A Review: The Bioactivities and Pharmacological Applications of Phellinus linteus
Abstract
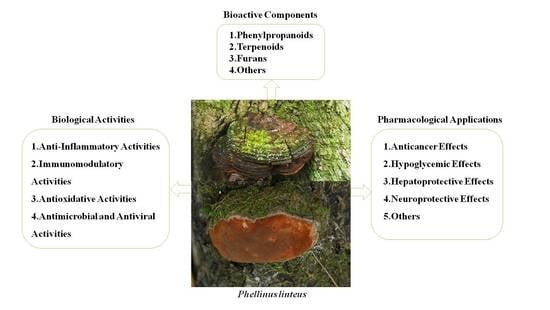
:1. Introduction
2. Bioactive Components
2.1. Phenylpropanoids
2.2. Terpenoids
2.3. Furans
2.4. Others
3. Biological Activities
3.1. Anti-Inflammatory Activities
3.2. Immunomodulatory Activities
3.3. Antioxidative Activities
3.4. Antimicrobial and Antiviral Activities
4. Pharmacological Applications
4.1. Anticancer Effects
4.1.1. The Anticancer Effect of Polysaccharides
4.1.2. The Anticancer Effect of Hispolon
4.1.3. The Anticancer Effect of Others
4.2. Hypoglycemic Effects
4.3. Hepatoprotective Effects
4.4. Neuroprotective Effects
4.5. Others
5. Safety
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| P. linteus | Phellinus linteus |
| 5-Fu | 5-Fluorouracil |
| ABCA1 | Adenosine 5′-triphosphate (ATP) binding cassette transporterA1 |
| ABCG1 | ATP-binding cassette G1 |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| ACOX1 | Acyl-CoA oxidase 1 |
| AKT | Protein kinase B |
| ALX | Alloxan |
| AMG | Aminoguanidine |
| AP-1 | Activator protein-1 |
| ARE | Antioxidant response element |
| BDEd | Bond dissociation enthalpy |
| CAT | Catalase |
| CDK2 | Cyclin-dependent kinases |
| CHOP | Kinase-homologous protein |
| DMN | Dimethylnitrosamine |
| DOX | Doxorubicin |
| Cox-2 | Cyclooxygenase-2 |
| CPT 11 | Camptothecin 11 |
| DBL | 3,4-dihydroxybenzalacetone |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DSS | Dextran sodium sulfate |
| EMT | epithelial–mesenchymal transition |
| EPA | Epalrestat |
| ERK | Extracellular regulated protein kinase |
| FAK | Focal adhesion kinase |
| FRAP | Ferric reducing ability of plasma |
| Gab2 | GRB2-associated-binding protein 2 |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| GSK3 β | Glycogen synthase kinase-3beta |
| HCC | Hepatocellular carcinoma |
| HFD | High-fat high-fructose diet |
| HO-1 | Heme oxygenase-1 |
| HSCs | Hepatic stellate cells |
| IC50 | Half maximal inhibitory concentration |
| IFN-γ | Interferon-gamma |
| IgE | Immunoglobulin E |
| IL-1β | Interleukin IL-1β |
| IL-6 | Interleukin IL-6 |
| iNOS | Nitric oxide synthase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LNCaP | Prostate cancer metastasized to lymph node |
| LPS | Lipopolysaccharides |
| LPA | Lipoteichoic acid |
| MAPKs | Mitogen-activated protein kinases |
| MDA | Malondialdehyde |
| MITF | Microphthalmia-associated transcription factor |
| MIC | Minimum inhibition concentration |
| MMP-9 | Matrix metalloproteinase-9 |
| α-MSH | α-melanocyte-stimulating hormone |
| NO | Nitric oxide |
| Nrf2 | NF-E2-related factor 2 |
| NF-kB | Nuclear factor kappa beta |
| PA | Palmitate |
| PARP | Poly ADP-Ribose Polymerase |
| PC-3 | Prostate cancer metastasized to bone |
| PCET | Proton-coupled electron transfer |
| PEMs | Peritoneal exudate macrophages |
| PI3K | Phosphoinositide-3-kinase |
| PKC | Protein kinase C |
| PKCδ | Phosphorylation of protein kinase Cδ |
| PPAR | Peroxisome proliferator-activated receptor |
| PPI | Protein–protein interaction |
| PTK | Protein tyrosine kinase |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| Syk | Spleen tyrosine kinase |
| TAA | Thioacetamide |
| TB | Tumor-bearing |
| TCM | Traditional Chinese medicine |
| TEAC | Trolox-equivalent antioxidant capacity |
| TGF-β | Transforming growth factor β |
| Th1 | T helper 1 |
| Th2 | T helper 2 |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
References
- Zhu, T.; Kim, S.H.; Chen, C.Y. A medicinal mushroom: Phellinus Linteus. Curr. Med. Chem. 2008, 15, 1330–1335. [Google Scholar] [CrossRef]
- Luan, Y.; Hou, W.S. Notes on Shen Nong’s Herbal Classic; People’s Military Medical Publishing House: Beijing, China, 2010; pp. 218–219. [Google Scholar]
- Su, J. New Compendium of Materia Medica; Anhui Science & Technology Publishing House: Hefei, China, 1981; p. 353. [Google Scholar]
- Li, S.H. Compendium of Materia Medica; People’s Medical Publishing House: Beijing, China, 2004; pp. 1713–1714. [Google Scholar]
- Dai, Y.C.; Xu, M.Q. Studies on the medicinal polypore, Phellinus baumii and its kin, Phellinus linteus. Mycotaxon 1998, 67, 191–200. [Google Scholar]
- Kim, B.C.; Jeon, W.K.; Hong, H.Y.; Jeon, K.B.; Hahn, J.H.; Kim, Y.M.; Numazawa, S.; Yosida, T.; Park, E.H.; Lim, C.J. The anti-inflammatory activity of Phellinus linteus (Berk. & M.A. Curt.) is mediated through the PKCδ/Nrf2/ARE signaling to up-regulation of heme oxygenase-1. J. Ethnopharmacol. 2007, 113, 240–247. [Google Scholar] [PubMed]
