecological surgery: An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2015, 1, CD011254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lishko, V.K.; Burke, T.; Ugarova, T. Antiadhesive effect of fibrinogen: A safeguard for thrombus stability. Blood 2006, 109, 1541–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zheng, X.; Fan, D.; Yu, S.; Wu, D.; Fan, C.; Cui, W.; Ruan, H. Release of celecoxib from a bi-layer biomimetic tendon sheath to prevent tissue adhesion. Mater. Sci. Eng. C 2016, 61, 220–226. [Google Scholar] [CrossRef]
- Rodgers, K.; Johns, D.B.; Girgis, W.; Dizerega, G.S. Prevention of adhesion formation with intraperitoneal administration of tolmetin and hyaluronic acid. J. Investig. Surg. 1998, 10, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Kim, J.W.; Park, J.S.; Kim, Y.S.; Choi, Y.S.; Kim, B.G.; Cha, S.J.; Park, S.J.; Chang, I.T.; Park, E.S.; et al. Effects of Three Different Types of Anti-adhesive Agents in a Rat Abdominal Wall Defect Model. J. Korean Surg. Soc. 2009, 77, 7. [Google Scholar] [CrossRef] [Green Version]
- Pross, M.; Lippert, H.; Misselwitz, F.; Nestler, G.; Krüger, S.; Langer, H.; Halangk, W.; Schulz, H.-U. Low-molecular-weight heparin (reviparin) diminishes tumor cell adhesion and invasion in vitro, and decreases intraperitoneal growth of colonadeno-carcinoma cells in rats after laparoscopy. Thromb. Res. 2003, 110, 215–220. [Google Scholar] [CrossRef]
- Cashman, J.D.; Kennah, E.; Shuto, A.; Winternitz, C.; Springate, C.M. Fucoidan Film Safely Inhibits Surgical Adhesions in a Rat Model. J. Surg. Res. 2011, 171, 495–503. [Google Scholar] [CrossRef]
- Imai, A.; Takagi, H.; Matsunami, K.; Suzuki, N. Non-barrier agents for postoperative adhesion prevention: Clinical and preclinical aspects. Arch. Gynecol. Obstet. 2010, 282, 269–275. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Anti-Inflammatory Mechanisms in the Vascular Wall. Circ. Res. 2001, 88, 877–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacharia, E.; Papageorgiou, N.; Ioannou, A.; Siasos, G.; Papaioannou, S.; Vavuranakis, M.; Latsios, G.; Vlachopoulos, C.; Toutouzas, K.; Deftereos, S.; et al. Inflammatory Biomarkers in Atrial Fibrillation. Curr. Med. Chem. 2019, 26, 837–854. [Google Scholar] [CrossRef]
- Kraft, F.; Schmidt, C.; Van Aken, H.; Zarbock, A. Inflammatory response and extracorporeal circulation. Best Prac. Res. Clin. Anaesthesiol. 2015, 29, 113–123. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Vermeire, S.; Van Assche, G. Biological Therapies for Inflammatory Bowel Diseases. Gastroenterology 2009, 136, 1182–1197. [Google Scholar] [CrossRef]
- Liu, S.; Hu, C.; Li, F.; Li, X.; Cui, W.; Fan, C.-Y. Prevention of Peritendinous Adhesions with Electrospun Ibuprofen-Loaded Poly(l-Lactic Acid)-Polyethylene Glycol Fibrous Membranes. Tissue Eng. Part A 2013, 19, 529–537. [Google Scholar] [CrossRef]
- Miller, J.A.; Ferguson, R.L.; Powers, D.L.; Burns, J.W.; Shalaby, S.W. Efficacy of hyaluronic acid/nonsteroidal anti-inflammatory drug systems in preventing postsurgical tendon adhesions. J. Biomed. Mater. Res. 1997, 38, 25–33. [Google Scholar] [CrossRef]
- Golan, A.; Winograd, I.; Bukovsky, I.; Maymon, R. Prevention of post-surgical adhesion formation using aspirin in a rodent model: A preliminary report. Hum. Reprod. 1995, 10, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Parsaei, P.; Karimi, M.; Asadi, S.Y.; Rafieian-Kopaei, M. Bioactive components and preventive effect of green tea (Camellia sinensis) extract on post-laparotomy intra-abdominal adhesion in rats. Int. J. Surg. 2013, 11, 811–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semeraro, N.; Locati, D.; Colucci, M. Stimulation of Human Platelet Coagulant Activity by Endotoxin: A New Leukocyte-Mediated Pathway. Oral Present. 1981, 46, 233. [Google Scholar] [CrossRef]
