Improved Removal Capacity and Equilibrium Time of Maghemite Nanoparticles Growth in Zeolite Type 5A for Pb(II) Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Adsorbent Synthesis
2.2.1. Synthesis 1
2.2.2. Synthesis 2
2.2.3. Synthesis 3
2.3. Characterization of Structural, Surface Area, and Magnetic Properties
2.4. Heavy Metal Adsorption Experiments
3. Results and Discussion
3.1. Structural and Morphological Properties
3.2. Surface and Textural Properties
3.3. Magnetic Properties
3.4. Effect of Variable Parameters on the Pb (II) Adsorption Process
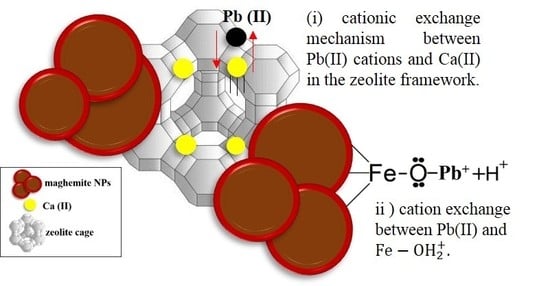
3.5. Effect of pH and Adsorption Mechanism
3.6. Effect of the Amount of NPZEO3 on the Adsorption Process
3.7. Adsorption Kinetics
3.8. Adsorption Isotherm
3.9. Interferent Ions and Reusability
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singh, S.; Barick, K.C.; Bahadur, D. Functional Oxide Nanomaterials and Nanocomposites for the Removal of Heavy Metals and Dyes. Nanomater. Nanotechnol. 2013, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Astruc, D. Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- World Health Organization. Lead in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Deng, X.; Lü, L.; Li, H.; Luo, F. The adsorption properties of Pb(II) and Cd(II) on functionalized graphene prepared by electrolysis method. J. Hazard. Mater. 2010, 183, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Petrusevski, B.; Amy, G. Chromium removal from water: A review. J. Water Supply Res. Technol. Aqua. 2008, 57, 541. [Google Scholar] [CrossRef]
- Baig, S.A.; Sheng, T.; Zhu, J.; Xu, X. Performance of an Integrated Treatment System for Clean Drinking Water Production. CLEAN Soil Air Water. 2015, 43, 13–26. [Google Scholar] [CrossRef]
- Reviews in Mineralogy and Geochemistry. In Natural Zeolites: Occurrence, Properties, Applications; Bish, D.L.; Ming, D.W. (Eds.) Mineralogical Society of America: Washington, DC, USA, 2001; ISBN 0-939950-57-X. [Google Scholar]
- Yang, R.T. Nanostructured adsorbents. Nanostructured Materials; Academic Press: Cambridge, MA, USA, 2001; p. 96. ISBN 0-12-008527-5. [Google Scholar]
- Jiang, N.; Shang, R.; Heijman, S.G.J.; Rietveld, L.C. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review. Water Res. 2018, 144, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Pittman, C.U.; Mohan, D. Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Irawan, C.; Nata, I.F.; Lee, C.-K. Removal of Pb(II) and As(V) using magnetic nanoparticles coated montmorillonite via one-pot solvothermal reaction as adsorbent. J. Environ. Chem. Eng. 2019, 7, 103000. [Google Scholar] [CrossRef]
- Levin, C.S.; Hofmann, C.; Ali, T.A.; Kelly, A.T.; Morosan, E.; Nordlander, P.; Whitmire, K.H.; Halas, N.J. Magnetic-Plasmonic-core-shell nanoparticles. ACS Nano 2009, 3, 1379–1388. [Google Scholar] [CrossRef]
- Eljamal, O.; Shubair, T.; Tahara, A.; Sugihara, Y.; Matsunaga, N. Iron based nanoparticles-zeolite composites for the removal of cesium from aqueous solutions. J. Mol. Liq. 2019, 277, 613–623. [Google Scholar] [CrossRef]
- Eljamal, O.; Sasaki, K.; Hirajima, T. Sorption kinetic of arsenate as water contaminant on zero valent iron. JWARP 2013, 5, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Lu, Z.; Xiao, B.; Gu, P.; Yao, W.; Xing, J.; Asiri, A.M.; Alamry, K.A.; Wang, X.; Wang, S. Enhanced removal of lead ions from aqueous solution by iron oxide nanomaterials with cobalt and nickel doping. J. Clean. Prod. 2019, 211, 1250–1258. [Google Scholar] [CrossRef]
- Perez, T.; Pasquini, D.; de Faria Lima, A.; Rosa, E.V.; Sousa, M.H.; Cerqueira, D.A.; de Morais, L.C. Efficient removal of lead ions from water by magnetic nanosorbents based on manganese ferrite nanoparticles capped with thin layers of modified biopolymers. J. Environ. Chem. Eng. 2019, 7, 102892. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. Part. B Eng. 2019, 162, 538–568. [Google Scholar] [CrossRef]
- Match-Phase Identification from Powder Diffraction-Version 3, Crystal Impact-Dr. H. Putz; Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn Germany. Available online: http://www.crystalimpact.com/match (accessed on 8 April 2020).