- Lin, C.J.; Lien, H.M.; Chang, H.Y.; Huang, C.L.; Liu, J.J.; Chang, Y.C.; Chen, C.C.; Lai, C.H. Biological evaluation of Phellinus linteus-fermented broths as anti-inflammatory agents. J. Biosci. Bioeng. 2014, 118, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J.; Park, H.J. Anti-inflammatory effect of Phellinus linteus grown on germinated brown rice on dextran sodium sulfate-induced acute colitis in mice and LPS-activated macrophages. J. Ethnopharmacol. 2014, 154, 311–318. [Google Scholar] [CrossRef]
- Yang, L.Y.; Shen, S.C.; Cheng, K.T.; Subbaraju, G.V.; Chien, C.C.; Chen, Y.C. Hispolon inhibition of inflammatory apoptosis through reduction of iNOS/NO production via HO-1 induction in macrophages. J. Ethnopharmacol. 2014, 156, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Chien, C.C.; Cheng, K.T.; Subbaraju, G.V.; Chen, Y.C. Hispolon suppresses LPS- or LTA-induced iNOS/NO production and apoptosis in BV-2 microglial cells. Am. J. Chin. Med. 2017, 45, 1649–1666. [Google Scholar] [CrossRef]
- Park, H.J. Anti-allergic and anti-inflammatory activity of Phellinus linteus grown on panax ginseng. Food Sci. Biotechnol. 2017, 26, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.; Deng, J.S.; Huang, S.S.; Li, P.Y.; Liang, Y.C.; Huang, G.J. 3, 4-dihydroxybenzal-acetone attenuates lipopolysaccharide-induced inflammation in acute lung injury via down-regulation of MMP-2 and MMP-9 activities through suppressing ROS-mediated MAPK and PI3K/AKT signaling pathways. Int. Immunopharmacol. 2017, 50, 77–86. [Google Scholar]
- Hu, T.; Lin, Q.L.; Guo, T.; Yang, T.; Zhou, W.H.; Deng, X.F.; Yan, J.K.; Luo, Y.; Ju, Y.Y.; Luo, F.J. Polysaccharide isolated from Phellinus linteus mycelia exerts anti-inflammatory effects via MAPK and PPAR signaling pathways. Carbohydr. Polym. 2018, 200, 487–497. [Google Scholar] [CrossRef]
- Xie, Z.L.; Wang, Y.; Huang, J.Q.; Qian, N.; Shen, G.Z.; Chen, L.H. Anti-inflammatory activity of polysaccharides from Phellinus linteus by regulating the NF-κB translocation in LPS-stimulated RAW264.7 macrophages. Int. Biol. Macromol. 2019, 129, 61–67. [Google Scholar] [CrossRef]
- Kim, G.Y.; Park, S.K.; Lee, M.K.; Lee, S.H.; Oh, Y.H.; Kwak, J.Y.; Yoon, S.; Lee, J.D.; Park, Y.M. Proteoglycan isolated from Phellinus linteus activates murine B lymphocytes via protein kinase C and protein tyrosine kinase. Int. Immunopharmacol. 2003, 3, 1281–1292. [Google Scholar] [CrossRef]
- Oh, G.S.; Lee, M.S.; Pae, H.K.; Kwon, J.; Lee, S.S.; Jeong, J.G.; Shin, M.K.; Kwon, T.O.; Chung, H.T. Effects of oral administration of Phellinus Linteus on the production of Th1- and Th2-type cytokines in mice. Immunopharmacol. Immunotoxicol. 2006, 28, 281–293. [Google Scholar] [CrossRef]
- Kim, G.Y.; Lee, J.Y.; Lee, J.O.; Rru, C.H.; Choi, B.T.; Jeong, Y.K.; Lee, K.W.; Jeong, S.C.; Choi, Y.H. Partial characterization and imunostimulatory effect of a novel polysaccharide–protein complex extracted from Phellinus Linteus. Biosci. Biotechnol. Biochem. 2006, 70, 1218–1226. [Google Scholar] [CrossRef]
- Suabjakyong, P.; Nishimura, K.; Toida, T.; Van Griensven, L.J. Structural characterization and immunomodulatory effects of polysaccharides from Phellinus linteus and Phellinus igniarius on the IL-6/IL-10 cytokine balance of the mouse macrophage cell lines (RAW264.7). Food. Funct. 2015, 6, 2834–2844. [Google Scholar] [CrossRef]
- Lin, C.J.; Lien, H.M.; Lin, H.J.; Huang, C.L.; Kao, M.C.; Chen, Y.A.; Wang, C.K.; Chang, H.Y.; Chang, Y.K.; Wu, H.S.; et al. Modulation of T cell response by Phellinus linteus. J. Biosci. Bioeng. 2016, 121, 84–88. [Google Scholar] [CrossRef]
- Gao, C.P.; Zhong, L.F.; Jiang, L.P.; Geng, C.Y.; Yao, X.F.; Cao, J. Phellinus linteus mushroom protects against tacrine-induced mitochondrial impairment and oxidative stress in HepG2 cells. Phytomedicine 2013, 20, 705–709. [Google Scholar] [CrossRef]
- Wang, Z.B.; Pei, J.J.; Ma, H.L.; Cai, P.F.; Yan, J.K. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohydr. Polym. 2014, 109, 49–55. [Google Scholar] [CrossRef]
- Chen, W.; Shen, Y.; Su, H.M.; Zheng, X.D. Hispidin derived from Phellinus linteus affords protection against acrylamide-induced oxidative stress in Caco-2 cells. Chem. Biol. Inter. 2014, 219, 83–89. [Google Scholar] [CrossRef]
- Anouar, E.H.; Shah, S.A.; Hassan, N.B.; Moussaoui, N.E.; Ahmad, R.; Zulkefeli, M.; Weber, J.F. Antioxidant activity of hispidin oligomers from medicinal fungi: ADFT study. Molecules 2014, 19, 3489–3507. [Google Scholar] [CrossRef]
