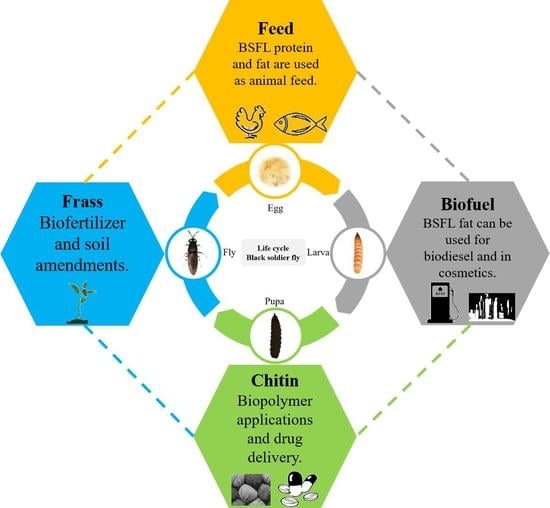
Larvae Mediated Valorization of Industrial, Agriculture and Food Wastes: Biorefinery Concept through Bioconversion, Processes, Procedures, and Products
Abstract
:1. Introduction
- Insect as biofactories—valorization of waste streams with insect bioconversion, BSFL for waste management and upcycling of nutrients to establish a circular economy is envisaged;
- Biorefining and fractionation—conventional, industrial-scale processing techniques for the retrieval of BSFL constituents with industrial patents as examples and other novel processing mechanisms are deliberated;
- Lipids—the oil content of BSFL reared on different substrates, the fatty acid profile, the lipid class compounds and its application in food, feed and other fields are discussed in detail;
- Proteins—the protein content of BSFL reared on different substrates, properties of partially and highly-defatted protein meal, the nitrogen-to-protein conversion ratio and feed applications;
- Amino acids—the essential amino acid index, apparent and ileal digestibility of various BSFL meal in vitro and in vivo, the limiting amino acids are covered;
- Chitosan—the extraction of chitin and chitin derivatives, the properties and applications of chitin and chitosan are discussed.
2. Insects as Biofactories: Nutrition Upcycling of Food Wastes
3. Biorefining and Fractionation of BSFL Biomass
3.1. Killing Methods
3.2. Fractionation
3.3. Biorefining
3.4. Lipids
3.4.1. Lipids in Human Nutrition
3.4.2. Lipids in Animal Nutrition
3.4.3. Non-Food Applications of BSFL Lipids: Biodiesel
3.4.4. Non-Food Applications of BSFL Lipids: Cosmetic Applications
3.5. Proteins
3.5.1. Protein Extraction
3.5.2. The Kp Conundrum
3.5.3. Essential Amino Acids and Their Digestibility
3.5.4. The Molecular Weight Distribution of BSFL Proteins
3.5.5. BSFL Protein Meal as Feed
3.6. Chitin
3.7. Properties and Applications of BSF Chitin
3.8. Minerals
3.9. Functional and Bioactive Peptides
3.10. Frass
4. BSFL Rearing
5. Legislation and Safety Aspects of BSF
6. Conclusion and Prospects
6.1. SWOT Analysis for Insect Processing
6.1.1. Strengths
6.1.2. Weaknesses
6.1.3. Opportunities
6.1.4. Threats
6.2. Insect Rearing: Sustainable Development Goals Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; ESA Working paper No. 12-03; FAO: Rome, Italy, 2012. [Google Scholar]
- Van Huis, A. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Huis, A. Edible insects contributing to food security? Agric. Food Secur. 2015, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Varelas, V. Food wastes as a potential new source for edible insect mass production for food and feed: A review. Fermentation 2019, 5, 81. [Google Scholar] [CrossRef] [Green Version]
- Diener, S.; Zurbrügg, C.; Roa Gutiérrez, F.; Nguyen, D.H.; Morel, A.; Koottatep, T.; Tockner, K. Black soldier fly larvae for organic waste treatment–prospects and constraints. In Proceedings of the Waste Safe 2011—2nd International Solid Waste Management in the Developing Countries, Khulna, Bangladesh, 13–15 February 2011; pp. 52–59. [Google Scholar]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects—Future Prospects for Food and Feed Security; FAO Forestry Paper 171; FAO: Rome, Italy, 2013. [Google Scholar]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Ravi, H.K.; Vian, M.A.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative solvents for lipid extraction and their effect on protein quality in black soldier fly (Hermetia illucens) larvae. J. Clean. Prod. 2019, 238, 117861. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sánchez-Muros, M.-J.; Ramos, R.; Segura, M.; Morote, E.; Guil, J.-L.; Torres, A. Insects as food: Enrichment of larvae of Hermetia illucens with omega 3 fatty acids by means of dietary modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food Wastage Footprint: Impacts on Natural Resources; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U. Global Food Losses and Food Waste. Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: “Global Food Losses and Food Waste—Extent, Causes and Prevention—FAO; SIK—The Swedish Institute for Food and Biotechnology: Gothenburg, Sweden, 2013. [Google Scholar]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Corsini, A.; Boschi, N.; Galletti, A.M.R. Smart valorization of waste biomass: Exhausted lemon peels, coffee silverskins and paper wastes for the production of levulinic acid. Chem. Eng. Trans. 2018, 65, 637–642. [Google Scholar]
