Fish Hydrolysate Supplementation Containing n-3 Long Chain Polyunsaturated Fatty Acids and Peptides Prevents LPS-Induced Neuroinflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Quantitative Real Time PCR
2.3. Western Blot
2.4. Lipid Analysis
2.5. Oxylipin Quantification
2.6. Statistical Analysis
3. Results
3.1. Fish Hydrolysate Supplementation Decreases Proinflammatory Marker Expression and Prevents IκB Degradation in Response to LPS
3.2. Fish Hydrolysate Supplementation Modulates Microglial Activation Markers in Response to LPS
3.3. Fish Hydrolysate Supplementation Prevents LPS-Induced Changes in the Expression of Neurotrophins
3.4. Fish Hydrolysate and DHA Supplementations Affect Cortical Fatty Acid Composition
3.5. Fish Hydrolysate Supplementation Affects Hippocampal Oxylipin Concentration in LPS-Treated Animals
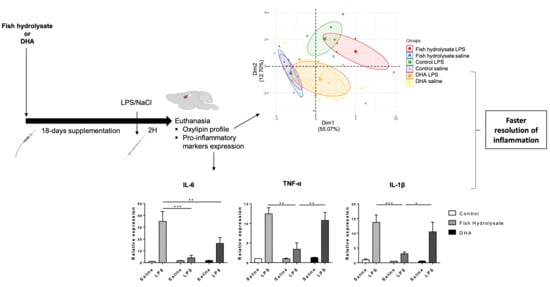
3.6. Multivariate Analysis Highlights the Separation of Oxylipin Profile in Function of the Supplementation in LPS-Treated Animals
3.7. LPS-Induced Hippocampal Expression of Oxylipin Biosynthesis Enzymes Is Regulated by Supplementations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-oxoETE | 5-oxo-eicosatetraenoic acid |
| AA | arachidonic acid |
| ALA | α-linolenic acid |
| Arg1 | arginase 1 |
| BDNF | brain-derived neurotrophic factor |
| CD | cluster of differentiation |
| CNS | central nervous system |
| COX | cyclooxygenase |
| DHA | docosahexaenoic acid |
| EET | epoxy eicosatrienoic acid |
| EPA | eicosapentaenoic acid |
| HDoHE | hydroxy-docosahexaenoic acid |
| HETE | hydroxy-eicosatetraenoic acid |
| HODE | hydroxy-octadecadienoic acid |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| JNK | c-Jun N-terminal kinase |
| LA | linoleic acid |
| LC-PUFAs | long chain polyunsaturated fatty acids |
| LOX | lipoxygenase |
| LPS | lipopolysaccharide |
| Lx | lipoxin |
| MAPK | mitogen-activated protein kinase |
| MaR | maresin |
| MCP-1/CCL2 | monocyte chemoattractant protein-1 |
| NF-κB | nuclear factor kappa B |
| NGF | nerve growth factor |
| NO | nitric oxide |
| PG | prostaglandin |
| PUFAs | polyunsaturated fatty acids |
| SPMs | specialized proresolving mediators |
| SOCS | Suppressor of cytokine signalling |
| TGF | transforming growth factor |
| TLR | toll-like receptor |
| TNF | tumor necrosis factor |
| Trk | tyrosine receptor kinase |
| Tx | thromboxane |
References
- Calder, P.C.; Albers, R.; Antoine, J.-M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory Disease Processes and Interactions with Nutrition. Br. J. Nutr. 2009, 101 (Suppl. S1), S1–S45. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Long Chain Fatty Acids and Gene Expression in Inflammation and Immunity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 425–433. [Google Scholar] [CrossRef]
- Aloisi, F. Immune Function of Microglia. Glia 2001, 36, 165–179. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinel, A.-L.; André, C.; Aubert, A.; Ferreira, G.; Layé, S.; Castanon, N. Lipopolysaccharide-Induced Brain Activation of the Indoleamine 2,3-Dioxygenase and Depressive-like Behavior Are Impaired in a Mouse Model of Metabolic Syndrome. Psychoneuroendocrinology 2014, 40, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Layé, S.; Parnet, P.; Goujon, E.; Dantzer, R. Peripheral Administration of Lipopolysaccharide Induces the Expression of Cytokine Transcripts in the Brain and Pituitary of Mice. Brain Res. Mol. Brain Res. 1994, 27, 157–162. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [Green Version]
- Layé, S. Polyunsaturated Fatty Acids, Neuroinflammation and Well Being. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 295–303. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Luheshi, G.N. Interleukin 1 in the Brain: Biology, Pathology and Therapeutic Target. Trends Neurosci. 2000, 23, 618–625. [Google Scholar] [CrossRef]
- Venters, H.D.; Dantzer, R.; Kelley, K.W. A New Concept in Neurodegeneration: TNFalpha Is a Silencer of Survival Signals. Trends Neurosci. 2000, 23, 175–180. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of Inflammation: A New Therapeutic Frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Dantzer, R. Cytokines and Depression: The Need for a New Paradigm. Brain Behav. Immun. 2003, 17 (Suppl. S1), S119–S124. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated Fatty Acids and Inflammation. Biochem. Soc. Trans. 2005, 33, 423–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.-L.; Serhan, C.N. Novel Docosatrienes and 17S-Resolvins Generated from Docosahexaenoic Acid in Murine Brain, Human Blood, and Glial Cells. Autacoids in Anti-Inflammation. J. Biol. Chem. 2003, 278, 14677–14687. [Google Scholar] [CrossRef] [Green Version]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid Mediator Class Switching during Acute Inflammation: Signals in Resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef]
