Synthesis of Highly Concentrated Suspensions of Silver Nanoparticles by Two Versions of the Chemical Reduction Method
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- Silver nitrate (J.A. Elmer, 99.9%, Lima, Peru)
- Sodium citrate (Movilab, 99.9%, Lima, Peru)
2.2. Equipment
- UV-VIS spectrophotometer, ISR 2600 plus (Shimadzu, Kyoto, Japan)
- Nanodrop 1000 Spectrophotometer (Thermo Fisher Scientific INC., Waltham, MA, USA)
- Dynamic light scattering (DLS) Möbiuζ® (Wyatt Technology, Santa Barbara, CA, USA)
- Vacuum Pump RV 3 (Edwards, West Sussex, UK)
3. Procedure
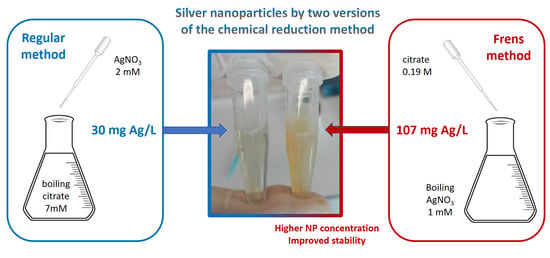
3.1. Synthesis of Silver Nanoparticles Using the Regular Method (Time for Completion: 1 h)
- Place the solution of AgNO3 2 mM on a dropper.
- Place 50 mL of sodium citrate 7mM in an Erlenmeyer flask with a magnetic pill (see Figure 1).
- Cover the flask with aluminum foil and heat it in a water bath until boiling point.
- When the solution of sodium citrate has reached the boiling point add dropwise 8.8 mL of AgNO3.
CRITICAL STEP: The solution of AgNO3 has to be added dropwise to obtain a good distribution of silver nanoparticles.
- Stir the solution for 40 min.
PAUSE STEP: After stirring, allow the suspension to cool down to ambient temperature.
- Centrifuge 1 mL of the solution at 2040 RCF (relative centrifugal force, following the recommendation of the company Citodiagnostics [11]) for 30 min, discard the supernatant and resuspend in 1 mL distilled water.
PAUSE STEP: The solution can be stored at 4 °C in the dark for up to 6 months.
3.2. Synthesis of Silver Nanoparticles Using the Frens Method (Time for Completion: 40 min)
- Place 50 mL of AgNO3 1mM in an Erlenmeyer flask with a magnetic pill (see Figure 1).
- Cover the flask with aluminum foil and heat it until boiling point.
- When the solution reaches the boiling point add 500 µL of sodium citrate 0.189 M (obtained from [12]).
CRITICAL STEP: To obtain a good distribution and small size of silver nanoparticles, the concentration of the sodium citrate has to be as mentioned above.
- Stir the solution for 20 min.
- Centrifuge 1 mL of the solution at 2040 RCF (relative centrifugal force) for 30 min, discard the supernatant and resuspend in 1 mL distilled water.
PAUSE STEP: The solution can be stored at 4 °C in the dark.
4. Results
4.1. Calculation of Nanoparticle Concentration
4.2. Characterization through Absorption Spectroscopy and Dynamic Light Scattering
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Xia, Y. Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Letters 2004, 4, 2047–2050. [Google Scholar] [CrossRef]
- Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, V.; Das, K.P. Interaction of silver nanoparticles with proteins: A characteristic protein concentration dependent profile of SPR signal. Colloids Surf. B 2013, 111, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.L.; Hatzakis, N.S.; Tshikhudo, T.R.; Razumas, V.; Patkar, S.; Vind, J.; Svendsen, A.; Nolte, R.J.M.; Rowan, A.E.; Brust, M. Bionanoconjugation via click chemistry: The creation of functional hybrids of lipases and gold nanoparticles. Bioconjugate Chem. 2006, 17, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Hamner, K.; Maye, M.M.; Ash, D.L. Quantification of Gold Nanoparticles Using the Thermo Scientific Nanodrop 2000 Spectrophotometer. Available online: http://www.thermo.com.cn/Resources/201305/3195042984.pdf (accessed on 12 November 2010).
- Pinedo, A.; Alcazar, B.; Rodriguez-Reyes, J.C.F. Protocol for the Synthesis of Silver Nanoparticles Using Sodium Citrate and Sodium Borohydride as Reducing Agents. Available online: https://www.researchgate.net/publication/323676186_Protocol_for_the_synthesis_of_silver_nanoparticles_using_sodium_citrate_and_sodium_borohydride_as_reducing_agents (accessed on 30 December 2016).
- Cytodiagnostics. Silver Nanoparticle Handling and Storage. Available online: http://www.cytodiagnostics. com/store/pc/Silver-Nanoparticle-Handling-and-Storage-d13.htm (accessed on 5 June 2017).
- Xu, S.; Shi, J.; Feng, D.; Yang, L.; Cao, S. Hollow hierarchical hydroxyapatite/Au/polyelectrolyte hybrid microparticles for multi-responsive drug delivery. J. Mater. Chem. B 2014, 2, 6500–6507. [Google Scholar] [CrossRef]



| Peak Position (nm) | Intensity (a.u.) | FWHM (nm) | ||||
|---|---|---|---|---|---|---|
| (Nanodrop) | (UV-Vis) | (Nanodrop) | (UV-Vis) | (Nanodrop) | (UV-Vis) | |
| Frens method | 418 ± 4 | 419 ± 3 | 0.9 ± 0.1 | 0.6 ± 0.5 * | 116 ± 10 | 106 ± 5 |
| Regular metho | 427 ± 7 | 429 ± 5 | 0.2 ± 0.1 | 1.5 ± 0.6 | 123 ± 7 | 118 ± 6.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gakiya-Teruya, M.; Palomino-Marcelo, L.; Rodriguez-Reyes, J.C.F. Synthesis of Highly Concentrated Suspensions of Silver Nanoparticles by Two Versions of the Chemical Reduction Method. Methods Protoc. 2019, 2, 3. https://doi.org/10.3390/mps2010003
Gakiya-Teruya M, Palomino-Marcelo L, Rodriguez-Reyes JCF. Synthesis of Highly Concentrated Suspensions of Silver Nanoparticles by Two Versions of the Chemical Reduction Method. Methods and Protocols. 2019; 2(1):3. https://doi.org/10.3390/mps2010003
Chicago/Turabian StyleGakiya-Teruya, Miguel, Luis Palomino-Marcelo, and Juan Carlos F. Rodriguez-Reyes. 2019. "Synthesis of Highly Concentrated Suspensions of Silver Nanoparticles by Two Versions of the Chemical Reduction Method" Methods and Protocols 2, no. 1: 3. https://doi.org/10.3390/mps2010003