- Pecharromán, C.; Gonzalez-Carreno, T.; Iglesias, J.E. The infrared dielectric properties of maghemite, γ-Fe2O3, from reflectance measurement on pressed powders. Phys. Chem. Miner. 1995, 22, 21–29. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Venturelli, P. In situ study of the goethite-hematite phase transformation by real time synchrotron powder diffraction. Am. Mineral. 1999, 84, 895–904. [Google Scholar] [CrossRef]
- Porcher, F.; Souhassou, M.; Graafsma, H.; Puig-Molina, A.; Dusausoy, Y.; Lecomte, C. Refinement of framework disorder in dehydrated CaA zeolite from single-crystal synchrotron data. Acta Crystallogr. Sect. B Struct. Sci. 2000, 56, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carvajal, J. Software Manual: An Introduction to the Program FullProf 2000 (Version July 2001). 2001. Available online: https://www.psi.ch/sites/default/files/import/sinq/dmc/ManualsEN/fullprof.pdf (accessed on 8 April 2020).
- Maamoun, I.; Eljamal, O.; Eljamal, R.; Falyouna, O.; Sugihara, Y. Promoting aqueous and transport characteristics of highly reactive nanoscale zerovalent iron via different layered hydroxide coatings. Appl. Surf. Sci. 2020, 506, 145018. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Science+Business Media: New York, NY, USA, 2004; ISBN 978-90-481-6633-6. [Google Scholar]
- Falyouna, O.; Eljamal, O.; Maamoun, I.; Tahara, A.; Sugihara, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interface Sci. 2020, 571, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Zak, A.K.; Majid, W.H.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson Hall and size-strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar]
- Jamal, M.; Asadabadi, S.J.; Ahmad, I.; Aliabad, H.A.R. Elastic constants of cubic crystals. Comput. Mater. Sci. 2014, 95, 592–599. [Google Scholar]
- Guivar, J.A.R.; Sadrollahi, E.; Menzel, D.; Fernandes, E.G.R.; López, E.O.; Torres, M.M.; Arsuaga, J.M.; Arencibia, A.; Litterst, F.J. Magnetic, structural and surface properties of functionalized maghemite nanoparticles for copper and lead adsorption. RSC Adv. 2017, 7, 28763–28779. [Google Scholar] [CrossRef] [Green Version]
- Maamoun, I.; Eljamal, O.; Falyouna, O.; Eljamal, R.; Sugihara, Y. Stimulating effect of magnesium hydroxide on aqueous characteristics of iron nanocomposites. Water Sci. Technol. 2019, 80, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Guivar, J.A.R.; Sanches, E.A.; Bruns, F.; Sadrollahi, E.; Morales, M.A.; López, E.O.; Litterst, F.J. Vacancy ordered γ-Fe2O3 nanoparticles with nanohydroxyapatite: XRD, FTIR, XPS and Mössbauer studies. Appl. Surf. Sci. 2016, 389, 721–734. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef] [Green Version]
- Hamed, H.H. Oxygen separation from air using zeolite type 5A. Int. J. Sci. Eng. Res. 2015, 6, 597–602. [Google Scholar]
- Guivar, J.A.R.; Bustamante, A.; Gonzalez, J.C.; Sanches, E.A.; Morales, M.A.; Raez, J.M.; López-Muñoz, M.-J.; Arencibia, A. Adsorption of arsenite and arsenate on binary and ternary magnetic nanocomposites with high iron oxide content. Appl. Surf. Sci. 2018, 454, 87–100. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lv, Z.; Xie, Y.; Shu, J.; Alsaedi, A.; Hayat, T.; Chen, C. Exploration of the adsorption performance and mechanism of zeolitic imidazolate framework-8@graphene oxide for Pb (II) and 1-naphthylamine from aqueous solution. J. Colloid Interface Sci. 2019, 542, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Dongsheng, Z.; Wenqiang, G.; Guozhang, C.; Shuai, L.; Weizhou, J.; Youzhi, L. Removal of heavy metal lead(II) using nanoscale zero-valent iron with different preservation methods. Adv. Powder Technol. 