- Rey, C.; Nadjar, A.; Buaud, B.; Vaysse, C.; Aubert, A.; Pallet, V.; Layé, S.; Joffre, C. Resolvin D1 and E1 Promote Resolution of Inflammation in Microglial Cells in Vitro. Brain Behav. Immun. 2016, 55, 249–259. [Google Scholar] [CrossRef]
- Rey, C.; Delpech, J.C.; Madore, C.; Nadjar, A.; Greenhalgh, A.D.; Amadieu, C.; Aubert, A.; Pallet, V.; Vaysse, C.; Layé, S.; et al. Dietary N-3 Long Chain PUFA Supplementation Promotes a pro-Resolving Oxylipin Profile in the Brain. Brain Behav. Immun. 2019, 76, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2–Nonsteroidal Antiinflammatory Drugs and Transcellular Processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and Maresins: New pro-Resolving Families of Mediators in Acute Inflammation and Resolution Bioactive Metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balvers, M.G.J.; Verhoeckx, K.C.M.; Bijlsma, S.; Rubingh, C.M.; Meijerink, J.; Wortelboer, H.M.; Witkamp, R.F. Fish Oil and Inflammatory Status Alter the N-3 to n-6 Balance of the Endocannabinoid and Oxylipin Metabolomes in Mouse Plasma and Tissues. Metabolomics 2012, 8, 1130–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, M.; Katakura, M.; Tanabe, Y.; Al Mamun, A.; Inoue, T.; Hossain, S.; Arita, M.; Shido, O. N-3 Fatty Acids Effectively Improve the Reference Memory-Related Learning Ability Associated with Increased Brain Docosahexaenoic Acid-Derived Docosanoids in Aged Rats. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2015, 1851, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Balvers, M.G.J.; Verhoeckx, K.C.M.; Meijerink, J.; Bijlsma, S.; Rubingh, C.M.; Wortelboer, H.M.; Witkamp, R.F. Time-Dependent Effect of in Vivo Inflammation on Eicosanoid and Endocannabinoid Levels in Plasma, Liver, Ileum and Adipose Tissue in C57BL/6 Mice Fed a Fish-Oil Diet. Int. Immunopharmacol. 2012, 13, 204–214. [Google Scholar] [CrossRef]
- Birnie, M.; Morrison, R.; Camara, R.; Strauss, K.I. Temporal Changes of Cytochrome P450 (Cyp) and Eicosanoid-Related Gene Expression in the Rat Brain after Traumatic Brain Injury. BMC Genom. 2013, 14, 303. [Google Scholar] [CrossRef] [Green Version]
- Rosenberger, T.A.; Villacreses, N.E.; Hovda, J.T.; Bosetti, F.; Weerasinghe, G.; Wine, R.N.; Harry, G.J.; Rapoport, S.I. Rat Brain Arachidonic Acid Metabolism Is Increased by a 6-Day Intracerebral Ventricular Infusion of Bacterial Lipopolysaccharide. J. Neurochem. 2004, 88, 1168–1178. [Google Scholar] [CrossRef]
- Taha, A.Y.; Blanchard, H.C.; Cheon, Y.; Ramadan, E.; Chen, M.; Chang, L.; Rapoport, S.I. Dietary Linoleic Acid Lowering Reduces Lipopolysaccharide-Induced Increase in Brain Arachidonic Acid Metabolism. Mol. Neurobiol. 2017, 54, 4303–4315. [Google Scholar] [CrossRef] [PubMed]
- Willenberg, I.; Rund, K.; Rong, S.; Shushakova, N.; Gueler, F.; Schebb, N.H. Characterization of Changes in Plasma and Tissue Oxylipin Levels in LPS and CLP Induced Murine Sepsis. Inflamm. Res. 2016, 65, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Rao, J.S.; Rapoport, S.I.; Igarashi, M. Dietary N-6 PUFA Deprivation Downregulates Arachidonate but Upregulates Docosahexaenoate Metabolizing Enzymes in Rat Brain. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2011, 1811, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, S.; Winter, T.; Aukema, H.M. Dietary ALA, EPA and DHA Have Distinct Effects on Oxylipin Profiles in Female and Male Rat Kidney, Liver and Serum. J. Nutr. Biochem. 2018, 57, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S.; Ertley, R.N.; DeMar, J.C.; Rapoport, S.I.; Bazinet, R.P.; Lee, H.-J. Dietary N-3 PUFA Deprivation Alters Expression of Enzymes of the Arachidonic and Docosahexaenoic Acid Cascades in Rat Frontal Cortex. Mol. Psychiatry 2007, 12, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Mechanisms of Neuroprotective Effects of Peptides Derived from Natural Materials and Their Production and Assessment. Compr. Rev. Food Sci. Food Saf. 2019, 18, 923–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumder, K.; Mine, Y.; Wu, J. The Potential of Food Protein-Derived Anti-Inflammatory Peptides against Various Chronic Inflammatory Diseases. J. Sci. Food Agric. 2016, 96, 2303–2311. [Google Scholar] [CrossRef]