- Lee, M.S.; Hwang, B.S.; Lee, I.K.; Seo, G.S.; Yun, B.S. Chemical constituents of the culture broth of Phellinus linteus and their antioxidant activity. Mycobiology 2015, 43, 43–48. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, Y.Y.; Wang, Z.B.; Ma, H.L.; Pei, J.J.; Wu, J.Y. Structure and antioxidative property of a polysaccharide from an ammonium oxalate extract of Phellinus linteus. Inter. J. Biol. Macromol. 2016, 91, 92–123. [Google Scholar] [CrossRef]
- Kong, S.Z.; Li, J.C.; Li, S.D.; Liao, M.N.; Li, C.P.; Zheng, P.J.; Guo, M.H.; Tan, W.X.; Zheng, Z.H.; Hu, Z. Anti-aging effect of chitosan oligosaccharide on D-galactose-induced subacute aging in mice. Mar. Drugs 2018, 16, 181. [Google Scholar] [CrossRef]
- Hur, J.M.; Yang, C.H.; Han, S.H.; Lee, S.H.; You, Y.O.; Park, J.C.; Kim, K.J. Antibacterial effect of Phellinus linteus against methicillin-resistant Staphylococcus aureus. Fitoterapia 2004, 75, 603–605. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kwon, Y.J.; Sohn, M.J.; Seok, S.J.; Kim, W.G. Phellinstatin, a new inhibitor of enoyl-ACP reductase produced by the medicinal fungus Phellinus linteus. Bioorg. Med. Chem. Lett. 2011, 21, 1716–1718. [Google Scholar] [CrossRef]
- Ota, K.; Yamazaki, I.; Saigoku, T.; Fukui, M.; Miyata, T.; Kamaike, K.; Shirahata, T.; Mizuno, F.; Asada, Y.; Hirotani, M.; et al. Phellilane L, sesquiterpene metabolite of Phellinus linteus: Isolation, structure elucidation, and asymmetric total synthesis. J. Org. Chem. 2017, 82, 12377–12385. [Google Scholar] [CrossRef]
- Shirahata, T.; Ino, C.; Mizuno, F.; Asada, Y.; Hirotani, M.; Petersson, G.A.; Ōmura, S.; Yoshikawa, T.; Kobayashi, Y. γ-Ionylidene-type sesquiterpenoids possessing antimicrobial activity against Porphyromonas gingivalis from Phellinus linteus and their absolute structure determination. J. Antibiot. 2017, 70, 695–698. [Google Scholar] [CrossRef]
- Yeo, W.H.; Hwang, E.I.; So, S.H.; Lee, S.M. Phellinone, a new furanone derivative from the Phellinus linteus KT&G PL-2. Arch. Pharm. Res. 2007, 30, 924–926. [Google Scholar]
- Ichinohe, T.; Ainai, A.; Nakamura, T.; Akiyama, Y.; Maeyama, J.J.; Odagiri, T.; Tashiro, M.; Takahashi, H.; Sawa, H.; Tamura, S.I.; et al. Induction of cross-protective immunity against influenza a virus H5N1 by an intranasal vaccine with extracts of mushroom mycelia. J. Med. Virol. 2010, 82, 128–137. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, M.S.; Lee, S.W.; Lee, I.K.; Seo, G.S.; Choi, H.J.; Yun, B.S. Neuraminidase inhibitors from the fermentation broth of Phellinus linteus. Mycobiology 2014, 42, 189–192. [Google Scholar] [CrossRef]
- Zhong, S.; Ji, D.F.; Li, Y.G.; Lin, T.B.; Lv, Z.Q.; Chen, H.P. Activation of P27kip1-cyclin D1/E-CDK2 pathway by polysaccharide from Phellinus linteus leads to S-phase arrest in HT-29 cells. Chem. Biol. Interact. 2013, 206, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Ji, D.F.; Zhong, S.; Liu, P.G.; Lv, Z.Q.; Zhu, J.X.; Chen, J.E.; Chen, H.P. Polysaccharide from Phellinus linteus induces S-phase arrest in HepG2 cells by decreasing calreticulin expression and activating the P27kip1–cyclin A/D1/E–CDK2 pathway. J. Ethnopharmacol. 2013, 150, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.J.; Wang, Z.B.; Ma, H.L.; Yan, J.K. Structural features and antitumor activity of a novel polysaccharide from alkaline extract of Phellinus linteus mycelia. Carbohyd. Polym. 2015, 115, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.X.; Zhu, H.; Hu, Q.M.; Liu, Y.Y.; Zhao, S.M.; Peng, N.; Liang, Y.X. A novel polysaccharide from mycelia of cultured Phellinus linteus displays antitumor activity through apoptosis. Carbohyd. Polym. 2015, 124, 90–97. [Google Scholar] [CrossRef]
- Chai, Y.Y.; Wang, G.B.; Fan, L.L.; Zhao, M. A proteomic analysis of mushroom polysaccharide-treated HepG2 cells. Sci. Rep. 2016, 6, 23565–23576. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Q.; Ganapathy, S.; Shen, L.; Peng, B.; Kim, S.H.; Makriyannis, A.; Chen, C.Y. A lethal synergy induced by phellinus linteus and camptothecin11 in colon cancer cells. Oncotarget 2018, 9, 6308–6319. [Google Scholar] [CrossRef]
