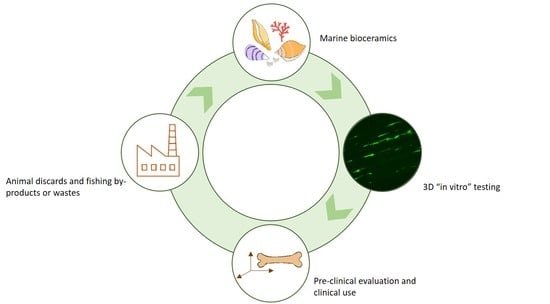
Current Stage of Marine Ceramic Grafts for 3D Bone Tissue Regeneration
Abstract
:1. Introduction
2. Bone Grafts: Limitations of Auto and Allografts
3. Bioceramic Xenografts: Mammalian Origin
Mammalian Xenografts in Research
4. Bioceramic Xenografts: Marine Origin
Marine Xenografts in Research
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Hoarau-Vechot, J.; Rafii, A.; Touboul, C. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology (Bethesda) 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, J.; Bertero, A.; Coccini, T.; Baderna, D.; Buzanska, L.; Caloni, F. Organoids are promising tools for species-specific in vitro toxicological studies. J. Appl. Toxicol. JAT 2019. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, P.; Erndt-Marino, J.; Chen, H.; Diaz-Quiroz, J.F.; Samavedi, S.; Hahn, M.S. A Bioengineered in vitro osteoarthritis model with tunable inflammatory environments indicates context-dependent therapeutic potential of human mesenchymal stem cells. Regen. Eng. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, R.I. Multi-Parametric Live Cell Microscopy of 3D Tissue Models; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Sánchez, M.; Landin, M. Drug-Loaded Biomimetic Ceramics for Tissue Engineering. Pharmaceutics 2018, 10, 272. [Google Scholar] [CrossRef]
- Crane, M.M.; Kaeberlein, M. The paths of mortality: How understanding the biology of aging can help explain systems behavior of single cells. Curr. Opin. Syst. Biol. 2018, 8, 25–31. [Google Scholar] [CrossRef]
- Lalzawmliana, V.A.A.; Mukherjee, P.; Chaudhuri, S.; Kundu, B.; Nandi, S.K.; Thakur, N.L. Marine organisms as a source of natural matrix for bone tissue engineering. Ceram. Int. 2019, 45A, 1469–1481. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- MediPoint. Available online: https://www.reportlinker.com/p02013605/MediPoint-Bone-Grafts-and-Substitutes-Global-Analysis-and-Market-Forecasts.html (accessed on 09 July 2019).
- Fernández-Hernández, O. Terapias para el hueso: Sustitutivos óseos. Mon. Act. Soc. Esp. Med. Cir. Pie Tobillo. 2017, 8, 45–53. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013, 25, S101–S103. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.A.; Cook, J.J. Bone graft substitutes and allografts for reconstruction of the foot and ankle. Clin. Podiatr. Med. Surg. 2009, 26, 589–605. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002. [Google Scholar] [CrossRef]
- Tatay Díaz, A.; Pérez Sánchez, J.M.; RiberaZabalbeascoa, J.; Cordero Fernández, J.A.; Mella Sousa, M. Sustitutos óseos. Revista de la Sociedad Andaluza de Traumatología y Ortopedia 2008, 26, 2–13. [Google Scholar]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Tortolini, P.; Rubio, S. Diferentes alternativas de rellenos óseos. Avances en Periodoncia e Implantología Oral 2012, 24, 133–138. [Google Scholar] [CrossRef]
- Müller, S.A.; Barg, A.; Vavken, P.; Valderrabano, V.; Müller, A.M. Autograft versus sterilized allograft for lateral calcaneal lengthening osteotomies: Comparison of 50 patients. Medicine (Baltimore) 2016, 95, e4343. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.S.; Ferreira, J.M.F. Synthetic and Marine-Derived Porous Scaffolds for Bone Tissue Engineering. Materials (Basel) 2018, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Tomford, W.W.; Bloem, R.M.; Mankin, H.J. Osteoarticular allografts. Acta Orthop. Belg. 1991, 57, 98–102. [Google Scholar] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Yeoh, J.C.; Taylor, B.A. Osseous Healing in Foot and Ankle Surgery with Autograft, Allograft, and Other Orthobiologics. Orthop. Clin. North Am. 2017, 48, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, D.; Singh, A. Radiation sterilization of tissue allografts: A review. World J. Radiol. 2016, 8, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Shehadi, J.A.; Elzein, S.M. Review of commercially available demineralized bone matrix products for spinal fusions: A selection paradigm. Surg. Neurol. Int. 2017, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, R.W.; Wood, G.W.; Sadasivian, K.K.; Stubbs, H.A.; Block, J.E. Grafting long bone fractures with demineralized bone matrix putty enriched with bone marrow: Pilot findings. Orthopedics 2006, 29, 939–941. [Google Scholar]