- Aihara, K.; Ishii, H.; Yoshida, M. Casein-Derived Tripeptide, Val-Pro-Pro (VPP), Modulates Monocyte Adhesion to Vascular Endothelium. J. Atheroscler. Thromb. 2009, 16, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Majumder, K.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Structure and Activity Study of Egg Protein Ovotransferrin Derived Peptides (IRW and IQW) on Endothelial Inflammatory Response and Oxidative Stress. J. Agric. Food Chem. 2013, 61, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Hirota, T.; Mizushima, K.; Ohki, K.; Naito, Y.; Yamamoto, N.; Yoshikawa, T. Milk-Derived Peptides, Val-Pro-Pro and Ile-Pro-Pro, Attenuate Atherosclerosis Development in Apolipoprotein E–Deficient Mice: A Preliminary Study. J. Med. Food 2013, 16, 396–403. [Google Scholar] [CrossRef]
- Zhang, H.; Kovacs-Nolan, J.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl Cysteine and γ-Glutamyl Valine Inhibit TNF-α Signaling in Intestinal Epithelial Cells and Reduce Inflammation in a Mouse Model of Colitis via Allosteric Activation of the Calcium-Sensing Receptor. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2015, 1852, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Min, L.-J.; Kobayashi, Y.; Mogi, M.; Tsukuda, K.; Yamada, A.; Yamauchi, K.; Abe, F.; Iwanami, J.; Xiao, J.-Z.; Horiuchi, M. Administration of Bovine Casein-Derived Peptide Prevents Cognitive Decline in Alzheimer Disease Model Mice. PLoS ONE 2017, 12, e0171515. [Google Scholar] [CrossRef] [Green Version]
- Chai, H.-J.; Wu, C.-J.; Yang, S.-H.; Li, T.-L.; Pan, B.-S. Peptides from Hydrolysate of Lantern Fish (Benthosema Pterotum) Proved Neuroprotective In Vitro and In Vivo. J. Funct. Foods 2016, 24, 438–449. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Cho, Y.-S.; Je, J.-Y. Purification and Anti-Inflammatory Action of Tripeptide from Salmon Pectoral Fin Byproduct Protein Hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef]
- Montserrat de la Paz, S.; Lemus-Conejo, A.; Toscano, R.; Pedroche, J.; Millan, F.; Millan-Linares, M.C. GPETAFLR, an Octapeptide Isolated from Lupinus Angustifolius L. Protein Hydrolysate, Promotes the Skewing to the M2 Phenotype in Human Primary Monocytes. Food Funct. 2019, 10, 3303–3311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs-Nolan, J.; Zhang, H.; Ibuki, M.; Nakamori, T.; Yoshiura, K.; Turner, P.V.; Matsui, T.; Mine, Y. The PepT1-Transportable Soy Tripeptide VPY Reduces Intestinal Inflammation. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Chataigner, M.; Mortessagne, P.; Lucas, C.; Pallet, V.; Layé, S.; Mehaignerie, A.; Bouvret, E.; Dinel, A.L.; Joffre, C. Dietary Fish Hydrolysate Supplementation Containing N-3 LC-PUFAs and Peptides Prevents Short-Term Memory and Stress Response Deficits in Aged Mice. Brain Behav. Immun. 2020, 91, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, N.; Desor, F.; Gleizes, C.; Denis, F.M.; Arab-Tehrany, E.; Soulimani, R.; Linder, M. Anxiolytic-like Effect of a Salmon Phospholipopeptidic Complex Composed of Polyunsaturated Fatty Acids and Bioactive Peptides. Mar. Drugs 2013, 11, 4294–4317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Poncin-Séac’h, M.; Le Poncin-Lafitte, C. Effets Sur Les Fonctions Cognitives et Mnésiques de Sujets Sains d’un Hydrolysat de Protéines Xde Poisson (FPHD!Àà): Étude Comparative Avec Le Ginkgo Biloba (EGB 761®). Méd. Longév. 2010, 2, 57–67. [Google Scholar] [CrossRef]
- Mingam, R.; Moranis, A.; Bluthé, R.-M.; De Smedt-Peyrusse, V.; Kelley, K.W.; Guesnet, P.; Lavialle, M.; Dantzer, R.; Layé, S. Uncoupling of Interleukin-6 from Its Signalling Pathway by Dietary n-3-Polyunsaturated Fatty Acid Deprivation Alters Sickness Behaviour in Mice. Eur. J. Neurosci. 2008, 28, 1877–1886. [Google Scholar] [CrossRef] [Green Version]
- Simões, A.E.; Pereira, D.M.; Amaral, J.D.; Nunes, A.F.; Gomes, S.E.; Rodrigues, P.M.; Lo, A.C.; D’Hooge, R.; Steer, C.J.; Thibodeau, S.N.; et al. Efficient Recovery of Proteins from Multiple Source Samples after Trizol® or Trizol®LS RNA Extraction and Long-Term Storage. BMC Genom. 2013, 14, 181. [Google Scholar] [CrossRef] [Green Version]
- Labrousse, V.F.; Nadjar, A.; Joffre, C.; Costes, L.; Aubert, A.; Grégoire, S.; Bretillon, L.; Layé, S. Short-Term Long Chain Omega3 Diet Protects from Neuroinflammatory Processes and Memory Impairment in Aged Mice. PLoS ONE 2012, 7, e36861. [Google Scholar] [CrossRef]
- Lafourcade, M.; Larrieu, T.; Mato, S.; Duffaud, A.; Sepers, M.; Matias, I.; De Smedt-Peyrusse, V.; Labrousse, V.F.; Bretillon, L.; Matute, C.; et al. Nutritional Omega-3 Deficiency Abolishes Endocannabinoid-Mediated Neuronal Functions. Nat. Neurosci. 2011, 14, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Larrieu, T.; Madore, C.; Joffre, C.; Layé, S. Nutritional N-3 Polyunsaturated Fatty Acids Deficiency Alters Cannabinoid Receptor Signaling Pathway in the Brain and Associated Anxiety-like Behavior in Mice. J. Physiol. Biochem. 2012, 68, 671–681. [Google Scholar] [CrossRef]