- Yu, I.K.M.; Tsang, D.C.W.; Chen, S.S.; Wang, L.; Hunt, A.J.; Sherwood, J.; De Oliveira Vigier, K.; Jérôme, F.; Ok, Y.S.; Poon, C.S. Polar aprotic solvent-water mixture as the medium for catalytic production of hydroxymethylfurfural (HMF) from bread waste. Bioresour. Technol. 2017, 245, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Karmee, S.K. Liquid biofuels from food waste: Current trends, prospect and limitation. Renew. Sustain. Energy Rev. 2016, 53, 945–953. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, S.P.M. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of other Value-Added Products; FAO: Rome, Italy, 2013; ISBN 978-92-5-107631-6. [Google Scholar]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2020, 102, 319–329. [Google Scholar] [CrossRef]
- Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed 2020, 6, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Van Hal, O.; de Boer, I.J.M.; Muller, A.; de Vries, S.; Erb, K.H.; Schader, C.; Gerrits, W.J.J.; van Zanten, H.H.E. Upcycling food leftovers and grass resources through livestock: Impact of livestock system and productivity. J. Clean. Prod. 2019, 219, 485–496. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, K.; Zhong, W.; Liu, N.; Wu, X.; Li, W.; Zheng, L.; Yu, Z.; Zhang, J. Bioconversion-Composting of Golden Needle Mushroom (Flammulina velutipes) Root Waste by Black Soldier Fly (Hermetia illucens, Diptera: Stratiomyidae) Larvae, to Obtain Added-Value Biomass and Fertilizer. Waste Biomass Valoriz. 2019, 10, 265–273. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Larouche, J.; Deschamps, M.-H.; Saucier, L.; Lebeuf, Y.; Doyen, A.; Vandenberg, G.W. Effects of Killing Methods on Lipid Oxidation, Colour and Microbial Load of Black Soldier Fly (Hermetia illucens) Larvae. Animals 2019, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- Leni, G.; Caligiani, A.; Sforza, S. Killing method affects the browning and the quality of the protein fraction of Black Soldier Fly (Hermetia illucens) prepupae: A metabolomics and proteomic insight. Food Res. Int. 2019, 115, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Marseglia, A.; Sorci, A.; Bonzanini, F.; Lolli, V.; Maistrello, L.; Sforza, S. Influence of the killing method of the black soldier fly on its lipid composition. Food Res. Int. 2018, 116, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Arsiwalla, T.; Aarts, K. Method to Convert Insects or Worms into Nutrient Streams and Compositions Obtained Thereby. US Patent 2015/0223508A1, 13 August 2015. [Google Scholar]
- Leni, G.; Soetemans, L.; Jacobs, J.; Depraetere, S.; Gianotten, N.; Bastiaens, L.; Caligiani, A.; Sforza, S. Protein hydrolysates from Alphitobius diaperinus and Hermetia illucens larvae treated with commercial proteases. J. Insects Food Feed 2020, 1–12, in press. [Google Scholar] [CrossRef]
- Stefaan, D.; Johan, J. A Method for Separating Larvae in a Pulp and a Liquid Fraction. Patent WO 2019/081067 A1, 2 May 2019. [Google Scholar]
- Soetemans, L.; Uyttebroek, M.; D’Hondt, E.; Bastiaens, L. Use of organic acids to improve fractionation of the black soldier fly larvae juice into lipid- and protein-enriched fractions. Eur. Food Res. Technol. 2019, 245, 2257–2267. [Google Scholar] [CrossRef] [Green Version]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential Extraction and Characterisation of Lipids, Proteins, and Chitin from Black Soldier Fly (Hermetia illucens) Larvae, Prepupae, and Pupae. Waste Biomass Valoriz. 2020. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.S.F. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, K.; Surendra, K.C.; Tomberlin, J.K.; Khanal, S.K. Insect-Based Biorefinery for Bioenergy and Bio-Based Products; Elsevier B.V.: Amsterdam, The Netherlands, 2018; ISBN 9780444639929. [Google Scholar]
- Keegan, D.; Kretschmer, B.; Elbersen, B.; Panoutsou, C. Cascading use: A systematic approach to biomass beyond the energy sector. Biofuels Bioprod. Biorefin. 2013, 7, 193–206. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.U.; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect biorefinery: A green approach for conversion of crop residues into biodiesel and protein. Biotechnol. Biofuels 2017, 10, 1–13. [Google Scholar]
- Win, S.S.; Ebner, J.H.; Brownell, S.A.; Pagano, S.S.; Cruz-Diloné, P.; Trabold, T.A. Anaerobic digestion of black solider fly larvae (BSFL) biomass as part of an integrated biorefinery. Renew. Energy 2018, 127, 705–712. [Google Scholar] [CrossRef]
- Smetana, S.; Schmitt, E.; Mathys, A. Sustainable use of Hermetia illucens insect biomass for feed and food: Attributional and consequential life cycle assessment. Resour. Conserv. Recycl. 2019, 144, 285–296. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, 1–23. [Google Scholar] [CrossRef]