- Ajiboye, R.M.; Eckardt, M.A.; Hamamoto, J.T.; Plotkin, B.; Daubs, M.D.; Wang, J.C. Outcomes of Demineralized Bone Matrix Enriched with Concentrated Bone Marrow Aspirate in Lumbar Fusion. Int. J. Spine Surg. 2016, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Al Kayal, T.; Panetta, D.; Canciani, B.; Losi, P.; Tripodi, M.; Burchielli, S.; Ottoni, P.; Salvadori, P.A.; Soldani, G. Evaluation of the effect of a gamma irradiated DBM-pluronic F127 composite on bone regeneration in Wistar rat. PLoS ONE 2015, 10, e0125110. [Google Scholar] [CrossRef] [PubMed]
- MedTronic. Available online: https://www.medtronic.com/us-en/healthcare-professionals/products/spinal-orthopaedic/bone-grafting/infuse-bone-graft.html (accessed on 09 July 2019).
- Kim, S.H.; Shin, J.W.; Park, S.A.; Kim, Y.K.; Park, M.S.; Mok, J.M.; Yang, W.I.; Lee, J.W. Chemical, structural properties, and osteoconductive effectiveness of bone block derived from porcine cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 68, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kemper, N.; Davison, N.; Fitzpatrick, D.; Marshall, R.; Lin, A.; Mundy, K.; Cobb, R.R. Characterization of the mechanical properties of bovine cortical bone treated with a novel tissue sterilization process. Cell Tissue Bank. 2011, 12, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Supronowicz, P.; Zhukauskas, R.; York-Ely, A.; Wicomb, W.; Thula, T.; Fleming, L.; Cobb, R.R. Immunologic analyses of bovine bone treated with a novel tissue sterilization process. Xenotransplantation 2008, 15, 398–406. [Google Scholar] [CrossRef]
- Alayan, J.; Ivanovski, S. A prospective controlled trial comparing xenograft/autogenous bone and collagen-stabilized xenograft for maxillary sinus augmentation-Complications, patient-reported outcomes and volumetric analysis. Clin. Oral Implant. Res. 2018, 29, 248–262. [Google Scholar] [CrossRef]
- Serrano, C.A.; Castellanos, P.; Botticelli, D. Use of Combination of Allografts and Xenografts for Alveolar Ridge Preservation Procedures: A Clinical and Histological Case Series. Implant. Dent. 2018, 27, 467–473. [Google Scholar] [CrossRef]
- Shibuya, N.; Jupiter, D.C. Bone graft substitute: Allograft and xenograft. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef]
- Calvo Guirado, J.L.; Ramirez Fernandez, M.P.; Negri, B.; Delgado Ruiz, R.A.; Mate Sanchez de-Val, J.E.; Gomez-Moreno, G. Experimental model of bone response to collagenized xenografts of porcine origin (OsteoBiol(R) mp3): A radiological and histomorphometric study. Clin. Implant Dent. Relat. Res. 2013, 15, 143–151. [Google Scholar] [CrossRef]
- Orsini, G.; Scarano, A.; Piattelli, M.; Piccirilli, M.; Caputi, S.; Piattelli, A. Histologic and ultrastructural analysis of regenerated bone in maxillary sinus augmentation using a porcine bone-derived biomaterial. J. Periodontol. 2006, 77, 1984–1990. [Google Scholar] [CrossRef]