- Le Faouder, P.; Baillif, V.; Spreadbury, I.; Motta, J.-P.; Rousset, P.; Chêne, G.; Guigné, C.; Tercé, F.; Vanner, S.; Vergnolle, N.; et al. LC-MS/MS Method for Rapid and Concomitant Quantification of pro-Inflammatory and pro-Resolving Polyunsaturated Fatty Acid Metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 932, 123–133. [Google Scholar] [CrossRef]
- Bara, O.; Day, J.; Djouadi, S.M. Nonlinear State Estimation for Complex Immune Responses. In Proceedings of the 52nd IEEE Conference on Decision and Control, Florence, Italy, 10–13 December 2013; pp. 3373–3378. [Google Scholar]
- Serhan, C.N. Pro-Resolving Lipid Mediators Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Savill, J. Resolution of Inflammation: The Beginning Programs the End. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Barnig, C.; Bezema, T.; Calder, P.C.; Charloux, A.; Frossard, N.; Garssen, J.; Haworth, O.; Dilevskaya, K.; Levi-Schaffer, F.; Lonsdorfer, E.; et al. Activation of Resolution Pathways to Prevent and Fight Chronic Inflammation: Lessons from Asthma and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 1699. [Google Scholar] [CrossRef] [Green Version]
- Doyle, S.L.; O’Neill, L.A.J. Toll-like Receptors: From the Discovery of NFkappaB to New Insights into Transcriptional Regulations in Innate Immunity. Biochem. Pharmacol. 2006, 72, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Weiner, H.L. Microglial Signatures and Their Role in Health and Disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 Microglia: The Good, the Bad, and the Inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanisch, U.-K. Functional Diversity of Microglia—How Heterogeneous Are They to Begin With? Front. Cell Neurosci. 2013, 7, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czapski, G.A.; Gajkowska, B.; Strosznajder, J.B. Systemic Administration of Lipopolysaccharide Induces Molecular and Morphological Alterations in the Hippocampus. Brain Res. 2010, 1356, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Modulation of Inflammation in Brain: A Matter of Fat. J. Neurochem. 2007, 101, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Nadjar, A.; Tridon, V.; May, M.J.; Ghosh, S.; Dantzer, R.; Amédée, T.; Parnet, P. NFkappaB Activates in Vivo the Synthesis of Inducible Cox-2 in the Brain. J. Cereb. Blood Flow Metab. 2005, 25, 1047–1059. [Google Scholar] [CrossRef] [Green Version]
- Rivest, S. Activation of the Nuclear Factor Kappa B (NF-KappaB) and Cyclooxygenase-2 (COX-2) Genes in Cerebral Blood Vessels in Response to Systemic Inflammation. Mol. Psychiatry 1999, 4, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Yamamoto, K.; Kurobe, H.; Yamashita, R.; Yamaguchi, H.; Ueda, N. Transcriptional Regulation of Fatty Acid Cyclooxygenases-1 and -2. Int. J. Tissue React. 1998, 20, 17–22. [Google Scholar] [PubMed]
- Delpech, J.-C.; Thomazeau, A.; Madore, C.; Bosch-Bouju, C.; Larrieu, T.; Lacabanne, C.; Remus-Borel, J.; Aubert, A.; Joffre, C.; Nadjar, A.; et al. Dietary N-3 PUFAs Deficiency Increases Vulnerability to Inflammation-Induced Spatial Memory Impairment. Neuropsychopharmacology 2015, 40, 2774–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcheselli, V.L.; Hong, S.; Lukiw, W.J.; Tian, X.H.; Gronert, K.; Musto, A.; Hardy, M.; Gimenez, J.M.; Chiang, N.; Serhan, C.N.; et al. Novel Docosanoids Inhibit Brain Ischemia-Reperfusion-Mediated Leukocyte Infiltration and Pro-Inflammatory Gene Expression. J. Biol. Chem. 2003, 278, 43807–43817. [Google Scholar] [CrossRef] [Green Version]
- Orr, S.K.; Palumbo, S.; Bosetti, F.; Mount, H.T.; Kang, J.X.; Greenwood, C.E.; Ma, D.W.L.; Serhan, C.N.; Bazinet, R.P. Unesterified Docosahexaenoic Acid Is Protective in Neuroinflammation. J. Neurochem. 2013, 127, 378–393. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Ren, H.; Huang, Z.; Peng, Y.; He, B.; Yao, X.; Yuan, T.-F.; Su, H. Fish Oil Prevents Lipopolysaccharide-Induced Depressive-Like Behavior by Inhibiting Neuroinflammation. Mol. Neurobiol. 2017, 54, 7327–7334. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Park, T.; Barnes, J.; Kevala, K.; Chen, H.; Kim, H.-Y. Reduced Acute Neuroinflammation and Improved Functional Recovery after Traumatic Brain Injury by α-Linolenic Acid Supplementation in Mice. J. Neuroinflamm. 2016, 13, 253. [Google Scholar] [CrossRef] [Green Version]