- Nam, G.S.; Nam, K.-S.; Park, H.-J. Caffeic Acid Diminishes the Production and Release of Thrombogenic Molecules in Human Platelets. Biotechnol. Bioprocess Eng. 2018, 23, 641–648. [Google Scholar] [CrossRef]
- Horie, S.; Hiraishi, S.; Hirata, Y.; Kazama, M.; Matsuda, J. Oxidized low-density lipoprotein impairs the anti-coagulant function of tissue-factor-pathway inhibitor through oxidative modification by its high association and accelerated degradation in cultured human endothelial cells. Biochem. J. 2000, 352, 277. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pendurthi, U.R.; Sen, P.; Rao, L.V.M. The Role of Putative Phosphatidylserine-Interactive Residues of Tissue Factor on Its Coagulant Activity at the Cell Surface. PLoS ONE 2016, 11, e0158377. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.I. Comparative Study for Preventive Effects of Intra-Abdominal Adhesion Using Cyclo-Oxygenase-2 Enzyme (COX-2) Inhibitor, Low Molecular Weight Heparin (LMWH), and Synthetic Barrier. Yonsei Med. J. 2013, 54, 1491–1497. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, K.E.; Girgis, W.; Campeau, J.D.; Dizerega, G.S. Reduction of Adhesion Formation by Intraperitoneal Administration of a Recombinant Hirudin Analog. J. Investig. Surg. 1996, 9, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Reyhan, E.; Irkorucu, O.; Surmelioglu, A.; Özkara, S.; Değer, K.C.; Aziret, M.; Erdem, H.; Cetinkunar, S.; Tilki, M.; Demirtürk, P.; et al. Abolition of anti-adhesiogenic effect of heparin by protamine sulfate. Int. J. Surg. 2014, 12, 729–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simsek, H.; Durmus, A.S.; Yildiz, H.; Özçelik, M. Surgery-Induced Changes in Erythrocyte and Plasma Lipid Peroxidation, Enzymatic and Non-Enzymatic Antioxidants of Female Rats: Protective Role of Heparin and Pentoxifylline. Acta Sci. Veter. 2018, 46, 9. [Google Scholar] [CrossRef]
- Gunay, E.; Abuoglu, H.H.; Uzunoglu, H.; Sunamak, O.; Akyuz, C. Efficacy level of dimethyl-sulfoxide (DMSO) in the prevention of peritoneal adhesions: An experimental rat model. Int. J. Clin. Exp. Med. 2019, 12, 705–711. [Google Scholar]
- Chanda, C.; Sarkar, A.; Chakrabarty, D. Thrombolytic protein from cobra venom with anti-adhesive properties. Arch. Biochem. Biophys. 2016, 590, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chapin, J.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2014, 29, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellgren, M. Hemostasis during Normal Pregnancy and Puerperium. Semin. Thromb. Hemost. 2003, 29, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Idell, S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit. Care Med. 2003, 31, S213–S220. [Google Scholar] [CrossRef]
- Chu, D.I.; Lim, R.; Heydrick, S.; Gainsbury, M.L.; Abdou, R.; D’Addese, L.; Reed, K.L.; Stucchi, A.; Becker, J.M. N-acetyl-l-cysteine decreases intra-abdominal adhesion formation through the upregulation of peritoneal fibrinolytic activity and antioxidant defenses. Surgery 2011, 149, 801–812. [Google Scholar] [CrossRef]