- Jeon, T.I.; Jung, C.H.; Cho, J.Y.; Park, D.K.; Moon, J.H. Identification of an anticancer compound against HT-29 cells from Phellinus linteus grown on germinated brown rice. Asian Pac. J. Trop. Biomed. 2013, 3, 785–789. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, H.Y.; Deng, J.S.; Chen, J.J.; Huang, S.S.; Lin, I.H.; Kuo, W.L.; Chao, W.; Huang, G.J. Hispolon from Phellinus linteus induces G0/G1 cell cycle arrest and apoptosis in NB4 human leukaemia cells. Am. J. Chin. Med. 2013, 41, 1439–1457. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lee, S.M.; Lin, C.C.; Liu, C.Y. Hispolon decreases melanin production and induces apoptosis in melanoma cells through the downregulation of tyrosinase and microphthalmia-associated transcription factor (MITF) expressions and the activation of Caspase-3, -8 and -9. Int. J. Mol. Sci. 2014, 15, 1201–1215. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Chien, S.Y.; Chou, Y.E.; Chen, C.J.; Chen, J.; Chen, M.K. Hispolon from Phellinus linteus possesses mediate caspases activation and induces human nasopharyngeal carcinomas cells apoptosis through ERK1/2, JNK1/2 and p38 MAPK pathway. Phytomedicine 2014, 21, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.H.; Jang, S.Y.; Cho, I.H.; Hong, D.; Jung, B.; Park, M.J.; Kim, J.K. Hispolon inhibits the growth of estrogen receptor positive human breast cancer cells through modulation of estrogen receptor alpha. Biochem. Biophys. Res. Commun. 2015, 463, 917–922. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.C.; PARK, B. Hispolon from Phellinus linteus induces apoptosis and sensitizes human cancer cells to the tumor necrosis factor-related apoptosis-inducing ligand through upregulation of death receptors. Oncol. Rep. 2016, 35, 1020–1026. [Google Scholar] [CrossRef]
- Hong, D.; Park, M.; Jang, E.H.; Jung, B.; Kim, N.J.; Kim, J.H. Hispolon as an inhibitor of TGF-β-induced epithelial-mesenchymal transition in human epithelial cancer cells by co- regulation of TGF-β-Snail/Twist axis. Oncol. Lett. 2017, 14, 4866–4872. [Google Scholar] [CrossRef]
- Arcella, A.; Oliva, M.A.; Sanchez, M.; Staffieri, S.; Esposito, V.; Giangaspero, F.; Cantore, G. Effects of hispolon on glioblastoma cell growth. Environ. Toxicol. 2017, 32, 2113–2123. [Google Scholar] [CrossRef]
- Park, H.J. CARI III inhibits tumor growth in a melanoma-bearing mouse model through induction of G0/G1 cell cycle arrest. Molecules 2014, 19, 14383–14395. [Google Scholar] [CrossRef]
- Konno, S.; Chu, K.; Feuer, N.; Phillips, J.; Choudhury, M. Potent anticancer effects of bioactive mushroom extracts (Phellinus linteus) on a variety of human cancer cells. J. Clin. Med. Res. 2015, 7, 76–82. [Google Scholar] [CrossRef]
- Lee, W.Y.; Hsu, K.F.; Chiang, T.A.; Chen, C.J. Phellinus Linteus extract induces autophagy and synergizes with 5-Fluorouracil to inhibit breast cancer cell growth. Nutr. Cancer 2015, 67, 275–284. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.B.; Lee, S.J.; Song, M.J. Phellinus linteus grown on germinated brown rice increases cetuximab sensitivity of KRAS-mutated colon cancer. Int. J. Mol. Sci. 2017, 18, 1746. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, H.K.; Kang, C.M.; Lee, W.J. Potential impact of Phellinus linteus on adherence to adjuvant treatment after curative resection of pancreatic ductal adenocarcinoma: Outcomes of a propensity score–matched analysis. Integr. Cancer Ther. 2019, 18, 1–9. [Google Scholar] [CrossRef]
- Chao, W.; Deng, J.S.; Li, P.Y.; Liang, Y.C.; Huang, G.J. 3,4-Dihydroxybenzalactone suppresses human non-small cell lung carcinoma cells metastasis via suppression of epithelial to mesenchymal transition, ROS-mediated PI3K/AKT/MAPK/MMP and NFκB signaling pathways. Molecules 2017, 22, 537. [Google Scholar] [CrossRef]
- Huang, S.C.; Kuo, P.C.; Hung, H.Y.; Pan, T.L.; Chen, F.A.; Wu, T.S. Ionone derivatives from the mycelium of Phellinus linteus and the inhibitory effect on activated rat hpatic stellate cells. Int. J. Mol. Sci. 2016, 17, 681. [Google Scholar] [CrossRef] [PubMed]
- Chandimali, N.; Huynh, D.L.; Jin, W.Y.; KWON, T. Combination effects of hispidin and gemcitabine via inhibition of stemness in pancreatic cancer stem cells. Anticancer Res. 2018, 38, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, A.; Itoh, S.; Tanaka, R.; Kato, S.; Haruna, M.; Kishimoto, K.; Hirayama, H.; Goda, Y.; Mizumasa, H.; Ogihara, Y. Identification of novel substituted fused aromatic compounds, meshimakobnol A and B, from natural Phellinus linteus fruit body. Tetrahedron. Lett. 2004, 45, 5931–5933. [Google Scholar] [CrossRef]