- Ajmone-Cat, M.A.; Salvatori, M.L.; Simone, R.D.; Mancini, M.; Biagioni, S.; Bernardo, A.; Cacci, E.; Minghetti, L. Docosahexaenoic Acid Modulates Inflammatory and Antineurogenic Functions of Activated Microglial Cells. J. Neurosci. Res. 2012, 90, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.K.-Y.; Khatchadourian, A.; McKinney, R.A.; Maysinger, D. Docosahexaenoic Acid (DHA): A Modulator of Microglia Activity and Dendritic Spine Morphology. J. Neuroinflamm. 2015, 12, 34. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wei, Y.; Chen, X.; Jiao, J.; Zhang, Y. Polyunsaturated Fatty Acids Ameliorate Aging via Redox-Telomere-Antioncogene Axis. Oncotarget 2017, 8, 7301–7314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smedt-Peyrusse, V.D.; Sargueil, F.; Moranis, A.; Harizi, H.; Mongrand, S.; Layé, S. Docosahexaenoic Acid Prevents Lipopolysaccharide-Induced Cytokine Production in Microglial Cells by Inhibiting Lipopolysaccharide Receptor Presentation but Not Its Membrane Subdomain Localization. J. Neurochem. 2008, 105, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, L.-X.; Cao, D.-L.; Gao, Y.-J. Spinal Injection of Docosahexaenoic Acid Attenuates Carrageenan-Induced Inflammatory Pain through Inhibition of Microglia-Mediated Neuroinflammation in the Spinal Cord. Neuroscience 2013, 241, 22–31. [Google Scholar] [CrossRef]
- Ma, Q.-L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. Beta-Amyloid Oligomers Induce Phosphorylation of Tau and Inactivation of Insulin Receptor Substrate via c-Jun N-Terminal Kinase Signaling: Suppression by Omega-3 Fatty Acids and Curcumin. J. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Khoutorova, L.; Atkins, K.D.; Eady, T.N.; Hong, S.; Lu, Y.; Obenaus, A.; Bazan, N.G. Docosahexaenoic Acid Therapy of Experimental Ischemic Stroke. Transl. Stroke Res. 2011, 2, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-Y.; Kuan, Y.-H.; Li, J.-R.; Chen, W.-Y.; Ou, Y.-C.; Pan, H.-C.; Liao, S.-L.; Raung, S.-L.; Chang, C.-J.; Chen, C.-J. Docosahexaenoic Acid Reduces Cellular Inflammatory Response Following Permanent Focal Cerebral Ischemia in Rats. J. Nutr. Biochem. 2013, 24, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, H.; Pu, H.; Wang, G.; Li, W.; Leak, R.K.; Chen, J.; Liou, A.K.; Hu, X. N-3 PUFA Supplementation Benefits Microglial Responses to Myelin Pathology. Sci. Rep. 2014, 4, 7458. [Google Scholar] [CrossRef]
- Eady, T.N.; Belayev, L.; Khoutorova, L.; Atkins, K.D.; Zhang, C.; Bazan, N.G. Docosahexaenoic Acid Signaling Modulates Cell Survival in Experimental Ischemic Stroke Penumbra and Initiates Long-Term Repair in Young and Aged Rats. PLoS ONE 2012, 7, e46151. [Google Scholar] [CrossRef] [Green Version]
- Eady, T.N.; Khoutorova, L.; Atkins, K.D.; Bazan, N.G.; Belayev, L. Docosahexaenoic Acid Complexed to Human Albumin in Experimental Stroke: Neuroprotective Efficacy with a Wide Therapeutic Window. Exp. Transl. Stroke Med. 2012, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Hjorth, E.; Zhu, M.; Toro, V.C.; Vedin, I.; Palmblad, J.; Cederholm, T.; Freund-Levi, Y.; Faxen-Irving, G.; Wahlund, L.-O.; Basun, H.; et al. Omega-3 Fatty Acids Enhance Phagocytosis of Alzheimer’s Disease-Related Amyloid-β 42 by Human Microglia and Decrease Inflammatory Markers. J. Alzheimer’s Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef] [Green Version]
- Manzhulo, O.; Tyrtyshnaia, A.; Kipryushina, Y.; Dyuizen, I.; Manzhulo, I. Docosahexaenoic Acid Induces Changes in Microglia/Macrophage Polarization after Spinal Cord Injury in Rats. Acta Histochem. 2018, 120, 741–747. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins: A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment that Counter Proinflammation Signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-Inflammatory and Proresolving Lipid Mediators. Annu. Rev. Pathol. 2008, 3, 279–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukiw, W.J.; Cui, J.-G.; Marcheselli, V.L.; Bodker, M.; Botkjaer, A.; Gotlinger, K.; Serhan, C.N.; Bazan, N.G. A Role for Docosahexaenoic Acid-Derived Neuroprotectin D1 in Neural Cell Survival and Alzheimer Disease. J. Clin. Investig. 2005, 115, 2774–2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, S.K.; Bazinet, R.P. The Emerging Role of Docosahexaenoic Acid in Neuroinflammation. Curr. Opin. Investig. Drugs 2008, 9, 735–743. [Google Scholar]
- Dalli, J.; Zhu, M.; Vlasenko, N.A.; Deng, B.; Haeggström, J.Z.; Petasis, N.A.; Serhan, C.N. The Novel 13S,14S-Epoxy-Maresin Is Converted by Human Macrophages to Maresin 1 (MaR1), Inhibits Leukotriene A4 Hydrolase (LTA4H), and Shifts Macrophage Phenotype. FASEB J. 2013, 27, 2573–2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel Macrophage Mediators with Potent Antiinflammatory and Proresolving Actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Francos-Quijorna, I.; Santos-Nogueira, E.; Gronert, K.; Sullivan, A.B.; Kopp, M.A.; Brommer, B.; David, S.; Schwab, J.M.; López-Vales, R. Maresin 1 Promotes Inflammatory Resolution, Neuroprotection, and Functional Neurological Recovery After Spinal Cord Injury. J. Neurosci. 2017, 37, 11731–11743. [Google Scholar] [CrossRef] [Green Version]