- Matthäus, B.; Piofczyk, T.; Katz, H.; Pudel, F. Renewable Resources from Insects: Exploitation, Properties, and Refining of Fat Obtained by Cold-Pressing from Hermetia illucens (Black Soldier Fly) Larvae. Eur. J. Lipid Sci. Technol. 2019, 121, 1–11. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sánchez-Muros, M.J.; Rincón, M.Á.; Rodriguez-Rodriguez, M.; Fabrikov, D.; Morote, E.; Guil-Guerrero, J.L. Production of n-3-rich insects by bioaccumulation of fishery waste. J. Food Compos. Anal. 2019, 82, 103237. [Google Scholar] [CrossRef]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Mai, H.C.; Dao, N.D.; Lam, T.D.; Nguyen, B.V.; Nguyen, D.C.; Bach, L.G. Purification Process, Physicochemical Properties, and Fatty Acid Composition of Black Soldier Fly (Hermetia illucens Linnaeus) Larvae Oil. J. Am. Oil Chem. Soc. 2019, 96, 1303–1311. [Google Scholar] [CrossRef]
- Delicato, C.; Schouteten, J.J.; Dewettinck, K.; Gellynck, X.; Tzompa-Sosa, D.A. Consumers’ perception of bakery products with insect fat as partial butter replacement. Food Qual. Prefer. 2020, 79, 103755. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wu, L.; Li, B.; Zhang, D. Reproductive potential and nutritional composition of hermetia illucens (Diptera: Stratiomyidae) prepupae reared on different organic wastes. J. Econ. Entomol. 2020, 113, 527–537. [Google Scholar] [CrossRef]
- Starcevic, K.; Lozica, L.; Gavrilovic, A.; Heruc, Z.; Masek, T. Fatty acid plasticity of black soldier fly (Hermetia illucens) larvae reared on alternative feeding media: Crude olive cake and processed animal protein. J. Anim. Feed Sci. 2019, 28, 374–382. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Giannetto, A.; Oliva, S.; Ceccon Lanes, C.F.; de Araújo Pedron, F.; Savastano, D.; Baviera, C.; Parrino, V.; Lo Paro, G.; Spanò, N.C.; Cappello, T.; et al. Hermetia illucens (Diptera: Stratiomydae) larvae and prepupae: Biomass production, fatty acid profile and expression of key genes involved in lipid metabolism. J. Biotechnol. 2020, 307, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Acuti, G.; Marangon, A.; Dalle Zotte, A. Black soldier fly as dietary protein source for broiler quails: Meat proximate composition, fatty acid and amino acid profile, oxidative status and sensory traits. Animal 2018, 12, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Leonhardt, L.; Kauppi, S.M.; Pajic, A.; Heinz, V. Insect margarine: Processing, sustainability and design. J. Clean. Prod. 2020, 264, 121670. [Google Scholar] [CrossRef]
- Kim, W.; Bae, S.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y. Biochemical characterization of digestive enzymes in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). J. Asia-Pac. Entomol. 2011, 14, 11–14. [Google Scholar] [CrossRef]
- Wang, T.; Shen, Q.; Feng, W.; Wang, C.; Yang, F. Aqueous ethyl acetate as a novel solvent for the degreasing of black soldier fly (Hermetia illucens L.) larvae: Degreasing rate, nutritional value evaluation of the degreased meal, and thermal properties. J. Sci. Food Agric. 2020, 100, 1204–1212. [Google Scholar] [CrossRef]
- Cullere, M.; Schiavone, A.; Dabbou, S.; Gasco, L.; Zotte, A.D. Meat quality and sensory traits of finisher broiler chickens fed with black soldier fly (Hermetia illucens L.) larvae fat as alternative fat source. Animals 2019, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Schiavone, A.; Dabbou, S.; De Marco, M.; Cullere, M.; Biasato, I.; Biasibetti, E.; Capucchio, M.T.; Bergagna, S.; Dezzutto, D.; Meneguz, M.; et al. Black soldier fly larva fat inclusion in finisher broiler chicken diet as an alternative fat source. Animal 2018, 12, 2032–2039. [Google Scholar] [CrossRef]
- Gasco, L.; Dabbou, S.; Gai, F.; Brugiapaglia, A.; Schiavone, A.; Birolo, M.; Xiccato, G.; Trocino, A. Quality and consumer acceptance of meat from rabbits fed diets in which soybean oil is replaced with black soldier fly and yellow mealworm fats. Animals 2019, 9, 629. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Dumas, A.; Raggi, T.; Barkhouse, J.; Lewis, E.; Weltzien, E. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 492, 24–34. [Google Scholar] [CrossRef]
- Belghit, I.; Waagbø, R.; Lock, E.J.; Liland, N.S. Insect-based diets high in lauric acid reduce liver lipids in freshwater Atlantic salmon. Aquac. Nutr. 2019, 25, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Sypniewski, J.; Kierończyk, B.; Benzertiha, A.; Mikołajczak, Z.; Pruszyńska-Oszmałek, E.; Kołodziejski, P.; Sassek, M.; Rawski, M.; Czekała, W.; Józefiak, D. Replacement of soybean oil by Hermetia illucens fat in turkey nutrition: Effect on performance, digestibility, microbial community, immune and physiological status and final product quality. Br. Poult. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Heugten, E.V.; Martinez, G.; McComb, A.; Koutsos, E. 285 Black soldier fly (Hermetia illucens) larvae oil improves growth performance of nursery pigs. J. Anim. Sci. 2019, 97 (Suppl. 3), 118. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Li, S.Y.; Su, C.H.; Chien, C.C.; Chen, Y.J.; Huong, D.T.M. Direct transesterification of black soldier fly larvae (Hermetia illucens) for biodiesel production. J. Taiwan Inst. Chem. Eng. 2018, 85, 165–169. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Q.; Zhang, J.; Yu, Z. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- Su, C.; Nguyen, H.C.; Bui, T.L.; Huang, D. Enzyme-assisted extraction of insect fat for biodiesel production. J. Clean. Prod. 2019, 223, 436–444. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Doan, T.T.; Su, C.H.; Yang, P.C. Lipase-catalyzed synthesis of biodiesel from black soldier fly (Hermetica illucens): Optimization by using response surface methodology. Energy Convers. Manag. 2017, 145, 335–342. [Google Scholar] [CrossRef]
- Manzano-Agugliaro, F.; Sanchez-Muros, M.J.; Barroso, F.G.; Martínez-Sánchez, A.; Rojo, S.; Pérez-Bañón, C. Insects for biodiesel production. Renew. Sustain. Energy Rev. 2012, 16, 3744–3753. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, G.R.; Ooms, T.; Vogels, L.; Vreysen, S.; Bovy, A.; Van Miert, S.; Meersman, F. Insects as an alternative source for the production of fats for cosmetics. J. Cosmet. Sci. 2018, 69, 187–202. [Google Scholar]
- Sangduan, C.; Sai, S. Skincare Products Containing Hermetia illucens Extract. US Patent 2018/0256483 A1, 13 September 2018. [Google Scholar]
- Zoller, U.; Sosis, P. Handbook of Detergents, Part F: Production; CRC Press, Taylor and Francis Group: New York, NY, USA, 2008. [Google Scholar]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; Van Huis, A.; Boekel, M.A.J.S.V. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Boekel, M.A.J.S.; Lakemond, C.M.M. Extracting Tenebrio molitor protein while preventing browning: Effect of pH and NaCl on protein yield. J. Insects Food Feed 2017, 3, 21–31. [Google Scholar] [CrossRef]
- Sugumaran, M. Comparative Biochemistry of Eumelanogenesis and the Protective Roles of Phenoloxidase and Melanin in Insects. Pigment Cell Res. 2002, 15, 2–9. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Lakemond, C.M.M.; Fogliano, V. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Cummins, V.C.; Rawles, S.D.; Thompson, K.R.; Velasquez, A.; Kobayashi, Y.; Hager, J.; Webster, C.D. Evaluation of black soldier fly (Hermetia illucens) larvae meal as partial or total replacement of marine fish meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2017, 473, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiab, K.K.M.; Subramanian, S.; Ekesi, S.; Van Huis, A.; Borgemeister, C. The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Fisher, H.J.; Collins, S.A.; Hanson, C.; Mason, B.; Colombo, S.M.; Anderson, D.M. Black soldier fly larvae meal as a protein source in low fish meal diets for Atlantic salmon (Salmo salar). Aquaculture 2020, 521, 734978. [Google Scholar] [CrossRef]
- Ullah, Z.; Ahmed, G.; Nisa, M.; Sarwar, M. Standardized Ileal amino acid digestibility of commonly used feed ingredients in growing broilers. Asian-Australas. J. Anim. Sci. 2016, 29, 1322–1330. [Google Scholar] [CrossRef] [Green Version]
- Mwaniki, Z.N.; Kiarie, E. Standardized ileal digestible amino acids and apparent metabolizable energy content in defatted black soldier fly larvae meal fed to broiler chickens. Can. J. Anim. Sci. 2019, 99, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Schiavone, A.; De Marco, M.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F.; et al. Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: Apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 1–9. [Google Scholar]
- Do, S.; Koutsos, L.; Utterback, P.; Parsons, C.; Godoy, M.; Swanson, K. Nutrient and AA digestibility of black soldier fly larvae differing in age using the precision-fed cecectomized rooster assay. J. Anim. Sci. 2020, 98, skz363. [Google Scholar] [CrossRef]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Rabani, V.; Cheatsazan, H.; Davani, S. Proteomics and Lipidomics of Black Soldier Fly (Diptera: Stratiomyidae) and Blow Fly (Diptera: Calliphoridae) Larvae. J. Insect Sci. 2019, 19, 29. [Google Scholar] [CrossRef]
- Miles, R.D.; Chapman, F.A. The Benefits of Fish Meal in Aquaculture Diets; IFAS Extension; University of Florida: Gainesville, FL, USA, 2006. [Google Scholar]
- Bosch, G.; Zhang, S.; Oonincx, D.G.A.B.; Hendriks, W.H. Protein quality of insects as potential ingredients for dog and cat foods. J. Nutr. Sci. 2014, 3, 1–4. [Google Scholar]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.H. Recent advances in role of insects as alternative protein source in poultry nutrition. J. Appl. Anim. Res. 2018, 46, 1144–1157. [Google Scholar] [CrossRef] [Green Version]