- Kojima, K.; Ohno, T.; Inoue, M.; Mizukami, H.; Nagatsu, A. Phellifuropyranone A: A new furopyranone compound isolated from fruit bodies of wild Phellinus linteus. Chem. Pharm. Bull. 2008, 56, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kang, J.S.; Kim, J.Y.; Park, S.K.; Kim, H.S.; Lee, Y.J.; Yun, J.; Hong, J.T.; Kim, Y.S.; Han, S.B. Evaluation of antidiabetic activity of polysaccharide isolated from Phellinus linteus in non-obese diabetic mouse. Int. Immunopharmacol. 2010, 10, 72–78. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.S.; Kim, Y.R.; Jung, W.C.; Lee, K.E.; Lee, S.Y.; Hong, E.K. Hispidin isolated from Phellinus linteus protects against hydrogen peroxide–induced oxidative stress in pancreatic MIN6N b-cells. J. Med. Food 2011, 14, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liao, Z.S.; Wu, X.Q.; Liu, Y.L.; Liu, X.Y.; Lin, Z.X.; Huang, Y.F.; Liu, B. Isolation, purification, and structural features of a polysaccharide from Phellinus linteus and its hypoglycemic effect in alloxan-induced diabetic mice. J. Food. Sci. 2014, 79, 1002–1010. [Google Scholar]
- Park, J.M.; Lee, J.S.; Song, J.E.; Sim, Y.C.; Ha, S.J.; Hong, E.K. Cytoprotective effect of hispidin against palmitate-induced lipotoxicity in C2C12 myotubes. Molecules 2015, 20, 5456–5467. [Google Scholar] [CrossRef]
- Song, T.Y.; Yang, N.C.; Chen, C.L.; Thi, T.L. Protective effects and possible mechanisms of ergothioneine and hispidin against methylglyoxal-induced injuries in rat pheochromocytoma cells. Oxid. Med. Cell. Longev. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, S.J.; Man-Fan Wan, J.; Gui, L.F.; Ruan, M.C.; Li, N.; Zhang, H.Y.; Liu, Z.G.; Wang, H.L. Polysaccharides extracted from Phellinus linteus ameliorate high-fat high-fructose diet induced insulin resistance in mice. Carbohydr. Polym. 2018, 200, 144–153. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, C.R.; Li, J.S.; Mei, Y.X.; Liang, Y.X. Hypoglycemic and hypolipidemic effects of Phellinus Linteus mycelial extract from solid-state culture in a rat model of type 2 diabetes. Nutrients 2019, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kang, Y.H.; Jung, J.Y.; Kang, I.J.; Han, S.N.; Chung, J.S.; Shin, H.K.; Lim, S.S. Inhibitory constituents of aldose reductase in the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008, 31, 765–768. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jang, Y.H.; Jung, J.Y.; Lee, S.; Ohuchi, K.; Shin, K.H.; Kang, I.J.; Park, J.H.; Shin, H.K.; Lim, S.S. Protein glycation inhibitors from the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008, 31, 1968–1972. [Google Scholar] [CrossRef]
- Kang, H.S.; Choi, J.H.; Cho, W.K.; Park, J.C.; Choi, J.S. A sphingolipid and tryrosinase inhibitors from the fruiting body of Phellinus linteus. Arch. Pharm. Res. 2004, 27, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, G.; Park, H.J.; Jiang, P.P.; Sit, W.H.; Van Griensven, L.J.; Wan, J.M. Protective effect of Phellinus linteus polysaccharide extracts against thioacetamide-induced liver fibrosis in rats: A proteomics analysis. Chin. Med. 2012, 7, 23–32. [Google Scholar] [CrossRef]
- Huang, S.C.; Wang, P.W.; Kuo, P.C.; Hung, H.Y.; Pan, T.L. Hepatoprotective principles and other chemical constituents from the mycelium of Phellinus linteus. Molecules 2018, 23, 1705. [Google Scholar] [CrossRef]
- Griensven, L.J.V.; Verhoeven, H.A. Phellinus linteus polysaccharide extracts increase the mitochondrialmembrane potential and cause apoptotic death of THP-1 monocytes. Chin. Med. 2013, 8, 25–37. [Google Scholar] [CrossRef]
- Choi, D.J.; Cho, S.; Seo, J.Y.; Lee, H.B.; Park, Y. Neuroprotective effects of the Phellinus linteus ethyl acetate extract against H2O2-induced apoptotic cell death of SK-N-MC cells. Nutr. Res. 2016, 36, 31–43. [Google Scholar] [CrossRef]
- Lemieszek, M.; Rzeski, W. Anticancer properties of polysaccharides isolated from fungi of the basidiomycetes class. Contemp. Oncol. 2012, 4, 285–289. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohyd. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Sliva, D. Medicinal mushroom Phellinus linteus as an alternative cancer therapy (Review). Exp. Ther. Med. 2010, 1, 407–411. [Google Scholar] [CrossRef]
- Lee, I.K.; Yun, B.S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Hsieh, P.W.; Wu, J.B.; Wu, Y.C. Chemistry and biology of Phellinus linteus. BioMedicine 2013, 3, 106–113. [Google Scholar] [CrossRef]
- Chen, H.; Tian, T.; Miao, H.; Zhao, Y.Y. Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: A review. Fitoterapia 2016, 113, 6–26. [Google Scholar] [CrossRef]