- Xian, W.; Wu, Y.; Xiong, W.; Li, L.; Li, T.; Pan, S.; Song, L.; Hu, L.; Pei, L.; Yao, S.; et al. The Pro-Resolving Lipid Mediator Maresin 1 Protects against Cerebral Ischemia/Reperfusion Injury by Attenuating the pro-Inflammatory Response. Biochem. Biophys. Res. Commun. 2016, 472, 175–181. [Google Scholar] [CrossRef]
- Xian, W.; Li, T.; Li, L.; Hu, L.; Cao, J. Maresin 1 Attenuates the Inflammatory Response and Mitochondrial Damage in Mice with Cerebral Ischemia/Reperfusion in a SIRT1-Dependent Manner. Brain Res. 2019, 1711, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Calon, F.; Julien, C.; Winkler, J.W.; Petasis, N.A.; Lukiw, W.J.; Bazan, N.G. Docosahexaenoic Acid-Derived Neuroprotectin D1 Induces Neuronal Survival via Secretase- and PPARγ-Mediated Mechanisms in Alzheimer’s Disease Models. PLoS ONE 2011, 6, e15816. [Google Scholar] [CrossRef] [Green Version]
- Abdelmoaty, S.; Wigerblad, G.; Bas, D.B.; Codeluppi, S.; Fernandez-Zafra, T.; El-Awady, E.-S.; Moustafa, Y.; Abdelhamid, A.E.S.; Brodin, E.; Svensson, C.I. Spinal Actions of Lipoxin A4 and 17(R)-Resolvin D1 Attenuate Inflammation-Induced Mechanical Hypersensitivity and Spinal TNF Release. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.-L.; Li, Q.-Q.; Chen, X.-P.; Zhang, X.-M.; Li, L.-L.; Li, B.-X.; Zhao, Z.-Q.; Tao, L.-Y. Lipoxin A4 Attenuates Brain Damage and Downregulates the Production of Pro-Inflammatory Cytokines and Phosphorylated Mitogen-Activated Protein Kinases in a Mouse Model of Traumatic Brain Injury. Brain Res. 2013, 1502, 1–10. [Google Scholar] [CrossRef]
- Medeiros, R.; Kitazawa, M.; Passos, G.F.; Baglietto-Vargas, D.; Cheng, D.; Cribbs, D.H.; LaFerla, F.M. Aspirin-Triggered Lipoxin A4 Stimulates Alternative Activation of Microglia and Reduces Alzheimer Disease-Like Pathology in Mice. Am. J. Pathol. 2013, 182, 1780–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.; Dichter, E.; Lacorte, G.; Kerner, D.; Spur, B.; Rodriguez, A.; Yin, K. Lipoxin A4 Increases Survival by Decreasing Systemic Inflammation and Bacterial Load in Sepsis. Shock 2011, 36, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Hu, Q.; Xu, L.; Guo, Z.-N.; Ou, Y.; He, Y.; Yin, C.; Sun, X.; Tang, J.; Zhang, J.H. Lipoxin A4 Reduces Inflammation through Formyl Peptide Receptor 2/P38 MAPK Signaling Pathway in Subarachnoid Hemorrhage Rats. Stroke 2016, 47, 490–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, A.C.; Berta, T.; Forner, S.; Chen, G.; Bento, A.F.; Ji, R.-R.; Rae, G.A. Lipoxin A4 Inhibits Microglial Activation and Reduces Neuroinflammation and Neuropathic Pain after Spinal Cord Hemisection. J. Neuroinflamm. 2016, 13, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-P.; Wu, Y.; Li, L.-Y.; Zheng, J.; Liu, R.-G.; Zhou, J.-P.; Yuan, S.-Y.; Shang, Y.; Yao, S.-L. Aspirin-Triggered Lipoxin A4 Attenuates LPS-Induced pro-Inflammatory Responses by Inhibiting Activation of NF-ΚB and MAPKs in BV-2 Microglial Cells. J. Neuroinflamm. 2011, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Hampson, A.J.; Grimaldi, M. 12-Hydroxyeicosatetrenoate (12-HETE) Attenuates AMPA Receptor-Mediated Neurotoxicity: Evidence for a G-Protein-Coupled HETE Receptor. J. Neurosci. 2002, 22, 257–264. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Y.-W.; Han, J.; Liang, H.; Wang, N.; Cheng, Y. 12/15-Lipoxygenase Metabolites of Arachidonic Acid Activate PPARγ: A Possible Neuroprotective Effect in Ischemic Brain. J. Lipid Res. 2015, 56, 502–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Control | Hydrolysate | DHA | |
|---|---|---|---|
| Water (µL/day) | 100 | - | 100 |
| Peanut oil (µL/day) | 50 | 50 | - |
| Fish hydrolysate group (µL/day) | - | 100 | - |
| Peptides (mg/day) | - | 5 | - |
| DHA (mg/day) | - | 0.143 | - |
| DHA supplementation group (µL/day) | - | - | 50 |
| DHA (mg/day) | - | - | 10 |
| Fatty Acid (% of Total Fatty Acids) | Control | Hydrolysate | DHA | Statistical Effect | |||||
|---|---|---|---|---|---|---|---|---|---|
| Saline | LPS | Saline | LPS | Saline | LPS | Supplementation | LPS | Supplementation × LPS | |
| 14:0 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.12 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 | - | - | - |
| 15:0 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.01 | 0.04 ± 0.00 | - | - | - |
| 16:0 | 21.43 ± 0.22 | 21.36 ± 0.31 | 21.36 ± 0.15 | 21.49 ± 0.12 | 21.27 ± 0.07 | 21.10 ± 0.15 | - | - | - |