- Lee, J.H.; Yi, G.S. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodontal. Implant Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliani, L.; Andersson, P.; Lanza, M.; Nappo, A.; Verrocchi, D.; Volpe, S.; Sennerby, L. A collagenated porcine bone substitute for augmentation at Neoss implant sites: A prospective 1-year multicenter case series study with histology. Clin. Implant Dent. Relat. Res. 2012, 14, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Mayer, Y.; Ginesin, O.; Khutaba, A.; Machtei, E.E.; Zigdon Giladi, H. Biocompatibility and osteoconductivity of PLCL coated and noncoated xenografts: An in vitro and preclinical trial. Clin. Implant Dent. Relat. Res. 2018, 20, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Calvo-Guirado, J.L.; Negri, B.; Di Felice, R.; Covani, U. Volumetric analysis of remodelling pattern after ridge preservation comparing use of two types of xenografts. A multicentre randomized clinical trial. Clin. Oral Implants Res. 2016, 27, e105–e115. [Google Scholar] [CrossRef]
- de Azambuja Carvalho, P.H.; Dos Santos Trento, G.; Moura, L.B.; Cunha, G.; Gabrielli, M.A.C.; Pereira-Filho, V.A. Horizontal ridge augmentation using xenogenous bone graft-systematic review. Oral Maxillofac. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Ko, H.J.; Jang, J.H.; Kang, H.; Suh, J.Y. Increased new bone formation with a surface magnesium-incorporated deproteinized porcine bone substitute in rabbit calvarial defects. J. Biomed. Mater. Res. Part A 2012, 100, 834–840. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, R.; Li, Z.; Luo, X.; Huang, B.; Liu, Q.; Chen, Z. Contribution of the in situ release of endogenous cations from xenograft bone driven by fluoride incorporation toward enhanced bone regeneration. Biomater. Sci. 2018, 6, 2951–2964. [Google Scholar] [CrossRef]
- Cingolani, A.; Grottoli, C.F.; Esposito, R.; Villa, T.; Rossi, F.; Perale, G. Improving Bovine Bone Mechanical Characteristics for the Development of Xenohybrid Bone Grafts. Curr. Pharm. Biotechnol. 2018, 19, 1005–1013. [Google Scholar] [CrossRef]
- Arpag, O.F.; Damlar, I.; Altan, A.; Tatli, U.; Gunay, A. To what extent does hyaluronic acid affect healing of xenografts? A histomorphometric study in a rabbit model. J. Appl. Oral Sci. Rev. FOB 2018, 26, e20170004. [Google Scholar] [CrossRef]
- Santos Kotake, B.G.; Gonzaga, M.G.; Coutinho-Netto, J.; Ervolino, E.; de Figueiredo, F.A.T.; Issa, J.P.M. Bone repair of critical-sized defects in Wistar rats treated with autogenic, allogenic or xenogenic bone grafts alone or in combination with natural latex fraction F1. Biomed. Mater. (Bristol Engl.) 2018, 13, 025022. [Google Scholar] [CrossRef]
- Damlar, I.; Arpag, O.F.; Tatli, U.; Altan, A. Effects of Hypericum perforatum on the healing of xenografts: A histomorphometric study in rabbits. Br. J. Oral Maxillofac. Surg. 2017, 55, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, Q.; Liu, H.; Heng, B.C.; Peng, H.; Song, Y.; Yang, Z.; Deng, X. Osteoconductive effectiveness of bone graft derived from antler cancellous bone: An experimental study in the rabbit mandible defect model. Int. J. Oral Maxillofac. Surg. 2012, 41, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, U.; Stoffels, D.; Kierdorf, H. Element concentrations and element ratios in antler and pedicle bone of yearling red deer (Cervus elaphus) stags-a quantitative X-ray fluorescence study. Biol. Trace Elem. Res. 2014, 162, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.J. 2-the Diversity of Deer. In Deer Antlers; Goss, R.J., Ed.; Academic Press: San Diego, CA, USA, 1983; pp. 6–51. [Google Scholar]
- Gaspar-López, E.; Landete-Castillejos, T.; Estevez, J.; Ceacero, F.; Gallego, L.; García, A. Biometrics, Testosterone, Cortisol and Antler Growth Cycle in Iberian Red Deer Stags (Cervus elaphus hispanicus). Reprod. Domest. Anim. 2010, 45, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, M.; Song, L.; Wei, Y.; Lin, Y.; Liu, W.; Heng, B.C.; Peng, H.; Wang, Y.; Deng, X. Effects of compatibility of deproteinized antler cancellous bone with various bioactive factors on their osteogenic potential. Biomaterials 2013, 34, 9103–9114. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhang, X.; Xu, M.; Heng, B.C.; Dai, X.; Mo, X.; Wei, J.; Wei, Y.; Deng, X. Effects of deer age on the physicochemical properties of deproteinized antler cancellous bone: An approach to optimize osteoconductivity of bone graft. Biomed. Mater. (Bristol Engl.) 2015, 10, 035006. [Google Scholar] [CrossRef] [PubMed]