- Kim, D.E.; Kim, B.; Shin, H.S.; Kwon, H.J.; Park, E.S. The protective effect of hispidin against hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblast cells through Akt/GSK-3β and ERK1/2 signaling pathway. Exp. Cell Res. 2014, 327, 264–275. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, H.J.; Kim, B.W. Protective effect of 4-(3,4-Dihydroxyphenyl)-3-Buten-2-One from Phellinus linteus on naproxen-induced gastric antral ulcers in rats. J. Microbiol. Biotechnol. 2016, 26, 823–828. [Google Scholar] [CrossRef]
- Min, B.S.; Yun, B.S.; Lee, H.K.; Jung, H.J.; Jung, H.A.; Choi, J.S. Two novel furan derivatives from Phellinus linteus with anticomplement activity. Bioorg. Med. Chem. Lett. 2006, 16, 3255–3257. [Google Scholar] [CrossRef]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841–805855. [Google Scholar] [CrossRef]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food. Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef]
- Joseph, T.P.; Chanda, W.; Padhiar, A.A.; Batool, S.; Qun, S.L.; Zhong, M.T.; Huang, M. A preclinical evaluation of the antitumor activities of edible and medicinal mushrooms: A molecular insight. Integr. Cancer Ther. 2017, 17, 200–209. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.H.; Ding, Z.Y. Bioactive mushroom polysaccharides: A review on monosaccharide composition, biosynthesis and regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Tech. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Necyk, C.; Zubach-Cassano, L. Natural health products and diabetes: A practical review. Can. J. Diabetes 2017, 41, 642–647. [Google Scholar] [CrossRef]
- Cai, L.L.; Wan, D.W.; Yi, F.L.; Luan, L.B. Purification, preliminary characterization and hepatoprotective effects of polysaccharides from dandelion root. Molecules 2017, 22, 1409–1423. [Google Scholar]
- Kim, Y.N.; Kim, M.S.; Chun, S.S.; Choi, J.H. Effect of Phellius linteus water extract on benign prostatic hyperplasia. Nutr. Res. Pract. 2013, 7, 172–177. [Google Scholar] [CrossRef]
- Li, X.H.; Li, Y.; Cheng, Z.Y.; Cai, X.G.; Wang, H.M. The effects of Phellinus linteus polysaccharide extracts on cholesterol efflux in oxidized low-density lipoprotein–loaded THP-1 macrophages. J. Investig. Med. 2015, 63, 752–757. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Choo, Y.M.; Cho, Y.S. Anti-pigmentation effects of eight Phellinus linteus-fermented traditional crude herbal extracts on brown guinea pigs of ultraviolet B-induced hyperpigmentation. J. Microbiol. Biotechnol. 2018, 28, 375–380. [Google Scholar] [CrossRef]






| No. | Compound Name | Classification | Origin | Biological Activity | References |
|---|---|---|---|---|---|
| 1 | 3,4-Dihydroxybenzalacetone | Phenylpropanoid | Fruiting body of P. linteus | Anti-inflammatory, antitumor | [12,53] |
| 2 | Hispidin | Phenylpropanoid | Mycelium of P. linteus | Antioxidative, antitumor, antidiabetic, cardioprotective | [22,23,55,59,61,62,78] |
| 3 | Inotilone | Phenylpropanoid | Mycelium of P. linteus | Antioxidative, antiviral | [24,33] |
| 4 | 4-(3,4-Dihydroxyphenyl)-3-buten-2-one | Phenylpropanoid | Mycelium of P. linteus | Antioxidative, antiviral, gastroprotective | [24,33,79] |
| 5 | Phellinstatin | Phenylpropanoid | Mycelium of P. linteus | Antimicrobial | [28] |
| 6 | Meshimakobnol A | Phenylpropanoid | Fruiting body of P. linteus | Antitumor | [56,57] |
| 7 | Meshimakobnol B | Phenylpropanoid | Fruiting body of P. linteus | Antitumor | [56,57] |
| 8 | Phellifuropyranone A | Phenylpropanoid | Fruiting body of P. linteus | Antitumor | [57] |
| 9 | Phelligridimer A | Phenylpropanoid | Fruiting body of P. linteus | Antidiabetic | [65] |
| 10 | Hypholomine B | Phenylpropanoid | Fruiting body of P. linteus | Antidiabetic | [65] |
| 11 | Interfungin A | Phenylpropanoid | Fruiting body of P. linteus | Antidiabetic | [65] |
| 12 | Protocatechualdehyde | Phenylpropanoid | Fruiting body of P. linteus | Antidiabetic | [65,66] |
| 13 | Davallialactone | Phenylpropanoid | Fruiting body of P. linteus | Antidiabetic | [65,66] |
| 14 | Inoscavin A | Phenylpropanoid | Fruiting body of P. linteus | Antidiabetic | [65,66] |
| 15 | Caffeic acid | Phenylpropanoid | Mycelium of P. linteus | Antioxidative | [24] |
| 16 | Phellilane L | Terpenoid | Mycelium of P. linteus | Antimicrobial | [29] |
| 17 | Phellidene E | Terpenoid | Mycelium of P. linteus | Antimicrobial | [30] |
| 18 | (−)-trans-γ-Monocyclofarnesol | Terpenoid | Mycelium of P. linteus | Antimicrobial | [30] |
