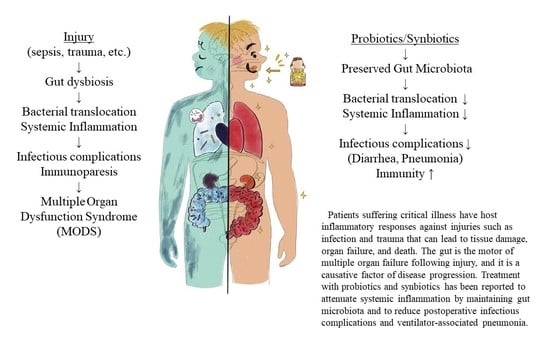
Gut Microbiota and Probiotics/Synbiotics for Modulation of Immunity in Critically Ill Patients
Abstract
:1. Introduction
2. Gut Origin of Sepsis and Systemic Inflammation
3. Gut Microbiota in Critically Ill Patients
4. Immune Reactions through Gut Microbiota
5. Effects of Probiotics and Synbiotics during Critical Illness
5.1. Influence of Probiotics on Gut and Immunity
5.2. Effectiveness of Probiotics and Synbiotics for Diarrhea
5.3. Prophylactic Effect of Probiotics and Synbiotics on Critical Illness
5.4. Gut Dysmotility and Limitations for Intestinal Therapy
6. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, R.M.; Walton, M.A.; Carter, P.M. The Major Causes of Death in Children and Adolescents in the United States. N. Engl. J. Med. 2018, 379, 2468–2475. [Google Scholar] [CrossRef]
- Stoecklein, V.M.; Osuka, A.; Lederer, J.A. Trauma equals danger—Damage control by the immune system. J. Leukoc. Biol. 2012, 92, 539–551. [Google Scholar] [CrossRef]
- Rosenthal, M.D.; Moore, F.A. Persistent Inflammation, Immunosuppression, and Catabolism: Evolution of Multiple Organ Dysfunction. Surg. Infect. (Larchmt) 2016, 17, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Otani, S.; Coopersmith, C.M. Gut integrity in critical illness. J. Intensiv. Care 2019, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.A.; Coopersmith, C.M. Intestinal crosstalk: A new paradigm for understanding the gut as the “motor” of critical illness. Shock 2007, 28, 384–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, E.E.; Moore, F.A.; Franciose, R.J.; Kim, F.J.; Biffl, W.L.; Banerjee, A. The Postischemic Gut Serves as a Priming Bed for Circulating Neutrophils that Provoke Multiple Organ Failure. J. Trauma Inj. Infect. Crit. Care 1994, 37, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Eaves-Pyles, T.; Alexander, J.W. Rapid and Prolonged Impairment of Gut Barrier Function after Thermal Injury in Mice. Shock 1998, 9, 95–100. [Google Scholar] [CrossRef]
- Magnotti, L.J.; Upperman, J.S.; Xu, D.-Z.; Lu, Q.; Deitch, E.A. Gut-Derived Mesenteric Lymph but not Portal Blood Increases Endothelial Cell Permeability and Promotes Lung Injury After Hemorrhagic Shock. Ann. Surg. 1998, 228, 518–527. [Google Scholar] [CrossRef] [Green Version]
- I Ramzy, P.; E Wolf, S.; Irtun, O.; Hart, D.W.; Thompson, J.C.; Herndon, D.N. Gut epithelial apoptosis after severe burn: Effects of gut hypoperfusion11No competing interests declared. J. Am. Coll. Surg. 2000, 190, 281–287. [Google Scholar] [CrossRef]
- Coopersmith, C.M.; Stromberg, P.E.; Dunne, W.M.; Davis, C.G.; Amiot, D.M., II; Buchman, T.G.; Karl, I.E.; Hotchkiss, R.S. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA 2002, 287, 1716–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoseph, B.P.; Klingensmith, N.J.; Liang, Z.; Breed, E.; Burd, E.M.; Mittal, R.; Dominguez, J.A.; Petrie, B.; Ford, M.L.; Coopersmith, C.M. Mechanisms of Intestinal Barrier Dysfunction in Sepsis. Shock 2016, 46, 52–59. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, C.J.; MacFie, J.; Mitchell, C.J.; Johnstone, D.; Sagar, P.M.; Sedman, P.C. Microbiology of bacterial translocation in humans. Gut 1998, 42, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Yokoyama, Y.; Nishio, H.; Ebata, T.; Sugawara, G.; Asahara, T.; Nomoto, K.; Nagino, M. Intraoperative Bacterial Translocation Detected by Bacterium-Specific Ribosomal RNA-Targeted Reverse-Transcriptase Polymerase Chain Reaction for the Mesenteric Lymph Node Strongly Predicts Postoperative Infectious Complications After Major Hepatectomy for Biliary Malignancies. Ann. Surg. 2010, 252, 1013–1019. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Goto, M.; Asahara, T.; Nomoto, K.; Morotomi, M.; Yoshiya, K.; Matsushima, A.; Sumi, Y.; Kuwagata, Y.; et al. Altered Gut Flora and Environment in Patients with Severe SIRS. J. Trauma Inj. Infect. Crit. Care 2006, 60, 126–133. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Hamasaki, T.; Goto, M.; Tasaki, O.; Asahara, T.; Nomoto, K.; Morotomi, M.; Matsushima, A.; Kuwagata, Y.; et al. Altered Gut Flora Are Associated with Septic Complications and Death in Critically Ill Patients with Systemic Inflammatory Response Syndrome. Dig. Dis. Sci. 2010, 56, 1171–1177. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef] [Green Version]
- Ojima, M.; Motooka, D.; Shimizu, K.; Gotoh, K.; Shintani, A.; Yoshiya, K.; Nakamura, S.; Ogura, H.; Iida, T.; Shimazu, T. Metagenomic Analysis Reveals Dynamic Changes of Whole Gut Microbiota in the Acute Phase of Intensive Care Unit Patients. Dig. Dis. Sci. 2016, 61, 1628–1634. [Google Scholar] [CrossRef] [Green Version]
- HayakawaTakashi, M.; Asahara, T.; Henzan, N.; Murakami, H.; Yamamoto, H.; Mukai, N.; Minami, Y.; Sugano, M.; Kubota, N.; Uegaki, S.; et al. Dramatic Changes of the Gut Flora Immediately After Severe and Sudden Insults. Dig. Dis. Sci. 2011, 56, 2361–2365. [Google Scholar] [CrossRef] [Green Version]
- Ojima, M.; Shimizu, K.; Motooka, D.; Ishihara, T.; Nakamura, S.; Shintani, A.; Ogura, H.; Iida, T.; Yoshiya, K.; Shimazu, T. Gut Dysbiosis Associated with Antibiotics and Disease Severity and Its Relation to Mortality in Critically Ill Patients. Dig. Dis. Sci. 2021, 1–13. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Tomono, K.; Tasaki, O.; Asahara, T.; Nomoto, K.; Morotomi, M.; Matsushima, A.; Nakahori, Y.; Yamano, S.; et al. Patterns of Gram-Stained Fecal Flora as a Quick Diagnostic Marker in Patients with Severe SIRS. Dig. Dis. Sci. 2011, 56, 1782–1788. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.; Samuel, B.S.; Gordon, J.I.; Relman, D.; Fraser, C.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Vollaard, E.J.; Clasener, H.A. Colonization resistance. Antimicrob. Agents Chemother. 1994, 38, 409–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, C.A.T.N.Z.M.L.E. The microbiome and innate immunity. Nat. Cell Biol. 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nat. Cell Biol. 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic acid Bacteria; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Sanders, M.E.; Guarner, F.; Guerrant, R.; Holt, P.; Quigley, E.M.M.; Sartor, R.B.; Sherman, P.; A Mayer, E. An update on the use and investigation of probiotics in health and disease. Gut 2013, 62, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Morotomi, M.; Tasaki, O.; Matsushima, A.; Kuwagata, Y.; Shimazu, T.; Sugimoto, H. Probiotic/Synbiotic Therapy for Treating Critically Ill Patients from a Gut Microbiota Perspective. Dig. Dis. Sci. 2012, 58, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bron, P.A.; van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Genet. 2011, 10, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; Marchesi, J.R.; McDonald, J.A.; Pass, D.A.; Masetti, G.; Michael, D.R.; Plummer, S.; Jack, A.A.; Davies, T.S.; Hughes, T.R.; et al. Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: Should we be considering probiotics during viral pandemics? Gut Microbes 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Genet. 2010, 8, 171–184. [Google Scholar] [CrossRef]