| 17:0 | 0.14 ± 0.00 | 0.16 ± 0.01 | 0.14 ± 0.00 | 0.14 ± 0.00 | 0.16 ± 0.02 | 0.14 ± 0.00 | - | - | - |
| 18:0 | 20.03 ± 0.06 a | 19.65 ± 0.04 b | 20.06 ± 0.08 a | 19.66 ± 0.17 b | 19.74 ± 0.05 b | 19.78 ± 0.09 b | - | p = 0.0001 | p = 0.0034 |
| 20:0 | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.21 ±0.01 | 0.22 ± 0.01 | 0.23 ± 0.01 | - | - | - |
| 22:0 | 0.15 ± 0.01 | 0.17 ± 0.02 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.00 | - | - | - |
| 24:0 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.00 | 0.17 ± 0.01 | 0.18 ± 0.01 | - | - | - |
| SFAs | 42.31 ± 0.21 | 41.89 ± 0.32 | 42.27 ± 0.19 | 42.00 ± 0.15 | 41.89 ± 0.08 | 41.76 ± 0.20 | - | - | - |
| 16:1n-9 | 0.15 ± 0.00 | 0.16 ± 0.00 | 0.16 ± 0.00 | 0.16 ± 0.00 | 0.15 ± 0.00 | 0.15 ± 0.00 | p = 0.0047 | - | - |
| 16:1n-7 | 0.51 ± 0.01 | 0.53 ± 0.01 | 0.52 ± 0.00 | 0.53 ± 0.01 | 0.52 ± 0.01 | 0.52 ± 0.01 | - | - | - |
| 18:1t | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.07 ± 0.00 b | p = 0.0004 | - | p = 0.0114 |
| 18:1n-9 | 14.04 ± 0.11 | 14.17 ± 0.11 | 14.17 ± 0.11 | 14.12 ± 0.10 | 14.22 ± 0.18 | 14.42 ± 0.11 | - | - | - |
| 18:1n-7 | 3.51 ± 0.01 | 3.55 ± 0.04 | 3.53 ± 0.02 | 3.50 ± 0.02 | 3.49 ± 0.03 | 3.49 ± 0.01 | - | - | - |
| 20:1n-9 | 0.90 ± 0.03 | 0.94 ± 0.04 | 0.90 ± 0.03 | 0.90 ± 0.03 | 0.93 ± 0.06 | 0.96 ± 0.03 | - | - | - |
| 20:1n-7 | 0.24 ± 0.01 | 0.26 ± 0.01 | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.26 ± 0.02 | 0.26 ± 0.01 | - | - | - |
| 22:1n-9 | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.09 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.01 | 0.10 ± 0.00 | - | - | - |
| 24:1n-9 | 0.20 ± 0.01 a,b,c | 0.18 ± 0.01 b,e | 0.22 ± 0.01 a,d | 0.20 ± 0.01 b,d,e | 0.20 ± 0.01 a,e | 0.23 ± 0.01 c,d | - | - | p = 0.0473 |
| MUFAs | 19.68 ± 0.15 | 19.93 ± 0.18 | 19.86 ± 0.16 | 19.79 ± 0.15 | 19.90 ± 0.28 | 20.19 ± 0.14 | - | - | - |
| 18:2n-6 | 0.52 ± 0.01 | 0.47 ± 0.02 | 0.51 ± 0.01 | 0.52 ± 0.01 | 0.54 ± 0.01 | 0.52 ± 0.01 | - | - | - |
| 20:2n-6 | 0.13 ± 0.00 a | 0.12 ± 0.00 b | 0.12 ± 0.00 b,c | 0.13 ± 0.00 a,b | 0.13 ± 0.00 a | 0.13 ± 0.00 a,c | - | p = 0.0432 | p = 0.0089 |
| 20:3n-6 | 0.41 ± 0.01 | 0.39 ± 0.01 | 0.42 ± 0.00 | 0.42 ± 0.01 | 0.47 ± 0.01 | 0.46 ± 0.01 | p < 0.0001 | - | - |
| 20:4n-6 | 9.98 ± 0.11 | 10.15 ± 0.08 | 9.96 ± 0.09 | 9.95 ± 0.06 | 9.34 ± 0.16 | 9.43 ± 0.09 | p < 0.0001 | - | - |
| 22:4n-6 | 2.05 ± 0.03 | 2.17 ± 0.07 | 2.05 ± 0.02 | 2.14 ± 0.04 | 1.93 ± 0.04 | 2.02 ± 0.04 | p = 0.0069 | p = 0.0218 | - |
| 22:5n-6 | 0.30 ± 0.01 | 0.32 ± 0.02 | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.28 ± 0.02 | 0.27 ± 0.00 | p = 0.0001 | - | - |
| n-6 | 13.41 ± 0.13 | 13.61 ± 0.16 | 13.34 ± 0.08 | 13.45 ± 0.07 | 12.69 ± 0.19 | 12.81 ± 0.13 | p < 0.0001 | - | - |
| 20:5n-3 | 0.06 ± 0.01 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | p < 0.0001 | - | - |
| 22:5n-3 | 0.13 ± 0.00 | 0.14 ± 0.00 | 0.14 ± 0.00 | 0.14 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | p < 0.0001 | - | - |
| 22:6n-3 | 15.85 ± 0.29 | 15.67 ± 0.25 | 15.69 ± 0.24 | 16.09 ± 0.24 | 16.74 ± 0.28 | 16.19 ± 0.18 | p = 0.0175 | - | - |
| n-3 | 16.05 ± 0.29 | 15.87 ± 0.24 | 15.89 ± 0.24 | 16.29 ± 0.24 | 16.99 ± 0.28 | 16.43 ± 0.18 | p = 0.01 | - | - |
| 20:3n-9 | 0.13 ± 0.02 a,b | 0.09 ± 0.01 c,d | 0.17 ± 0.03 a | 0.08 ± 0.00 c,d | 0.10 ± 0.00 b,c | 0.12 ± 0.01 b,d | - | p = 0.0066 | p = 0.0122 |
| PUFAs | 29.59 ± 0.31 | 29.56 ± 0.28 | 29.40 ± 0.26 | 29.81 ± 0.20 | 29.78 ± 0.38 | 29.36 ± 0.13 | - | - | - |
| dma16:0 | 2.23 ± 0.03 | 2.36 ± 0.03 | 2.27 ± 0.03 | 2.27 ± 0.05 | 2.24 ± 0.05 | 2.28 ± 0.02 | - | - | - |
| dma18:0 | 3.99 ± 0.03 | 3.99 ± 0.03 | 3.98 ± 0.03 | 3.92 ± 0.03 | 3.97 ± 0.05 | 4.04 ± 0.03 | p = 0.0448 | - | - |
| dma18:1n-9 | 1.19 ± 0.02 | 1.22 ± 0.03 | 1.20 ± 0.03 | 1.19 ± 0.02 | 1.19 ± 0.03 | 1.24 ± 0.02 | - | - | - |
| dma18:1n-7 | 1.02 ± 0.02 | 1.05 ± 0.03 | 1.03 ± 0.02 | 1.03 ± 0.03 | 1.04 ± 0.03 | 1.08 ± 0.02 | - | - | - |
| DMA | 8.43 ± 0.08 | 8.62 ± 0.08 | 8.48 ± 0.06 | 8.41 ± 0.13 | 8.43 ± 0.15 | 8.64 ± 0.09 | - | - | - |
| n-6/n-3 | 0.84 ± 0.02 | 0.86 ± 0.02 | 0.84 ± 0.01 | 0.83 ± 0.01 | 0.75 ± 0.01 | 0.78 ± 0.01 | p < 0.0001 | - | - |
| Oxylipin (pg /mg protein) | Control | Hydrolysate | DHA | Statistical Effect | |||||
|---|---|---|---|---|---|---|---|---|---|
| Saline | LPS | Saline | LPS | Saline | LPS | Supplementation | LPS | Supplementation × LPS | |
| AA-oxylipins | |||||||||
| Thromboxanes, prostaglandins | |||||||||
| TxB2 | 1584.36 ± 136.93 | 1739.60 ± 138.94 | 1729.72 ± 220.19 | 1495.16 ± 30.78 | 1781.36 ± 166.54 | 1747.47 ± 205.07 | - | - | - |