| 19 | Atractylenolide I | Terpenoid | Mycelium of P. linteus | Antitumor | [40] |
| 20 | Phellinulin D | Terpenoid | Mycelium of P. linteus | Hepatoprotective | [69] |
| 21 | Phellinulin E | Terpenoid | Mycelium of P. linteus | Hepatoprotective | [69] |
| 22 | Phellinulin F | Terpenoid | Mycelium of P. linteus | Hepatoprotective | [69] |
| 23 | Phellinulin G | Terpenoid | Mycelium of P. linteus | Hepatoprotective | [69] |
| 24 | Phellinulin H | Terpenoid | Mycelium of P. linteus | Hepatoprotective | [69] |
| 25 | Phellinulin I | Terpenoid | Mycelium of P. linteus | Hepatoprotective | [69] |
| 26 | Phellinulin K | Terpenoid | Mycelium of P. linteus | Hepatoprotective | [69] |
| 27 | Phellinulin M | Terpenoid | Mycelium of P. linteus | Hepatoprotective | [69] |
| 28 | Phellinulin N | Terpenoid | Mycelium of P. linteus | Hepatoprotective | [69] |
| 29 | Phellinone | Furan | Mycelium of P. linteus | Antimicrobial | [31] |
| 30 | Phellinusfuran A | Furan | Fruiting body of P. linteus | Anti-complementary | [80] |
| 31 | Phellinusfuran B | Furan | Fruiting body of P. linteus | Anti-complementary | [80] |
| 32 | 5-Hydroxymethyl-2-furaldehyde | Furan | Fruiting body of P. linteus | Antidiabetic | [67] |
| 33 | Ellagic acid | Other | Fruiting body of P. linteus | Antidiabetic | [65] |
| 34 | Phellilin C | Other | Mycelium of P. linteus | Hepatoprotective | [69] |
| 35 | γ-Ionylideneacetic acid | Other | Mycelium of P. linteus | Hepatoprotective | [69] |
| 36 | Phellinulin A | Other | Mycelium of P. linteus | Hepatoprotective | [54,69] |
| 37 | Hispolon | Other | Fruiting body and mycelium of P. linteus | Antitumor, anti-inflammatory | [7,9,41,42,43] |
| 38 | Ergothioneine | Other | Mycelium of P. linteus | Antidiabetic | [62] |
| No. | Polysaccharides, Hispolon, or Others | Model | Dose | Results | References |
|---|---|---|---|---|---|
| 1 | Polysaccharides | Human colorectal carcinoma (HT29) cells | 25, 50, 100, 200 μg/mL | Polysaccharides had an inhibitory effect on the proliferation of HT29 cells by blocking the cell cycle in S-phase and downregulation of the expression of cyclin D1, cyclin E, and cyclin-dependent kinases (CDK2), and increased upregulation of the expression of P27kip1 in vitro. | [34] |
| 2 | Polysaccharides | HepG2 cells | 50, 100, 200 μg/mL | Polysaccharides had an inhibitory effect on the proliferation of HepG2 cells by blocking tumor cells going into the S-phase, upregulating the expression of P27kip1 and cyclin A, and downregulating the expression of calreticulin, cyclin D1, cyclin E, and CDK2 in vitro. | [35] |
| 3 | Polysaccharide (PL-N1) | HepG2 cells | 50, 100, 200 μg/mL | Polysaccharides had an inhibitory effect on the growth of HepG2 cells. | [36] |
| 4 | Polysaccharide (PLPS1 and PLPS-2) | S-180 sarcoma cells | 25 μg/mL | Polysaccharides exhibited strong anticancer activity against S-180 sarcoma cells. | [37] |
| 5 | Polysaccharides | HepG2 cells | 0.5–2.0 mg/mL | These results provided information on significant proteins of hepatocellular carcinoma (HCC). | [38] |
| 6 | Polysaccharides | Colon cancer HCT116 and HT29 cells | 50 μg/mL | Polysaccharides could reduce the side effects of camptothecin 11 (CPT 11) (10 ng/ml) when they were used as drug combinations. | [39] |
| 7 | Ethanol extracts, ethyl acetate extracts, n-hexane fractions | HT29 cells | 149.9, 69.8, and 77.8 µg/mL | Fractions of ethanol, ethyl acetate, and n-hexane inhibited the growth of HT29 cell lines. | [40] |
| 8 | Hispolon | NB4 human leukemia cells | 10 μg/mL | Hispolon inhibited cell proliferation and promoted cell apoptosis through blocking G/G1 to S transition. | [41] |
| 9 | Hispolon | B16-F10 cells | 10 μg/mL | Hispolon could induce cell apoptosis by increasing the expression of caspase-3, -8, and -9. | [42] |
| 10 | Hispolon | HONE-1 and NPC-039 human nasopharyngeal carcinoma cells | 0–100 μM | Hispolon could inhibit cell proliferation and directly induce cell apoptosis by promoting the phosphorylation of JNK1/2, ERK1/2, and p38 MAPK to activate the Csp-3, Csp-8, Csp-9, and Poly ADP-Ribose Polymerase (PARP) expression in a dose- and time-dependent manner. | [43] |
| 11 | Hispolon | MCF7 and T47D human breast cancer cells | 0–100 μM | Hispolon could induce cell apoptosis through increasing PARP cleavage and decreasing the expression of Bcl-2 and inhibit cell proliferation by reducing the ER-α expression at the level of both mRNA and protein. | [44] |