- Macha, I.J.; Ben-Nissan, B. Marine Skeletons: Towards Hard Tissue Repair and Regeneration. Mar. Drugs 2018, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Lai, W.F.; Jung, H.S. Evolving marine biomimetics for regenerative dentistry. Mar. Drugs 2014, 12, 2877–2912. [Google Scholar] [CrossRef]
- Fu, K.; Xu, Q.; Czernuszka, J.; Triffitt, J.T.; Xia, Z. Characterization of a biodegradable coralline hydroxyapatite/calcium carbonate composite and its clinical implementation. Biomed. Mater. (Bristol Engl.) 2013, 8, 065007. [Google Scholar] [CrossRef]
- Korovessis, P.; Koureas, G.; Zacharatos, S.; Papazisis, Z.; Lambiris, E. Correlative radiological, self-assessment and clinical analysis of evolution in instrumented dorsal and lateral fusion for degenerative lumbar spine disease. Autograft versus coralline hydroxyapatite. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2005, 14, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Coughlin, M.J.; Grimes, J.S.; Kennedy, M.P. Coralline hydroxyapatite bone graft substitute in hindfoot surgery. Foot Ankle Int. 2006, 27, 19–22. [Google Scholar] [CrossRef]
- Messina, A.M.; Marini, L.; Oh, D.S.; Marini, E. A Step-by-Step Procedure for Bone Regeneration Using Calcium Phosphate Scaffolds: From Site Preparation to Graft Placement. J. Craniofacial Surg. 2019, 30, 149–153. [Google Scholar] [CrossRef]
- Schwartz, O.; Binderman, I. Coral Bone Graft Substitute. U.S. Patent 8936638B2, 2011. [Google Scholar]
- Damien, E.; Revell, P.A. Coralline hydroxyapatite bone graft substitute: A review of experimental studies and biomedical applications. J. Appl. Biomater. Biomech. JABB 2004, 2, 65–73. [Google Scholar]
- Schopper, C.; Moser, D.; Sabbas, A.; Lagogiannis, G.; Spassova, E.; Konig, F.; Donath, K.; Ewers, R. The fluorohydroxyapatite (FHA) FRIOS Algipore is a suitable biomaterial for the reconstruction of severely atrophic human maxillae. Clin. Oral Implants Res. 2003, 14, 743–749. [Google Scholar]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Ben-Nissan, B.; Milev, A.; Vago, R. Morphology of sol-gel derived nano-coated coralline hydroxyapatite. Biomaterials 2004, 25, 4971–4975. [Google Scholar] [CrossRef]
- Sivakumar, M.; Manjubala, I. Preparation of hydroxyapatite/fluoroapatite-zirconia composites using Indian corals for biomedical applications. Mater. Lett. 2001, 50, 199–205. [Google Scholar] [CrossRef]
- Sethmann, I.; Luft, C.; Kleebe, H.J. Development of Phosphatized Calcium Carbonate Biominerals as Bioactive Bone Graft Substitute Materials, Part I: Incorporation of Magnesium and Strontium Ions. J. Funct. Biomater. 2018, 9, 69. [Google Scholar] [CrossRef]
- Du, B.; Gao, Y.; Deng, Y.; Zhao, Y.; Lai, C.; Guo, Z.; Rong, M.; Zhou, L. Local delivery of rhVEGF165 through biocoated nHA/coral block grafts in critical-sized dog mandible defects: A histological study at the early stages of bone healing. Int. J. Clin. Exp. Med. 2015, 8, 4940–4953. [Google Scholar]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; de Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng.C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Lopez-Alvarez, M.; Perez-Davila, S.; Rodriguez-Valencia, C.; Gonzalez, P.; Serra, J. The improved biological response of shark tooth bioapatites in a comparative in vitro study with synthetic and bovine bone grafts. Biomed. Mater. (Bristol Engl.) 2016, 11, 035011. [Google Scholar] [CrossRef]