- Schlee, M.; Wehkamp, J.; Altenhoefer, A.; Oelschlaeger, T.A.; Stange, E.F.; Fellermann, K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect. Immun. 2007, 75, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahara, T.; Shimizu, K.; Nomoto, K.; Hamabata, T.; Ozawa, A.; Takeda, Y. Probiotic Bifidobacteria Protect Mice from Lethal Infection with Shiga Toxin-Producing Escherichia coli O157:H7. Infect. Immun. 2004, 72, 2240–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahara, T.; Takahashi, A.; Yuki, N.; Kaji, R.; Takahashi, T.; Nomoto, K. Protective Effect of a Synbiotic against Multidrug-Resistant Acinetobacter baumannii in a Murine Infection Model. Antimicrob. Agents Chemother. 2016, 60, 3041–3050. [Google Scholar] [CrossRef] [Green Version]
- Yasui, H.; Kiyoshima, J.; Hori, T. Reduction of Influenza Virus Titer and Protection against Influenza Virus Infection in Infant Mice Fed Lactobacillus casei Shirota. Clin. Vaccine Immunol. 2004, 11, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Hagihara, M.; Kuroki, Y.; Ariyoshi, T.; Higashi, S.; Fukuda, K.; Yamashita, R.; Matsumoto, A.; Mori, T.; Mimura, K.; Yamaguchi, N.; et al. Clostridium butyricum Modulates the Microbiome to Protect Intestinal Barrier Function in Mice with Antibiotic-Induced Dysbiosis. iScience 2020, 23, 100772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khailova, L.; Baird, C.H.; Rush, A.A.; McNamee, E.N.; Wischmeyer, P.E. Lactobacillus rhamnosus GG improves outcome in experimental pseudomonas aeruginosa pneumonia: Potential role of regulatory T cells. Shock 2013, 40, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Tirlapur, N.; Puthucheary, Z.A.; Cooper, J.A.; Sanders, J.; Coen, P.G.; Moonesinghe, S.R.; Wilson, A.P.; Mythen, M.G.; Montgomery, H.E. Diarrhoea in the critically ill is common, associated with poor outcome and rarely due to Clostridium difficile. Sci. Rep. 2016, 6, 24691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickson, M.; D’Souza, A.L.; Muthu, N.; Rogers, T.; Want, S.; Rajkumar, C.; Bulpitt, C.J. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: Randomised double blind placebo controlled trial. BMJ 2007, 335, 80. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, A.L.; Rajkumar, C.; Cooke, J.; Bulpitt, C.J. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ 2002, 324, 1361. [Google Scholar] [CrossRef] [Green Version]
- Bleichner, G.; Blehaut, H.; Mentec, H.; Moyse, D. Saccharomyces boulardii prevents diarrhea in critically ill tube-fed patients. Intensiv. Care Med. 1997, 23, 517–523. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Kabata, D.; Shintani, A.; Tasaki, O.; Ojima, M.; Ikeda, M.; Shimazu, T. Association of prophylactic synbiotics with reduction in diarrhea and pneumonia in mechanically ventilated critically ill patients: A propensity score analysis. J. Infect. Chemother. 2018, 24, 795–801. [Google Scholar] [CrossRef]