| 12 | Hispolon | Human colon cancer cells | 25 µM | Hispolon could induce cell apoptosis. | [45] |
| 13 | Hispolon | Human epithelial cancer cells | 1–500 mM | Hispolon could inhibit cell proliferation through repressing the transforming growth factor β (TGF-β)-Snail/Twist signaling pathway of epithelial–mesenchymal transition (EMT). | [46] |
| 14 | Hispolon | Glioblastoma U87MG cells | 25, 50 μM | Hispolon significantly inhibited the tumor cell proliferation and promoted cell apoptosis. | [47] |
| 15 | Ethanol extracts | B16F10 melanoma cells | 250–500 μg/mL | Ethanol extracts had antiproliferative activity against B16F10 melanoma cells through inducing G0/G1 cell cycle arrest through decreasing cyclin D1 and CDK2 expression and inducing p21. | [48] |
| 16 | Extracts | PC-3, DU-145, LNCaP, T24, ACHN, A549, MCF-7, AGS, HepG2, and U-87 cancer cells | 0–700 µg/mL | Extracts could induce apoptosis through oxidative stress by stimulating Csp-3 and Csp-9 in varieties of human malignancies, compared with the untreated control. | [49] |
| 17 | Aqueous extracts | MDA-MB-231 breast cancer cells | 40 mg/mL | Aqueous extracts exhibited an antiproliferative effect with an IC50 value of 40 mg/mL in a dose-dependent manner (control: 10 µg/mL 5-flurouracil (5-FU). | [50] |
| 18 | Ethanol extracts | G12VKRAS mutant colon cancer cells | 100 µg/mL | Ethanolic extracts and cetuximab (10, 30 µg/mL) were combined, and treatment for three days inhibited G12VKRAS mutant colon cancer cells by inducing apoptosis. | [51] |
| 19 | 3,4-Dihydroxybenzalactone | Human non-small cell lung carcinoma A 549 cells | 0, 6.25, 12.5, 25, 50 µM | 3,4-Dihydroxybenzalactone inhibited migratory and invasive abilities of cancer cells. | [53] |
| 20 | Phellinulin A | Rat hepatic stellate cells | 40 µM | Phellinulin A had significant inhibitory and therapeutic effects. | [54] |
| 21 | Atractylenolide I | HT29 human colon cancer cells | 0–100 µg/mL | Atractylenolide I had good preventive and therapeutic effects. | [40] |
| 22 | Hispidin | BxPC-3 pancreatic cancer cells and CSCs | 50, 100, 150 μM | Hispidin had therapeutic potential against BxPC-3 pancreatic cancer cells and Cancer stem cells (CSCs) by downregulating the expression of NF-ĸB, in vitro, in a dose-dependent manner. | [55] |
| 23 | Phellifuropyranone, meshimakobnol A and meshimakobnol B | Mouse melanoma cells and human lung cancer cells | 5.6–31.3 μM, 7.1–22.6 μM and 6.1–15.0 μM | Phellifuropyranone, meshimakobnol A, and meshimakobnol B exhibited antiproliferative activity against mouse melanoma cells and human lung cancer cells in vitro. | [56,57] |
| No. | Polysaccharides, Hispolon, or Others | Model | Dose | Results | References |
|---|---|---|---|---|---|
| 1 | Polysaccharides | HT29 cells -bearing mouse | 100, 200 mg/kg/d | Polysaccharides had an inhibitory effect on tumor growth in a human colorectal carcinoma cell (HT29)-bearing mouse in vivo. | [34] |
| 2 | Polysaccharides | HepG2 cells bearing mouse | 100, 200 mg/kg | Polysaccharides had an inhibitory effect on tumor growth in a HepG2 cell-bearing mouse in vivo. | [35] |
| 3 | Ethanol extracts | C57BL6 mice | 300 mg/kg/d | Ethanol extracts reduced tumor weight and increased life span (ILS% = 50.88%) compared with the tumor control group. | [48] |
| 4 | Ethanol extracts | Tumor-xenografted mouse | 400 mg/kg/d | Ethanol extracts could inhibit the proliferation with a tumor-xenografted mouse model compared with the cetuximab (10, 30 mg/kg/d) control group. | [51] |
| 5 | Water extracts | Pancreatic cancer patients | 1100 mg 3 times per day | Water extracts could assist the chemotherapy treatment of pancreatic ductal adenocarcinoma, which improved patient survival. | [52] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Tan, H.; Liu, Q.; Zheng, X.; Zhang, H.; Liu, Y.; Xu, L. A Review: The Bioactivities and Pharmacological Applications of Phellinus linteus. Molecules 2019, 24, 1888. https://doi.org/10.3390/molecules24101888
Chen W, Tan H, Liu Q, Zheng X, Zhang H, Liu Y, Xu L. A Review: The Bioactivities and Pharmacological Applications of Phellinus linteus. Molecules. 2019; 24(10):1888. https://doi.org/10.3390/molecules24101888
Chicago/Turabian StyleChen, Wenhua, Huiying Tan, Qian Liu, Xiaohua Zheng, Hua Zhang, Yuhong Liu, and Lingchuan Xu. 2019. "A Review: The Bioactivities and Pharmacological Applications of Phellinus linteus" Molecules 24, no. 10: 1888. https://doi.org/10.3390/molecules24101888