| 6kPGF1α | 151.68 ± 68.79 a | 505.96 ± 66.67 b | 246.65 ± 83.26 a | 78.59 ± 49.27 a | 210.93 ± 69.00 a | 226.75 ± 92.39 a | - | - | p = 0.0086 |
| PGF2α | 4429.79 ± 380.97 | 5550.45 ± 330.52 | 4582.04 ± 486.14 | 5708.87 ± 577.25 | 5656.33 ± 461.46 | 5155.75 ± 727.26 | - | - | - |
| PGE2 | 1428.02 ± 141.17 a | 2366.55 ± 121.53 b | 1410.87 ± 117.93 a | 2356.64 ± 224.38 b | 1883.66 ± 204.02 a,b | 1881.02 ± 257.90 a,b | - | p = 0.0003 | p = 0.0211 |
| PGD2 | 1741.08 ± 319.14 a | 3208.60 ± 445.99 b | 1639.05 ± 216.33 a | 3188.42 ± 282.95 b,c | 2301.83 ± 244.21 a,b | 2178.11 ± 411.06 a,c | - | p = 0.002 | p = 0.0314 |
| PGA1 | 0.00 ± 0.00 a | 28.65 ± 7.78 b | 0.00 ± 0.00 a | 34.75 ± 11.80 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | p = 0.0014 | p < 0.0001 | p = 0.0014 |
| 15dPGJ2 | 31.15 ± 19.37 | 71.23 ± 8.44 | 13.59 ± 13.59 | 89.69 ± 19.08 | 45.69 ± 23.73 | 53.66 ± 24.59 | - | p = 0.0145 | - |
| 8isoPGA2 | 464.81 ± 24.87 | 651.95 ± 76.86 | 500.41 ± 109.57 | 864.62 ± 133.33 | 682.20 ± 116.56 | 750.64 ± 92.68 | - | p = 0.0184 | - |
| Lipoxins | |||||||||
| LxA4 | 265.31 ± 31.85 a | 764.10 ± 117.00 c | 352.61 ± 37.66 a,b | 1126.31 ± 115.00 d | 631.05 ± 121.16 b,c | 614.25 ± 151.20 b,c | - | p < 0.0001 | p = 0.0041 |
| LxB4 | 119.60 ± 79.81 | 413.04 ± 100.91 | 488.09 ± 79.65 | 560.64 ± 67.12 | 342.60 ± 115.89 | 309.99 ± 112.83 | p = 0.0483 | - | - |
| Epoxy fatty acids | |||||||||
| 14,15-EET | 258.15 ± 55.32 | 218.68 ± 27.43 | 221.62 ± 44.03 | 339.82 ± 95.94 | 317.46 ± 68.49 | 321.12 ± 36.59 | - | - | - |
| 8,9-EET | 665.74 ± 86.14 | 717.35 ± 62.99 | 745.55 ± 88.82 | 1132.12 ± 251.53 | 984.20 ± 127.64 | 904.92 ± 119.39 | - | - | - |
| 5,6-EET | 510.40 ± 59.39 | 552.36 ± 22.88 | 607.26 ± 61.65 | 776.15 ± 152.98 | 730.88 ± 94.28 | 687.14 ± 90.15 | - | - | - |
| Hydroxy fatty acids | |||||||||
| 15-HETE | 5381.63 ± 491.85 a | 10236.55 ± 1133.60 b,c | 4986.08 ± 394. 85 a | 12370.92 ± 946.68 c | 8412.97 ± 1033.41 b | 8271.61 ± 1124.26 b | - | p < 0.0001 | p = 0.0014 |
| 8-HETE | 616.39 ± 59.62 a | 861.88 ± 71.18 b | 536.21 ± 32.63 a | 1094.93 ± 100.61 c | 825.45 ± 81.77 b | 823.81 ± 84.78 b | - | p = 0.0001 | p = 0.0033 |
| 12-HETE | 1496.97 ± 53.35 a,b | 2662.28 ± 394.98 c,d | 1188.01 ± 51.49 a | 3479.62 ± 252.58 d | 2076.89 ± 258.48 b,c | 2145.92 ± 439.72 b,c | - | p < 0.0001 | p = 0.0044 |
| 5-HETE | 5518.82 ± 671.54 | 6747.00 ± 403.13 | 5484.87 ± 486.33 | 8002.11 ± 382.00 | 7715.60 ± 1005.41 | 8939.82 ± 1212.00 | p = 0.0318 | p = 0.0262 | - |
| 5-oxoETE | 5524.57 ± 639.67 a | 6300.15 ± 474.39 a,c | 5286.41 ± 199.95 a | 9143.13 ± 785.06 b | 8623.43 ± 291.86 b | 7449.52 ± 981.74 b,c | p = 0.0072 | p = 0.0342 | p = 0.0022 |
| LA-oxylipins | |||||||||
| Hydroxy fatty acids | |||||||||
| 13-HODE | 1294.54 ± 41.33 a | 2091.36 ± 184.04 a | 1429.20 ± 192.49 a | 3655.32 ± 871.76 b | 2266.32 ± 315.64 a | 2212.73 ± 339.80 a | - | p = 0.0026 | p = 0.0134 |
| 9-HODE | 497.38 ± 19.99 a | 979.03 ± 109.81 b,c | 577.19 ± 76.69 a,b | 1628.82 ± 355.30 d | 967.76 ± 132.13 b,c | 950.78 ± 147.61 a,c | - | p = 0.0005 | p = 0.0066 |
| DHA-oxylipins | |||||||||
| Hydroxy fatty acids | |||||||||
| 17-HDoHE | 1208.83 ± 91.11 a,b | 2030.94 ± 216.72 c,d | 1002.70 ± 83.64 b | 2428.07 ± 154.48 c | 1717.12 ± 318.00 a,d | 1731.76 ± 194.06 a,d | - | p = 0.0001 | p = 0.0076 |
| 14-HDoHE | 819.92 ± 71.54 a,b | 1171.61 ± 168.81 b,c | 639.76 ± 45.18 a | 1683.52 ± 143.81 d | 1266.36 ± 167.27 c,d | 1290.20 ± 175.01 c,d | - | p = 0.0004 | p = 0.0056 |
| 7-MaR1 | 0.00 ± 0.00 a,b | 42.73 ± 11.82 b | 0.00 ± 0.00 a | 88.89 ± 50.81 c | 0.00 ± 0.00a | 0.00 ± 0.00a | p = 0.0289 | p = 0.0019 | p = 0.0289 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chataigner, M.; Martin, M.; Lucas, C.; Pallet, V.; Layé, S.; Mehaignerie, A.; Bouvret, E.; Dinel, A.-L.; Joffre, C. Fish Hydrolysate Supplementation Containing n-3 Long Chain Polyunsaturated Fatty Acids and Peptides Prevents LPS-Induced Neuroinflammation. Nutrients 2021, 13, 824. https://doi.org/10.3390/nu13030824
Chataigner M, Martin M, Lucas C, Pallet V, Layé S, Mehaignerie A, Bouvret E, Dinel A-L, Joffre C. Fish Hydrolysate Supplementation Containing n-3 Long Chain Polyunsaturated Fatty Acids and Peptides Prevents LPS-Induced Neuroinflammation. Nutrients. 2021; 13(3):824. https://doi.org/10.3390/nu13030824
Chicago/Turabian StyleChataigner, Mathilde, Marie Martin, Céline Lucas, Veronique Pallet, Sophie Layé, Alexis Mehaignerie, Elodie Bouvret, Anne-Laure Dinel, and Corinne Joffre. 2021. "Fish Hydrolysate Supplementation Containing n-3 Long Chain Polyunsaturated Fatty Acids and Peptides Prevents LPS-Induced Neuroinflammation" Nutrients 13, no. 3: 824. https://doi.org/10.3390/nu13030824