- Yagmurlu, A.; Barlas, M.; Gursel, I.; Gökçora, I.H. Reduction of Surgery-Induced Peritoneal Adhesions by Continuous Release of Streptokinase from a Drug Delivery System. Eur. Surg. Res. 2003, 35, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, D.H.; Van Der Wall, E.E.; Bosker, H.A.; Scheffer, E.; Macfarlane, J.D. Serum-Sickness-Like Illness as a Complication after Streptokinase Therapy for Acute Myocardial Infarction. Cardiology 1991, 78, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Nkere, U.U. Postoperative Adhesion Formation and the Use of Adhesion Preventing Techniques in Cardiac and General Surgery. ASAIO J. 2000, 46, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Aloia, T.A.; Cooper, A.B.; Shi, W.; Vauthey, J.-N.; Lee, J.E. Reoperative Surgery: A Critical Risk Factor for Complications Inadequately Captured by Operative Reporting and Coding of Lysis of Adhesions. J. Am. Coll. Surg. 2014, 219, 143–150. [Google Scholar] [CrossRef]
- Andersson, M.; Johansson, J.; Rising, A. Silk Spinning in Silkworms and Spiders. Int. J. Mol. Sci. 2016, 17, 1290. [Google Scholar] [CrossRef] [Green Version]
- Dobb, M.G.; Fraser, R.D.B.; Macrae, T.P. The Fine Structure of Silk Fibroin. J. Cell Biol. 1967, 32, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Blackledge, T.A.; Pérez-Rigueiro, J.; Plaza, G.R.; Perea, B.; Navarro, A.; Guinea, G.V.; Elices, M. Sequential origin in the high performance properties of orb spider dragline silk. Sci. Rep. 2012, 2, 782. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Zhang, Y.-Q. Postoperative anti-adhesion ability of a novel carboxymethyl chitosan from silkworm pupa in a rat cecal abrasion model. Mater. Sci. Eng. C 2016, 61, 387–395. [Google Scholar] [CrossRef]
- Vepari, C.P.; Kaplan, D.L. Surface Modification of Silk Fibroin Matrices with Poly (Ethylene Glycol) Useful as Anti-Adhesion Barriers and Anti-Thrombotic Materials. U.S. Patent 9,427,499, 30 August 2016. [Google Scholar]
- Zarrintaj, P.; Manouchehri, S.; Ahamadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Koontz, L. Agarose Gel Electrophoresis. In Methods in Enzymology; Elsevier BV: Amsterdam, The Netherlands, 2013; Volume 529, pp. 35–45. [Google Scholar]
- Tang, S.; Yang, W.; Mao, X. Agarose/collagen composite scaffold as an anti-adhesive sheet. Biomed. Mater. 2007, 2, S129–S134. [Google Scholar] [CrossRef]
- Yalcin, E.; Cavusoglu, K. Glutaraldehyde Cross-Linked Agarose Carriers: Design, Characterization and Insulin Release Behaviour. Turk. J. Biochem. 2008, 33, 148–153. [Google Scholar]
- Armoiry, X.; Viprey, M.; Constant, H.; Aulagner, G.; Roux, A.S.; Huot, L.; Roubertie, F.; Ninet, J.; Henaine, R. Potential interest of a new absorbable collagen membrane in the prevention of adhesions in paediatric cardiac surgery: A feasibility study. Arch. Cardiovasc. Dis. 2013, 106, 433–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holman, W.L.; Bourge, R.C.; Zorn, G.L.; Brantley, L.H.; Kirklin, J.K. Use of expanded polytetrafluoroethylene pericardial substitute with ventricular assist devices. Ann. Thorac. Surg. 1993, 55, 181–183. [Google Scholar] [CrossRef]
- Sologaistua, E.; Lladó, A.; Guimerà, J.; Marín, M. Expanded polytetrafluoroethylene membrane for the prevention of peridural fibrosis after spinal surgery: A clinical study. Eur. Spine J. 1999, 8, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.S.; Eskandari, M.K. Accidental discovery: The polytetrafluoroethylene graft. Surgery 2012, 151, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, T.; Hayashi, R.; Ebihara, M.; Miyazaki, M.; Tomioka, T. Mucosal Defect Repair with a Polyglycolic Acid Sheet. Jpn. J. Clin. Oncol. 2012, 43, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Makadia, H.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Xu, G.; Lin, S. Functional Modification of Polypropylene. J. Macromol. Sci. Part C 1994, 34, 555–606. [Google Scholar] [CrossRef]