- De Souza-Vilela, J.; Andrew, N.R.; Ruhnke, I. Insect protein in animal nutrition. Anim. Prod. Sci. 2019, 59, 2029–2036. [Google Scholar] [CrossRef]
- Nogales-Mérida, S.; Gobbi, P.; Józefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kierończyk, B.; Józefiak, A. Insect meals in fish nutrition. Rev. Aquac. 2019, 11, 1080–1103. [Google Scholar] [CrossRef]
- Ferrer Llagostera, P.; Kallas, Z.; Reig, L.; Amores de Gea, D. The use of insect meal as a sustainable feeding alternative in aquaculture: Current situation, Spanish consumers’ perceptions and willingness to pay. J. Clean. Prod. 2019, 229, 10–21. [Google Scholar] [CrossRef]
- Gasco, L.; Biasato, I.; Dabbou, S.; Schiavone, A.; Gai, F. Animals fed insect-based diets: State-of-the-art on digestibility, performance and product quality. Animals 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Abdel-Latif, H.M.R. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Li, Y.; Kortner, T.M.; Chikwati, E.M.; Belghit, I.; Lock, E.J.; Krogdahl, Å. Total replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal does not compromise the gut health of Atlantic salmon (Salmo salar). Aquaculture 2020, 520, 734967. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Truzzi, C.; Giorgini, E.; Marcellucci, C.; Vargas-Abúndez, J.A.; Zimbelli, A.; Annibaldi, A.; Parisi, G.; Tulli, F.; et al. A six-months study on Black Soldier Fly (Hermetia illucens) based diets in zebrafish. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Alam, M.R.; Scampicchio, M.; Angeli, S.; Ferrentino, G. Effect of hot melt extrusion on physical and functional properties of insect based extruded products. J. Food Eng. 2019, 259, 44–51. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Hahn, T.; Zibek, S. Sewage polluted water treatment via chitosan: A review. In Chitin—Chitosan Myriad Functionalities in Science and Technology; Dongre, R., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Stegmaier, T.; Wunderlich, W.; Hager, T.; Siddique, A.B.; Sarsour, J.; Planck, H. Chitosan—A sizing agent in fabric production—Development and ecological evaluation. Clean SoilAirWater 2008, 36, 279–286. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, D.; Sarkar, S. Physicochemical Structure Analysis of Chitin Extracted from Pupa Exuviae and Dead Imago of Wild Black Soldier Fly (Hermetia illucens). J. Polym. Environ. 2020, 28, 445–457. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Ji, R.; Schmitt, E.; Zibek, S. Chitosan production with larval exoskeletons derived from the insect protein production. J. Biotechnol. 2020, 310, 62–67. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Yan, T.; Wang, X.; Huang, J.; Kuang, Z.; Ye, M.; Pan, M. Selectivity of deproteinization and demineralization using natural deep eutectic solvents for production of insect chitin (Hermetia illucens). Carbohydr. Polym. 2019, 225, 115255. [Google Scholar] [CrossRef]
- Wang, H.; ur Rehman, K.; Feng, W.; Yang, D.; ur Rehman, R.; Cai, M.; Zhang, J.; Yu, Z.; Zheng, L. Physicochemical structure of chitin in the developing stages of black soldier fly. Int. J. Biol. Macromol. 2020, 149, 901–907. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Ottenheim, C.; Phua, J.W.; Caligiani, A.; Dritsas, S.; Fernandez, J.G. Circular manufacturing of chitinous bio-composites via bioconversion of urban refuse. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- De Souza, P.R.; do Carmo Ribeiro, T.M.; Lôbo, A.P.; Tokumoto, M.S.; de Jesus, R.M.; Lôbo, I.P. Removal of bromophenol blue anionic dye from water using a modified exuviae of Hermetia illucens larvae as biosorbent. Environ. Monit. Assess. 2020, 192, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Irungu, F.G.; Mutungi, C.M.; Faraj, A.K.; Affognon, H.; Tanga, C.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Minerals content of extruded fish feeds containing cricket (Acheta domesticus) and black soldier fly larvae (Hermetia illucens) fractions. Int. Aquat. Res. 2018, 10, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.C. Insect Diets: Science and Technology; CRC Press: London, UK, 2003. [Google Scholar]
- Schmitt, E.; Belghit, I.; Johansen, J.; Leushuis, R.; Lock, E.J.; Melsen, D.; Ramasamy Shanmugam, R.K.; Van Loon, J.; Paul, A. Growth and safety assessment of feed streams for black soldier fly larvae: A case study with aquaculture sludge. Animals 2019, 9, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.S.; Park, S.I. Novel attacin from Hermetia illucens: cDNA cloning, characterization, and antibacterial properties. Prep. Biochem. Biotechnol. 2019, 49, 279–285. [Google Scholar] [CrossRef]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperstad, S.V.; Haug, T.; Paulsen, V.; Rode, T.M.; Strandskog, G.; Solem, S.T.; Styrvold, O.B.; Stensvåg, K. Characterization of crustins from the hemocytes of the spider crab, Hyas araneus, and the red king crab, Paralithodes camtschaticus. Dev. Comp. Immunol. 2009, 33, 583–591. [Google Scholar] [CrossRef]