- Alagna, L.; Haak, B.W.; Gori, A. Fecal microbiota transplantation in the ICU: Perspectives on future implementations. Intensiv. Care Med. 2019, 45, 998–1001. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; E Coffin, S.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Wurm, P.; Spindelboeck, W.; Krause, R.; Plank, J.; Fuchs, G.; Bashir, M.; Petritsch, W.; Halwachs, B.; Langner, C.; Hogenauer, C.; et al. Antibiotic-Associated Apoptotic Enterocolitis in the Absence of a Defined Pathogen: The Role of Intestinal Microbiota Depletion. Crit. Care Med. 2017, 45, e600–e606. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Hiroshi, O.; Goto, M.; Tasaki, O.; Kuwagata, Y.; Tanaka, H.; Shimazu, T.; Sugimoto, H.; Asahara, T.; Nomoto, K.; et al. Synbiotics Reduce the Septic Complications in Patients with Severe Sirs. Crit. Care Med. 2005, 33, A9. [Google Scholar] [CrossRef]
- Wu, X.D.; Liu, M.M.; Liang, X.; Hu, N.; Huang, W. Effects of perioperative supplementation with pro-/synbiotics on clinical outcomes in surgical patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.J.; Deng, T.; Gong, Y.Z.; Jing, R.; Liu, J.C. The effects of probiotics in early enteral nutrition on the outcomes of trauma: A meta-analysis of randomized controlled trials. JPEN J. Parenter. Enteral Nutr. 2013, 37, 310–317. [Google Scholar] [CrossRef]
- Sugawara, G.; Nagino, M.; Nishio, H.; Ebata, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Nimura, Y. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: A randomized controlled trial. Ann. Surg. 2006, 244, 706–714. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Goto, M.; Asahara, T.; Nomoto, K.; Morotomi, M.; Matsushima, A.; Tasaki, O.; Fujita, K.; Hosotsubo, H.; et al. Synbiotics Decrease the Incidence of Septic Complications in Patients with Severe SIRS: A Preliminary Report. Dig. Dis. Sci. 2008, 54, 1071–1078. [Google Scholar] [CrossRef]
- Shimizu, K.; Yamada, T.; Ogura, H.; Mohri, T.; Kiguchi, T.; Fujimi, S.; Asahara, T.; Ojima, M.; Ikeda, M.; Shimazu, T. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: A randomized controlled trial. Crit. Care 2018, 22, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batra, P.; Soni, K.D.; Mathur, P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: An updated systematic review and meta-analysis of randomized control trials. J. Intensive Care 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nat. Cell Biol. 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.C.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Borrazzo, C.; Pinacchio, C.; Santinelli, L.; Innocenti, G.P.; Cavallari, E.N.; Celani, L.; Marazzato, M.; Alessandri, F.; Ruberto, F.; et al. Oral Bacteriotherapy in Patients With COVID-19: A Retrospective Cohort Study. Front. Nutr. 2021, 7, 613928. [Google Scholar] [CrossRef] [PubMed]
- Fruhwald, S.; Holzer, P.; Metzler, H. Intestinal motility disturbances in intensive care patients pathogenesis and clinical impact. Intensiv. Care Med. 2006, 33, 36–44. [Google Scholar] [CrossRef]
- Caddell, K.A.; Martindale, R.; McClave, S.A.; Miller, K. Can the Intestinal Dysmotility of Critical Illness be Differentiated from Postoperative Ileus? Curr. Gastroenterol. Rep. 2011, 13, 358–367. [Google Scholar] [CrossRef]
- Montejo, J.C. Enteral nutrition-related gastrointestinal complications in critically ill patients: A multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit. Care Med. 1999, 27, 1447–1453. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Morotomi, M.; Nakahori, Y.; Osuka, A.; Yamano, S.; Goto, M.; Matsushima, A.; et al. Gastrointestinal dysmotility is associated with altered gut flora and septic mortality in patients with severe systemic inflammatory response syndrome: A preliminary study. Neurogastroenterol. Motil. 2010, 23, 330-e157. [Google Scholar] [CrossRef]