- Lopez-Alvarez, M.; Vigo, E.; Rodriguez-Valencia, C.; Outeirino-Iglesias, V.; Gonzalez, P.; Serra, J. In vivo evaluation of shark teeth-derived bioapatites. Clin. Oral Implants Res. 2017, 28, e91–e100. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Garcia-Triñanes, P.; Echezarreta López, M.M.; Santoveña, A.; Landin, M. Mineralized alginate hydrogels using marine carbonates for bone tissue engineering applications. Carbohydr. Polym. 2018, 195, 235–242. [Google Scholar] [CrossRef]
- Maldonado, M.; Navarro, L.; Grasa, A.; Gonzalez, A.; Vaquerizo, I. Silicon uptake by sponges: A twist to understanding nutrient cycling on continental margins. Sci. Rep. 2011, 1, 30. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, M.; Lopez, E.; Stempfle, P.; Brendle, M.; Franke, L.; Guette, A.; Naslain, R.; Bourrat, X. Multiscale structure of sheet nacre. Biomaterials 2005, 26, 6254–6262. [Google Scholar] [CrossRef] [Green Version]
- Lamghari, M.; Huet, H.; Laurent, A.; Berland, S.; Lopez, E. A model for evaluating injectable bone replacements in the vertebrae of sheep: Radiological and histological study. Biomaterials 1999, 20, 2107–2114. [Google Scholar] [CrossRef]
- Balmain, J.; Hannoyer, B.; Lopez, E. Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction analyses of mineral and organic matrix during heating of mother of pearl (nacre) from the shell of the mollusc Pinctada maxima. J. Biomed. Mater. Res. 1999, 48, 749–754. [Google Scholar] [CrossRef]
- Zhang, G.; Brion, A.; Willemin, A.S.; Piet, M.H.; Moby, V.; Bianchi, A.; Mainard, D.; Galois, L.; Gillet, P.; Rousseau, M. Nacre, a natural, multi-use and timely biomaterial for bone graft substitution. J. Biomed. Mater. Res. Part A 2017, 105, 662–671. [Google Scholar] [CrossRef]
- Atlan, G.; Balmain, N.; Berland, S.; Vidal, B.; Lopez, E. Reconstruction of human maxillary defects with nacre powder: Histological evidence for bone regeneration. Comptes Rendus De l’Academie Des Sci. Ser. III Sci. De La Vie 1997, 320, 253–258. [Google Scholar] [CrossRef]
- Pascaretti-Grizon, F.; Libouban, H.; Camprasse, G.; Camprasse, S.; Mallet, R.; Chappard, D. The interface between nacre and bone after implantation in the sheep: A nanotomographic and Raman study. J. Raman Spectrosc. 2014, 45, 558–564. [Google Scholar] [CrossRef]
- Du, G.; Mao, A.; Yu, J.; Hou, J.; Zhao, N.; Han, J.; Zhao, Q.; Gao, W.; Xie, T.; Bai, H. Nacre-mimetic composite with intrinsic self-healing and shape-programming capability. Nat. Commun. 2019, 10, 800. [Google Scholar] [CrossRef]
- Enax, J.; Prymak, O.; Raabe, D.; Epple, M. Structure, composition, and mechanical properties of shark teeth. J. Struct. Biol. 2012, 178, 290–299. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Chemical and crystallographic events in the caries process. J. Dent. Res. 1990, 69, 567–574. [Google Scholar] [CrossRef]
- Ellingsen, J.E.; Thomsen, P.; Lyngstadaas, S.P. Advances in dental implant materials and tissue regeneration. Periodontol. 2000 2006, 41, 13–156. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, M.E.; Canalejo, A.; Herencia, C.; Martinez-Moreno, J.M.; Peralta-Ramirez, A.; Perez-Martinez, P.; Navarro-Gonzalez, J.F.; Rodriguez, M.; Peter, M.; Gundlach, K.; et al. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol. Dial. Transplant. Off. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2014, 29, 282–289. [Google Scholar] [CrossRef]

| Brand Name | Company | Compositional Details | Intended Use |
|---|---|---|---|
| GraftonTM DBM | Medtronic | DBM with bone fibers, instead of particles, to create a physical network with pathways for the cells | Spine, pelvis, extremities; augment dental intraosseous, oral, and cranio-maxillofacial defects |
| MagnifuseTM DBM Bone Graft | Medtronic | DBM fibers with surface-demineralized cortical chips in a resorbable mesh system | Spine, pelvis, extremities; Magnifuse™ II Bone Graft only for posterolateral spine and pelvis |