- Choi, W.H.; Yun, J.H.; Chu, J.P.; Chu, K.B. Antibacterial effect of extracts of hermetia illucens (diptera: Stratiomyidae) larvae against gram-negative bacteria. Entomol. Res. 2012, 42, 219–226. [Google Scholar] [CrossRef]
- Alvarez, D.; Wilkinson, K.A.; Treilhou, M.; Téné, N.; Castillo, D.; Sauvain, M. Prospecting Peptides Isolated from Black Soldier Fly (Diptera: Stratiomyidae) with Antimicrobial Activity against Helicobacter pylori (Campylobacterales: Helicobacteraceae). J. Insect Sci. 2019, 19, 1–5. [Google Scholar] [CrossRef]
- Choi, W.H.; Choi, H.; Goo, T.W.; Quan, F. Novel antibacterial peptides induced by probiotics in Hermetia illucens (Diptera: Stratiomyidae) larvae. Entomol. Res. 2018, 48, 237–247. [Google Scholar] [CrossRef]
- Park, S.I.; Yoe, S.M. A novel cecropin-like peptide from black soldier fly, Hermetia illucens: Isolation, structural and functional characterization. Entomol. Res. 2017, 47, 115–124. [Google Scholar] [CrossRef]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2018, 78, 141–148. [Google Scholar] [CrossRef]
- Elhag, O.; Zhou, D.; Song, Q.; Soomro, A.A.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Screening, expression, purification and functional characterization of novel antimicrobial peptide genes from Hermetia illucens (L.). PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Huang, X.; Tu, F.; Wang, C.; Yang, F. Preparation, antioxidant activity evaluation, and identification of antioxidant peptide from black soldier fly (Hermetia illucens L.) larvae. J. Food Biochem. 2020, 44, 1–9. [Google Scholar] [CrossRef]
- Schmitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; van Loon, J.J.; Dicke, M. Insects for sustainable animal feed: Inclusive business models involving smallholder farmers. Curr. Opin. Environ. Sustain. 2019, 41, 23–30. [Google Scholar] [CrossRef]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm Frass as a Potential Biofertilizer and Abiotic Stress Tolerance-Inductor in Plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Pakpahan, A.; Widowati, R.; Suryadinata, A. Black soldier fly liquid biofertilizer in Bunga Mayang sugarcane plantation: From experiment to policy implications. MOJ Eco Environ. Sci. 2020, 5, 89–98. [Google Scholar]
- Wu, X.; Cai, R.; Wang, X.; Wu, N.; Xu, X. Study on Effects of Black Soldier Fly Feces on Rice Growth. IOP Conf. Ser. Earth Environ. Sci. 2020, 450, 012099. [Google Scholar] [CrossRef]
- Alattar, M.; Alattar, F.; Popa, R. Effects of microaerobic fermentation and black soldier fly larvae food scrap processing residues on the growth of corn plants (Zea mays). Plant. Sci. Today 2016, 3, 57–62. [Google Scholar] [CrossRef]
- Dortmans, B.M.A.; Diener, S.; Verstappen, B.M.; Zurbrügg, C. Black Soldier Fly Biowaste Processing—A Step-by-Step Guide; Eawag, Swiss Federal Institute of Aquatic Science and Technology: Dübendorf, Switzerland, 2017. [Google Scholar]
- Meneguz, M.; Gasco, L.; Tomberlin, J.K. Impact of pH and feeding system on black soldier fly (Hermetia illucens, L.; Diptera: Stratiomyidae) larval development. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.M.; Niassy, S.; Van Loon, J.J.A.; Dicke, M.; et al. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS ONE 2018, 13, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, L.A.; Vanlaerhoven, S.L.; Tomberlin, J.K. Relative Humidity Effects on the Life History of Hermetia illucens (Diptera: Stratiomyidae). Environ. Entomol. 2012, 41, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Cammack, J.A.; Tomberlin, J.K. The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Purschke, B.; Scheibelberger, R.; Axmann, S.; Adler, A.; Jäger, H. Impact of substrate contamination with mycotoxins, heavy metals and pesticides on the growth performance and composition of black soldier fly larvae (Hermetia illucens) for use in the feed and food value chain. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 1410–1420. [Google Scholar] [CrossRef]
- Somroo, A.A.; ur Rehman, K.; Zheng, L.; Cai, M.; Xiao, X.; Hu, S.; Mathys, A.; Gold, M.; Yu, Z.; Zhang, J. Influence of Lactobacillus buchneri on soybean curd residue co-conversion by black soldier fly larvae (Hermetia illucens) for food and feedstock production. Waste Manag. 2019, 86, 114–122. [Google Scholar] [CrossRef]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Wong, C.Y.; Lim, J.W.; Chong, F.K.; Lam, M.K.; Uemura, Y.; Tan, W.N.; Bashir, M.J.K.; Lam, S.M.; Sin, J.C.; Lam, S.S. Valorization of exo-microbial fermented coconut endosperm waste by black soldier fly larvae for simultaneous biodiesel and protein productions. Environ. Res. 2020, 185, 109458. [Google Scholar] [CrossRef]