- Shimojima, N.; Nakaki, T.; Morikawa, Y.; Hoshino, K.; Ozaki, H.; Hori, M.; Kitajima, M. Interstitial Cells of Cajal in Dysmotility in Intestinal Ischemia and Reperfusion Injury in Rats. J. Surg. Res. 2006, 135, 255–261. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Matsumoto, N.; Ikeda, M.; Yamamoto, H.; Mori, M.; Morii, E.; Shimazu, T. Interstitial cells of Cajal are diminished in critically ill patients: Autopsy cases. Nutrition 2020, 70, 110591. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, Z.; Wu, K.; Wang, B.; Yuang, Y. Gastrointestinal dysmotility in patients with acute pancreatitis. J. Gastroenterol. Hepatol. 2003, 18, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Oláh, A.; Belágyi, T.; Issekutz, Á.; Gamal, M.E.; Bengmark, S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. BJS 2002, 89, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, T. Dutch probiotics study is criticised for its “design, approval, and conduct”. BMJ 2010, 340, c77. [Google Scholar] [CrossRef] [PubMed]
- Expression of concern–Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 875–876. [CrossRef]
- Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Matsushima, A.; Hayakawa, K.; Ikegawa, H.; Tasaki, O.; Kuwagata, Y.; Shimazu, T. Gut microbiota and environment in patients with major burns–A preliminary report. Burns 2015, 41, e28–e33. [Google Scholar] [CrossRef] [PubMed]






| SIRS Patients | Normal | |
|---|---|---|
| Total obligate anaerobes | 8.3 ± 2.3 * | 10.5 ± 0.5 |
| Bacteroidaceae | 7.3 ± 3.0 * | 10.1 ± 0.4 |
| Bifidobacterium | 4.8 ± 3.3 * | 9.6 ± 0.7 |
| Clostridium | 2.1 ± 1.0 | 2.1 ± 0.7 |
| Veillonella | 3.1 ± 1.8 * | 7.0 ± 1.2 |
| Total facultative anaerobes | 7.8 ± 1.4 | 7.5 ± 0.4 |
| Lactobacillus | 2.7 ± 1.5 * | 5.0 ± 1.0 |
| Enterobacteriaceae | 4.1 ± 2.7 * | 7.4 ± 0.8 |
| Enterococcus | 6.4 ± 2.5 | 7.0 ± 0.9 |
| Staphylococcus | 5.3 ± 1.7 * | 2.7 ± 0.8 |
| Pseudomonas | 2.8 ± 1.4 * | ND |
| Candida | 2.5 ± 1.0 | 2.0 ± 0.5 |
| SIRS Patients | Normal | |
|---|---|---|
| Total organic acid | 30.3 ± 20.3 * | 88.4 ± 21.2 |
| Succinic acid | 2.0 ± 2.5 | 0.9 ± 1.2 |
| Lactic acid | 3.8 ± 5.5 | 0.5 ± 0.3 |
| Formic acid | 1.7 ± 2.9 | 0.4 ± 0.3 |
| Acetic acid | 18.7 ± 15.9 * | 50.8 ± 13.1 |
| Propionic acid | 2.5 ± 4.6 * | 18.7 ± 6.8 |
| Isobutyric acid | 0.1 ± 0.5 | 1.1 ± 0.3 |
| Butyric acid | 0.9 ± 2.3 * | 16.6 ± 6.7 |
| Isovaleric acid | 0.5 ± 1.9 | 1.4 ± 0.7 |
| Valeric acid | 0.1 ± 0.7 | 0.6 ± 0.4 |
| pH | 7.4 ± 0.6 * | 6.6 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, K.; Ojima, M.; Ogura, H. Gut Microbiota and Probiotics/Synbiotics for Modulation of Immunity in Critically Ill Patients. Nutrients 2021, 13, 2439. https://doi.org/10.3390/nu13072439
Shimizu K, Ojima M, Ogura H. Gut Microbiota and Probiotics/Synbiotics for Modulation of Immunity in Critically Ill Patients. Nutrients. 2021; 13(7):2439. https://doi.org/10.3390/nu13072439
Chicago/Turabian StyleShimizu, Kentaro, Masahiro Ojima, and Hiroshi Ogura. 2021. "Gut Microbiota and Probiotics/Synbiotics for Modulation of Immunity in Critically Ill Patients" Nutrients 13, no. 7: 2439. https://doi.org/10.3390/nu13072439