| DBX® Demineralized Bone Matrix | DePuy Synthes | DBM with sodium hyaluronate, natural derived material not of animal origin, biocompatible and biodegradable | Trauma, mandibular maxillary reconstruction, alveolar ridges, oral/maxillofacial/dental intraosseous defects, osseous defects in the cranium |
| Allomatrix® | Wright Medical Technology | DBM with cancellous chips containing surgical-grade calcium sulfate OsteosetTM | Skeletal system (i.e., extremities, spine, pelvis) |
| Ignite® | Wright Medical Technology | Biologic solutions that can be combined with bone marrow aspirate. Injectable | Rigid non unions. Soft and/or hard tissue repair |
| Alphagraft® DBM | Alphatec Spine | DBM reverse phase medium: thickens at body temperature resists irrigation to minimize the likelihood of migration from the surgical site | Designed to supplement other Alphatec Spine products |
| Stryker DBM | Stryker | DBM reverse phase media carrier and cancellous chips (putty plus) | Spinal procedures; oral and maxillofacial defects |
| io DBM | Stryker | Contains cancellous, cortical bone, and periosteum. Reverse phase medium carrier for gel, putty, and putty plus | Bone void filler or extender in posterolateral fusion procedures |
| AlloFuse® | AlloSource | DBM reverse phase medium: matrix easily moldable and with higher viscosity becoming thicker at warmer temperatures (human body) | General bone grafting applications in orthopedic and spinal fusion procedures |
| Accell Total Bone Matrix® | SeaSpine | 100% DBM processed from ground cortical bone | Skeletal system as bone graft extender in spine, extremities, and pelvis, or as a bone void filler in extremities and pelvis |
| InterGro® DBM | Zimmer Biomet | DBM Plus with porous granules of calcium carbonate with outer layer of calcium phosphate | Extremities and pelvis, spine, craniofacial defects, craniotomies not larger than 25 cm2 |
| Equivabone® | Zimmer Biomet | DBM with Etex nanocrystalline calcium phosphate technology | Bone voids or defects that are not intrinsic to the stability of the bony structure |
| Puros® Allograft | Zimmer Biomet | DBM in reverse phase medium. RPM Putty: with cancellous bone chips | Spinal fusion procedures and dental applications |
| StaGraft® Fiber | Zimmer Biomet | 100% cortical fiber DBM | Orthopedic, spinal, reconstructive craniomaxillofacial, periodontal bone grafting procedures |
| Opteform® | Exactech | DBM with cortical cancellous bone chips (osteoconductive) and gelatin carrier | Oncology, joints, foot and ankle, hand, sports medicine, trauma, long bone fractures |
| Optefil® | Exactech | DBM and gelatin carrier | Oncology, joints, foot and ankle, hand, sports medicine, trauma, long bone fractures |
| OsteoSelect® | Xtant Medical | OsteoSelect Plus: DMB putty with demineralized cortical chips | Standalone bone graft in spinal procedures |
| OsteoSponge® | Xtant Medical | OsteoSponge: DBM made from 100% cancellous bone with malleable properties and shape memory | Spinal fusion devices, in arthrodesis, or in fracture sites |
| Progenix Putty® | Umg Uysal Medikal | DBM (70%) with type I bovine collagen (11%) as carrier and alginate (19%, dry weight) | Small or large intrabony defects through a precise 1 mm delivery syringe |
| Progenix Plus® | Umg Uysal Medikal | DBM and demineralized cortical chips (60%) with type I bovine collagen (5%) as carrier and alginate (35%) by dry weight. Demineralized chips provide osteoconductivity and access to growth factors | Progenix® Plus contains bone chips of approximately 2–4 mm for use in small defects |
| H-GeninTM | Berkeley Advanced Biomaterials | DBM produced from ground cortical bone | Cranio-facial surgery, spinal fusion, hand and foot surgery, fracture repair, joint reconstruction |