- Burdukova, E.; Li, H.; Ishida, N.; O’Shea, J.-P.; Franks, G.V. Temperature controlled surface hydrophobicity and interaction forces induced by poly (N-isopropylacrylamide). J. Colloid Interface Sci. 2010, 342, 586–592. [Google Scholar] [CrossRef]








| Biomaterial | Form | References |
|---|---|---|
| 2,2,6,6-tetramethylpiperidine-1-loxy (TEMPO)-oxidized nanocellulose | Hydrogel | [27] |
| Hyaluronic acid (HA)/ carboxymethyl cellulose (CMC) | Hydrogel | [28] |
| HA/CMC/Poly (D, L-lactide-co-glycolide) (PLGA) | Hydrogel | [29] |
| furfuryl hyaluronic acid | Film | [30] |
| Carboxymethyl chitosan (CMChi), CMC, collagen | Film | [31] |
| Chitosan (Chi) | Hydrogel | [32] |
| N, O-carboxymethyl chitosan (N, O-Chi)/oxidized regenerated cellulose (ORC) | Film | [33] |
| Polyethylene glycol (PEG)/Polylactic acid (PLA) | Film | [34] |
| silk fibroin protein (SFP)/ Polyvinyl alcohol (PVA), SFP/PEG, SFP/ polyethylene oxide (PEO) | Film | [35] |
| poly(anhydride-esters)/PEG | Hydrogel | [36] |
| poly (lactic-co-glycolic acid)-graft-polyvinylpyrrolidone/polyiodide (PLGA-g-PVP/I) | Film | [37] |
| PLGA/ poly(lactide-co-caprolactone) (PLCA)/poly (L-phenylalanine-co-p-dioxanone (PDPA) | Film | [38] |
| polypropylene (PP)/poly ε-caprolactone (PCL)/ ORC | Film | [39] |
| R-CPC copolymer (PCL−polypropylene glycol (PPG)−PEG−PPG−PCL) | Hydrogel | [40] |
| PVA | Hydrogel | [41] |
| poly(p-dioxanone-co-l-phenylalanine) (PDPA) | Film | [42] |
| Biomaterial | Product Name | Form | References |
|---|---|---|---|
| Oxidized regenerated cellulose (ORC) | Surgicel® | Film | [45] |
| ORC | Interceed® | Film | [46] |
| Carboxymethyl cellulose (CMC) | Seprafilm® | Film | [47] |
| Hyaluronic acid (HA)/CMC | Sepragel® | Solution | [48] |
| HA/CMC | Guardix-sol® | Hydrogel | [49] |
| HA/CMC | SeprasprayTM | Powder | [50] |
| HA derivate | Incert | Film | [51] |
| HA | Hyalobarrier® | Hydrogel | [52] |
| HA | Sepracoat | Solution | [53] |
| HA derivate | ACP gel | Solution | [54] |
| Ferric HA | Lubricoat | Solution | [55] |
| Ferric HA | Intergel® | Solution | [56] |
| HA derivate | Carbylan-SX | Film/spray | [57] |
| Icodextrin | Adept® | Solution | [58] |
| Dextran | Hyskon® | Hydrogel | [59] |
| Collagen | COVA+TM | Hydrogel | [60] |
| Polyethylene glycol (PEG) | SprayShieldTM | Spray | [61] |
| PEG | SprayGelTM | Spray | [62] |
| PEG | Coseal® | Hydrogel | [63] |
| PEG/CMC | Oxiplex® | Hydrogel | [64] |
| Polylactic acid (PLA)-PEG | REPEL-CV | Film | [65] |
| PLA | SurgiWrap® | Film | [66] |
| Polyvinyl alcohol (PVA)/CMC | A-part Gel® | Hydrogel | [67] |
| Poloxamer/alginate | Guardix-SG | Hydrogel | [68] |
| Expanded polytetrafluoroethylene (e-PTFE) | Gore®Preclude® | Film | [69] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Baek, S.; Kang, H.; Lee, D. Biomaterials to Prevent Post-Operative Adhesion. Materials 2020, 13, 3056. https://doi.org/10.3390/ma13143056
Park H, Baek S, Kang H, Lee D. Biomaterials to Prevent Post-Operative Adhesion. Materials. 2020; 13(14):3056. https://doi.org/10.3390/ma13143056
Chicago/Turabian StylePark, Heekyung, Seungho Baek, Hyun Kang, and Donghyun Lee. 2020. "Biomaterials to Prevent Post-Operative Adhesion" Materials 13, no. 14: 3056. https://doi.org/10.3390/ma13143056