- Hoc, B.; Noël, G.; Carpentier, J.; Francis, F.; Megido, R.C. Optimization of black soldier fly (Hermetia illucens) artificial reproduction. PLoS ONE 2019, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Heussler, C.D.; Walter, A.; Oberkofler, H.; Insam, H.; Arthofer, W.; Schlick-Steiner, B.C.; Steiner, F.M. Correction: Influence of three artificial light sources on oviposition and half-life of the Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae): Improving small-scale indoor rearing. PLoS ONE 2019, 14, 40–45. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Volk, N.; Diehl, J.J.E.; van Loon, J.J.A.; Belušič, G. Photoreceptor spectral sensitivity of the compound eyes of black soldier fly (Hermetia illucens) informing the design of LED-based illumination to enhance indoor reproduction. J. Insect Physiol. 2016, 95, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.C. Effects of light intensity on mating of the black soldier fly (Hermetia illucens, Diptera: Stratiomyidae). J. Insects Food Feed 2019, 6, 111–119. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No. 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No. 1774/2002 (Animal by-products Regulation). O. J. L 2009, 300/1, 1–33. [Google Scholar]
- Pinotti, L.; Giromini, C.; Ottoboni, M.; Tretola, M.; Marchis, D. Review: Insects and former foodstuffs for upgrading food waste biomasses/streams to feed ingredients for farm animals. Animal 2019, 13, 1365–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Fels-Klerx, H.J.; Camenzuli, L.; Belluco, S.; Meijer, N.; Ricci, A. Food Safety Issues Related to Uses of Insects for Feeds and Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1172–1183. [Google Scholar] [CrossRef] [Green Version]
- Proc, K.; Bulak, P.; Wiącek, D.; Bieganowski, A. Hermetia illucens exhibits bioaccumulative potential for 15 different elements—Implications for feed and food production. Sci. Total Environ. 2020, 723, 138125. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; De Rijk, T.C.; Oonincx, D.G.A.B. Aflatoxin B1 Tolerance and Accumulation in Black Soldier Fly Larvae (Hermetia illucens) and Yellow Mealworms (Tenebrio molitor). Toxins 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Camenzuli, L.; Van Dam, R.; De Rijk, T.; Andriessen, R.; Van Schelt, J.; Van der Fels-Klerx, H.J.I. Tolerance and Excretion of the Mycotoxins Aflatoxin B1, Zearalenone, Deoxynivalenol, and Ochratoxin A by Alphitobius diaperinus and Hermetia illucens from Contaminated Substrates. Toxins 2018, 10, 91. [Google Scholar] [CrossRef] [Green Version]
- Konietzny, U.; Greiner, R. Phytic Acid: Nutritional Impact. In Encyclopedia of Food Science and Nutrition; Caballero, B., Trugo, L., Finglas, P., Eds.; Elsevier: London, UK, 2003; pp. 4555–4563. [Google Scholar]
- Gasco, L.; Acuti, G.; Bani, P.; Dalle Zotte, A.; Danieli, P.P.; De Angelis, A.; Fortina, R.; Marino, R.; Parisi, G.; Piccolo, G.; et al. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Mancini, L.; Vidal Legaz, B.; Vizzarri, M.; Wittmer, D.; Grassi, G.; Pennington, D. Mapping the Role of Raw Materials in Sustainable Development Goals. A Preliminary Analysis of Links, Monitoring Indicators, and Related Policy Initiatives; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]














| Reference | [49] | [42] | [50] | [50] | [50] | [50] | [43] | [51] | [52] | [9] | [53] | [54] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid (%)/Substrate | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S1 | S10 | S11 | |
| C12 (Lauric) a | 47 | 51.8 | 56.24 | 56.41 | 56.94 | 54.18 | 40.6 | 23.9 | 11.85 | 61.4 | 42.27 | 28.1 | 42.8 |
| C14 (Myristic) a | 6.5 | 9.5 | 9.27 | 8.46 | 8.19 | 7.05 | 8.5 | 6.7 | 2.09 | 10.2 | 9.41 | 3.85 | 8.12 |
| C16 (Palmitic) a | 15 | 12.7 | 10.26 | 11.05 | 8.36 | 7.95 | 14.8 | 16.6 | 12.69 | 7.8 | 13.91 | 5.78 | 13.9 |
| C18:1n9 (Oleic) b | 14 | 12 | 7.06 | 7.15 | 6.97 | 6.35 | 8.8 | 17.9 | 54.12 | 7.8 | 11.84 | 4.27 | 10.4 |
| C16:1(Palmitoleic) b | 3.1 | 2.8 | 2.37 | 2.24 | 1.95 | 2.06 | 2 | 2.5 | 1.26 | 2.5 | 2.73 | 1.65 | 2.06 |
| C18:2n6 (Linoleic) c | 9.4 | 7.7 | 10.26 | 10.18 | 10.74 | 10.94 | 17.9 | 18.6 | 12.29 | 7.2 | 14.29 | 1.27 | 12.6 |
| C18:3n3 (Linolenic) c | 0.8 | 1.6 | - | - | - | - | 1.4 | 1.6 | 0.44 | 0.4 | 1.41 | 10.3 | 1.16 |
| Σ Fatty acids | 95.8 | 98.1 | 95.46 | 95.49 | 93.15 | 88.53 | 94 | 87.8 | 94.74 | 97.3 | 95.86 | 55.22 | 91.04 |
| Crude lipid | NA | 57.8 | 37.24 | 36.52 | 36.18 | 35.73 | 33.8 | 8.1 | NA | 28.4 | 32.51 | 32.97 | NA |
| Biodiesel Properties | Units | EN14214 | [66] | [72] | [68] | [69] | [70] |
|---|---|---|---|---|---|---|---|