2019, 30, 581–589. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Nilchi, A.; Hassani, A.H. Synthesis of novel surface-modified hematite nanoparticles for lead ions removal from aqueous solution. Mater. Chem. Phys. 2019, 227, 279–290. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Dai, C.; Shi, H.; Li, J. Adsorption of Pb2+ on amino-functionalized core–shell magnetic mesoporous SBA-15 silica composite. Chem. Eng. J. 2015, 262, 897–903. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthes, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]











| Refined Parameters | Zeo5A | NPZEO1 | NPZEO2 | |
|---|---|---|---|---|
| γ-Fe2O3 | goethite | γ-Fe2O3 | ||
| a (Å) | 24.5586 | 8.3506 | 9.9182 | 8.3022 |
| b (Å) | 24.5586 | 8.3506 | 3.0059 | 8.3022 |
| c (Å) | 24.5586 | 8.3506 | 4.5801 | 8.3022 |
| α (°) | 90 | 90 | 90 | 90 |
| β (°) | 90 | 90 | 90 | 90 |
| γ (°) | 90 | 90 | 90 | 90 |
| V (Å3) | 14,811.94 | 582 | 136.5 | 572 |
| global average size (nm) | 373 | 6.6 | 290 | 10.5 |
| Rp (%) | 16.9 | 55 | 53 | |
| Rwp (%) | 19.1 | 43.8 | 35.7 | |
| Rexp (%) | 16.8 | 24.8 | 27.9 | |
| χ2 | 1.3 | 3.1 | 1.6 | |
| Adsorbent | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Width (nm) |
|---|---|---|---|
| zeolite type 5A [33] | 571 | 0.18 | 0.5 |
| NPZEO1 | 167 | 0.40 | 3.2 |
| NPZEO2 | 178 | 0.35 | 1.4 |
| NPZEO3 | 194 | 0.13 | 2.8 |
| Adsorbent | k2 (mg g−1 min−1) | R (%) | t (min) | C0 (mg L−1) | Dose (g L−1) |
|---|---|---|---|---|---|
| Co-Fe2O3 [16] | 1.74 | 95 | 45 | 20 | 0.1 |
| ZIF-8@GO [35] | 5.89 × 10−3 | - | 100 | 30 | 0.15 |
| MNPs@carboxymethylated biopolymers [17] | 34 × 10−3 | 99.9 | 180 | 24 | 2 |
| nanoscale zero-valent irons (nZVI) [36] | 7.65 ×10−4 | ~90 | 200 | 100 | 0.35 |
| α-Fe2O3 NPs [37] | 12 ×10−4 | ~88 | 240 | 10 | 0.1 |
| Fe3O4@SBA-15 [38] | 11 × 10−3 | - | 720 | ||
| zeolite 5A (this work) | 35.5 | 100 | 15 | 50 | 0.5 |
| NPZEO3 (this work) | 0.9 | 100 | 5 | 50 | 0.5 |
| Model | qt,exp | qt,model | AIC |
|---|---|---|---|
| Pseudo second order model | 99.8 | 100 | −50.12 |
| Intraparticle difusion model | 99.8 | 99.91 | −40.28 |
| Elovich | 99.8 | 99.908 | −46.73 |
| Pb (II) Isotherm | ||
|---|---|---|
| Langmuir Parameters | Freundlich Parameters | Sips Parameters |
| Q0 (mg g−1) 252 (5) | KF (mg g−1) 122 (5) | (mg g−1) 265 (5) |
| b (mg−1 L) 0.99 (1) | 1/n 0.16 (5) | (L mg−1)m 0.25 (5) |
| R2 0.88 | R2 0.97 | 0.87 (5) |
| R2 0.98 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Guivar, J.A.; Taipe, K.; Schettino, M.A., Jr.; Silva, E.; Morales Torres, M.A.; Passamani, E.C.; Litterst, F.J. Improved Removal Capacity and Equilibrium Time of Maghemite Nanoparticles Growth in Zeolite Type 5A for Pb(II) Adsorption. Nanomaterials 2020, 10, 1668. https://doi.org/10.3390/nano10091668
Ramos-Guivar JA, Taipe K, Schettino MA Jr., Silva E, Morales Torres MA, Passamani EC, Litterst FJ. Improved Removal Capacity and Equilibrium Time of Maghemite Nanoparticles Growth in Zeolite Type 5A for Pb(II) Adsorption. Nanomaterials. 2020; 10(9):1668. https://doi.org/10.3390/nano10091668
Chicago/Turabian StyleRamos-Guivar, Juan A., Katterine Taipe, Miguel Angelo Schettino, Jr., Eloi Silva, Marco Antonio Morales Torres, Edson Caetano Passamani, and Fred Jochen Litterst. 2020. "Improved Removal Capacity and Equilibrium Time of Maghemite Nanoparticles Growth in Zeolite Type 5A for Pb(II) Adsorption" Nanomaterials 10, no. 9: 1668. https://doi.org/10.3390/nano10091668