| StimuBlast® | Arthrex | DBM in reverse phase medium giving moldable properties. It thickens up at body temperature and resists irrigation | Orthopaedic applications as filler for gaps or voids that are not intrinsic to the stability of the bony structure |
| Brand Name | Company | Compositional Details | Intended Use |
|---|---|---|---|
| Bio-Oss® | Geistlich | Bovine deproteinized bone | Periodontal, oral, and maxillofacial surgery |
| Orthoss® | Geistlich | Bovine derived bone substitute made from highly purified bone mineral | Filling of bone voids following trauma, reconstruction in orthopedics, and spinal surgery |
| Cerabone® | Botiss | Sintered bovine bone granules | Sinus lift, horizontal and vertical augmentation, ridge preservation, peri-implant defects, socket preservation, bone defect augmentation, periodontal intrabony defects, furcation defects |
| Endobon® | Biomet 3i LLC | Fully deproteinized bovine hydroxyapatite | Alveolar ridge augmentation, sinus elevation, filling bone defects after root resection, socket filling after tooth extraction |
| CopiOs® | Zimmer | Mineralized particulate cancellous bovine bone chips | Large and small bone defects |
| Bonefill® | Bioinnovations Inc | Natural bovine bone mineral extracted from bovine femur | Bone failure reconstructions where remodeling or bone neoformation is desired |
| InterOss® | Sigmagraft | Natural bovine hydroxyapatite | Bone defect filling |
| Apatos OsteoBiol® | Tecnoss | Heterologous cortico-cancellous bovine bone mix | Large maxillofacial bone defects, reconstruction, or corrections |
| GenOx Org® | Braumer | Lyophilized porous organic matrix extracted from the bovine cortical bone | Procedures of dental implant, Maxillofacial and bone surgery in general |
| Cerabone® | aap Implantate AG | Cancellous bovine bone | Permanent bone filling or reconstruction of aseptic bone defects |
| OssiGuide® | Collagen Matrix | Cancellous bovine bone | Filling bony voids or gaps of the skeletal system that are not intrinsic to the stability of the bony structure |
| THE Graft® | Purgo Biologics | Porcine cancellous granules | Extraction socket with intact socket, extraction socket with defective socket, minor bone, augmentation, major bone augmentation, sinus floor elevation, peri-implantitis |
| Gen-Os® | Tecnoss | Cortico-cancellous heterologous porcine or equine bone mix | Alveolar ridge preservation, lateral access maxillary sinus lift, dehiscence regeneration |
| MatrixOss® | Collagen Matrix | An organic porcine bone mineral with carbonate apatite structure | Bone filling |
| Sp-Block | Tecnoss | Equine cancellous bone | When a vertical gain in posterior mandible is required |
| BIO-GEN® BIO-GEN® MIX GEL BIO-GEN® PUTTY | BioTECK | Equine cortical bone or spongy bone | Bone regeneration surgery |
| Alpha-Bio’s Graft | Alpha-Bio Tec | Bovine cancellous bone + bioactive resorbable polymers | Open sinus floor augmentation, peridontal intrabony defects, peri-implant bony defects, socket preservation, vertical and horizontal bone augmentations |
| Bio-Oss Collagen® | Geistlich | Deproteinized bovine bone mineral small granules +10% porcine collagen | Extraction socket management, minor bone augmentation, periodontal regeneration |
| Gel 40 Putty mp3® | Tecnoss | Porcine or equine Cortico-cancellous heterologous bone mix + different proportions of Collagen gel | Lateral and crestal access sinus lift, deep and narrow peri-implant defects, three-wall intrabony defects, gingival recessions, post-extractive sockets, defects that present a self-contained cavity |
| OsteoBiol® GTO® | Tecnoss | Porcine or equine heterologous cortico-cancellous bone mix + OsteoBiol® TSV Gel | Horizontal augmentation procedures, socket preservation |