| Density | Kg/m3 | 860–900 | 885 | 872 | 860 | 875 | 872 |
| Viscosity at 40 °C | mm2/s | 1.9–6.0 | 5.8 | 4.5 | 4.9 | 5.3 | 5.2 |
| Sulfur content | wt.% | 0.05 | NR | NR | ND | 0.04 | ND |
| Ester content | % | 96.5 | 97.2 | 97.2 | 96.9 | 98.7 | 98.3 |
| Water content | mg/kg | <0.03 | 0.03 | NR | 0.02 | NR | 0.03 |
| Flash point | °C | 120 | 123 | 121 | 128 | 121 | 121 |
| Cetane index | - | 48–60 | 53 | NR | 58 | 50 | 50 |
| Acid number | mg KOH/g | <0.8 | 1.1 | 0.8 | 0.6 | <0.5 | <0.8 |
| Reference | [49] | [50] | [50] | [50] | [50] | [84] | [84] | [84] | [44] | [44] | [52] | [54] | [78] | [85] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | S1 | S2 | S3 | S4 | S5 | S4 | S6 | S7 | S8 | S9 | S1 | S10 | S10 | S10 |
| EAA | g/kg CP | g/kg DM | mg/g DM | % CP | g/kg DM | % BSFM | % DM | % BSFM | ||||||
| Arginine | 57.1 | 20.9 | 21.1 | 20.6 | 20.1 | 1.1 | 5 | 2.5 | 4.5 | 6.5 | 20.5 | 1.64 | 1.96 | 3 |
| Histidine | 33.4 | 14.2 | 13.9 | 13.9 | 13 | 3.5 | 3.3 | 4.7 | 2.8 | 2.7 | 31.6 | 0.47 | 1.17 | 1.9 |
| Isoleucine | 51.9 | 18.4 | 18.3 | 17.7 | 17.5 | 1.6 | 2.6 | 1.8 | 3.9 | 3.8 | 15.6 | 2.24 | 1.47 | 2.2 |
| Leucine | 86.3 | 28.6 | 28.1 | 28.1 | 27.4 | 3 | 2.9 | 3.7 | 6.4 | 6.2 | 27.1 | 3.3 | 2.7 | 4.2 |
| Lysine | 71.2 | 24 | 24.6 | 24.1 | 23.4 | 4.1 | 4.7 | 4.7 | 6.2 | 5.6 | 23.2 | 1.96 | 2.3 | 3.8 |
| Methionine | 21.8 | 8 | 8.1 | 7.9 | 7.5 | 6.1 | 7.9 | 7.4 | 1.7 | 1.4 | 22.4 | 0.62 | 0.6 | 1.2 |
| Phenylalanine | 38.9 | 18.1 | 18.5 | 18.3 | 17.2 | 1.9 | 4.6 | 2.4 | 4 | 3.2 | 18.6 | 1.69 | 1.3 | 2.3 |
| Threonine | 46.4 | 19.1 | 19.5 | 18.4 | 19.2 | - | - | - | 3.9 | 3.9 | 18.4 | 1.93 | 1.49 | 2.4 |
| Valine | 72.1 | 19.7 | 19.5 | 18.4 | 19.2 | 7.2 | 1.2 | 9.3 | 5.8 | 5.5 | 18.7 | 3.58 | 2.4 | 3.7 |
| Crude protein | 476 | 436.9 | 428.3 | 417.1 | 412.5 | 411 | 330 | 413 | 40 | 41.3 | 39.2 | 43.4 | 32 | 56.01 |
| Crude lipid | 118 | 372.4 | 365.2 | 361.8 | 357.3 | 301 | 343 | 310 | 33.8 | 8.1 | 28.4 | NA | 37.1 | 11.81 |
| Essential Amino Acids | Amino Acid Digestibility of BSFL Meals | ||||
|---|---|---|---|---|---|
| Reference | A [88] | B [88] | C [89] | D [90] | E [62] |
| Arginine | 0.79 | 0.8 | 0.91 | 0.83 | 0.92 |
| Histidine | 0.64 | 0.63 | 0.83 | 0.81 | 0.89 |
| Isoleucine | 0.83 | 0.87 | 0.87 | 0.45 | 0.88 |
| Leucine | 0.84 | 0.89 | 0.86 | 0.76 | 0.84 |
| Lysine | 0.8 | 0.8 | 0.9 | 0.46 | 0.9 |
| Methionine | 0.83 | 0.78 | 0.93 | 0.42 | 0.85 |
| Phenylalanine | 0.82 | 0.86 | 0.9 | 0.63 | 0.86 |
| Threonine | 0.73 | 0.77 | 0.88 | 0.75 | 0.87 |
| Tryptophan | - | - | 0.93 | - | 0.96 |
| Valine | 0.9 | 0.91 | 0.75 | 0.64 | - |
| Mean | 0.8 | 0.81 | 0.88 | 0.64 | 0.89 |
| Reference | [50] | [50] | [50] | [50] | [84] | [84] | [84] | [118] | [43] | [43] | [116] | [54] | [85] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | S1 | S2 | S3 | S4 | S3 | S5 | S6 | S7 | S8 | S9 | S10 | S10 | S10 |
| Minerals | g/kg dry matter | ||||||||||||
| Ca (Calcium) | 19.48 | 23.71 | 21.94 | 34.71 | 3.2 | 2 | 1.7 | 0.08 | 8.4 | 30 | 1.97 | 9.77 | 27.6 |
| K (Potassium) | 6.17 | 6.04 | 6.71 | 7.53 | 4.9 | 5.7 | 4.4 | 9.9 | 10.2 | 21.3 | 13.85 | 11.54 | 14 |
| P (Phosphorus) | 3.62 | 3.94 | 4.14 | 4.76 | 3.9 | 4.1 | 4.6 | 19.2 | 6.8 | 11.3 | 8.58 | 8.33 | 8.6 |
| Mg (Magnesium) | 2.71 | 2.35 | 2.82 | 2.11 | 4 | 3.3 | 3.5 | 4.1 | 2.1 | 6.2 | 3.54 | 2.51 | 3.9 |
| Fe (Iron) | 0.42 | 0.36 | 0.28 | 0.39 | 0.6 | 2.2 | 0.3 | 0.5 | 0.21 | 0.35 | 0.85 | 0.3 | - |
| Na (Sodium) | 0.59 | 0.51 | 0.47 | 0.39 | 2.4 | 2 | 2.6 | 2.2 | 1 | 12.3 | 22.55 | 1.81 | 1.9 |
| Mn (Manganese) | 0.13 | 0.17 | 0.16 | 0.2 | 1.4 | 0.9 | 1.1 | 0.2 | 0.19 | 0.17 | 1.76 | 0.17 | 0.26 |
| Zn (Zinc) | 0.1 | 0.11 | 0.14 | 0.18 | 0.3 | 0.3 | 0.3 | 0.3 | 0.07 | 0.15 | 0.58 | 0.1 | 0.14 |
| Cu (Copper) | 0.01 | 0.01 | 0.01 | 0.01 | 0.4 | 0.2 | 0.5 | - | 0.01 | 0.01 | 1.61 | 0.02 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravi, H.K.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Larvae Mediated Valorization of Industrial, Agriculture and Food Wastes: Biorefinery Concept through Bioconversion, Processes, Procedures, and Products. Processes 2020, 8, 857. https://doi.org/10.3390/pr8070857
Ravi HK, Degrou A, Costil J, Trespeuch C, Chemat F, Vian MA. Larvae Mediated Valorization of Industrial, Agriculture and Food Wastes: Biorefinery Concept through Bioconversion, Processes, Procedures, and Products. Processes. 2020; 8(7):857. https://doi.org/10.3390/pr8070857
Chicago/Turabian StyleRavi, Harish Karthikeyan, Antoine Degrou, Jérôme Costil, Christophe Trespeuch, Farid Chemat, and Maryline Abert Vian. 2020. "Larvae Mediated Valorization of Industrial, Agriculture and Food Wastes: Biorefinery Concept through Bioconversion, Processes, Procedures, and Products" Processes 8, no. 7: 857. https://doi.org/10.3390/pr8070857