| Brand Name | Company | Compositional Details | Intended Use |
|---|---|---|---|
| BioCoral® | BioCoral Inc | Natural coral calcium carbonate wholly mineral bone graft substitute (99% calcium carbonate) [68] | Spinal surgery, tibial osteotomies, hip fractures, trephine hole replacement, fracture repair, osteoporosis; maxillocraniofacial; reconstructive and cosmetic surgery, bone defects due to loss of teeth or periodontal disease |
| Pro-Osteon® 200R | Zimmer Biomet | Coral calcium carbonate matrix covered by outer layer 2–10 µm thickness of calcium phosphate. Pore size 190–230 µm. Significant resorption in 6–18 months. | Indicated for bony voids or gaps that are not intrinsic to the stability of the skeletal system |
| Pro-Osteon® 500R | Zimmer Biomet | Coral calcium carbonate matrix covered by outer layer 2–10 µm thickness of calcium phosphate. Median pore diameter 435 µm. Significant resorption in 6–18 months. | Indicated to be gently packed into bony voids or gaps of the skeletal system (i.e., the extremities, spine, and pelvis) as for cervical fusion. |
| CoreBone® Coross® | Corebone/DSI, Dental Solutions Israel | Coral calcium carbonate crystals (>95%) as aragonite enriched with silicon, strontium, and other non-organic substances. Ca, Si, and Sr play important roles in bone mineralization | Maxillofacial and orthopedic indications. Interconnected porosity allows 3D generation of bone with high fusion rates, without loss of strength |
| Frios® Algipore® | Dentsply Sirona | Algae-derived hydroxyapatite by hydrothermal conversion of original calcium carbonate of the algae Corallina officialis [69]. Particle sizes 0.3–2 mm; pores of 5–10 μm [70] | Bone augmentation in the atrophic maxilla, periimplantitis lesions, alveolar ridge alteration following tooth extraction |
| Bone Grafts | Advantages | Limitations | Clinical Application |
|---|---|---|---|
| Autografts | Osteoinductivity Osteoconductivity Biocompatibility Bone mechanical properties | Postoperative morbidity Limited volume Increase in surgical time Additional anesthetic procedure required | Gold standard in trauma and dentistry when possible |
| Allografts | Osteoinductivity Osteoconductivity Biocompatibility High availability Reduced surgical time | Lower osteoinductive capacity than autografts Inferior mechanical properties Costly and laborious processing Potential risk of diseases transmission | Osteoarticular reconstructive surgery Foot and ankle surgery |
| Mammalian xenografts | Bone tissue physiological similarities Osteogenic and bone inductive properties Excellent support for bone colonization | Low tissue remodeling Stay mainly unaltered on the host bone Batch variability | Filling of bone voids following trauma, reconstruction in orthopedics, spinal surgery, periodontal, oral, and maxillofacial surgery |
| Marine xenografts | Interconnected porosity Hierarchical structure Osteoconductivity Availability in large quantities | Weak mechanical properties Fast degradation Batch variability | Bone fillers in non-load bearing regions |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Rodriguez, P.; López-Álvarez, M.; Serra, J.; González, P.; Landín, M. Current Stage of Marine Ceramic Grafts for 3D Bone Tissue Regeneration. Mar. Drugs 2019, 17, 471. https://doi.org/10.3390/md17080471
Diaz-Rodriguez P, López-Álvarez M, Serra J, González P, Landín M. Current Stage of Marine Ceramic Grafts for 3D Bone Tissue Regeneration. Marine Drugs. 2019; 17(8):471. https://doi.org/10.3390/md17080471
Chicago/Turabian StyleDiaz-Rodriguez, Patricia, Miriam López-Álvarez, Julia Serra, Pío González, and Mariana Landín. 2019. "Current Stage of Marine Ceramic Grafts for 3D Bone Tissue Regeneration" Marine Drugs 17, no. 8: 471. https://doi.org/10.3390/md17080471